Cory:
Unlock Your AI Assistant Now!
Abstract
This manuscript proposes a novel implanter classification system for left atrial appendage (LAA) closure, aimed at overcoming the limitations of current anatomical classifications. By integrating essential anatomical and functional details, this new classification system strives to provide a comprehensive framework that is both user-friendly and effective in distinguishing between complex and standard LAA anatomies, facilitating a common language among implanters and imagers, and predicting procedural risks.
Over the last two decades, the field of transcatheter left atrial appendage (LAA) closure (LAAC) has evolved tremendously, with marked improvements in safety, efficacy, and device iterations1234567. As such, LAAC is becoming a pivotal intervention for stroke prevention, and was recently upgraded in the 2023 ACC/AHA/ACCP/HRS atrial fibrillation (AF) guidelines, moving from a Class 2b to a Class 2a indication for patients with a moderate-to-high risk of stroke and a contraindication to long-term oral anticoagulation (OAC), based on the accumulation of data supporting the efficacy and safety of the procedure8. However, LAAC remains challenging because of the variability of LAA anatomies, which necessitates accurate classification to guide clinicians for optimal procedural planning and risk assessment. In this setting, the current anatomical classifications of LAA into different types (broccoli, chicken wing, cactus and windsock) provide a visual understanding of LAA morphology but fall short in several clinical and procedural aspects910. Indeed, while these categories are helpful to the initial assessment, they lack specificity in predicting procedural complexities and potential complications such as peridevice leaks (PDL), device-related thrombus (DRT), device embolisation, and other procedural challenges. This clinical gap underscores the need for a novel and more pragmatic implanter classification system integrating essential anatomical and functional details that influence device choice, procedural planning and risk management.
Methods
Recognising the limitations of existing anatomical classifiÂcations, the board members of the European Left Atrial Appendage Closure Club (ELAACC) initiated a project to develop a novel and more pragmatic classification system. This new system aimed to be comprehensive, easily integrable into daily practice, and useful for distinguishing between standard and complex LAAC cases, as well as for anticipating various acute and long-term procedural complications.
Criteria proposal and initial feedback
The initial phase involved soliciting input on relevant anatomical and functional criteria from the board members (J.E. Nielsen-Kudsk, A. Aminian, O. De Backer, X. Iriart, S. Berti, R. Galea, X. Freixa, L. Räber, I. Cruz-Gonzalez, N.C. Wunderlich, P. Garot), who have developed extensive expertise in procedural and imaging aspects of LAAC. This consultative approach ensured that the proposed criteria were grounded in current clinical practice and reflected a wide range of physician experiences and perspectives.
The proposed criteria were first presented and discussed during the inaugural ELAACC meeting held in Barcelona in February 2024. The meeting served as a platform for robust discussion among practitioners and experts in the field. Feedback from this meeting highlighted the necessity for the classification system to focus on procedural determinants and be simple and practical at the same time. Opinions varied between the adoption of a numerical scoring system and a colour-coded system ranging from standard to complex, thus reflecting the diverse needs and preferences of practitioners.
Development and voting process
To refine the classification system, numerous consensus meetings were conducted within the board. These discussions aimed to balance scientific rigour with practical usability in clinical settings. Each criterion proposed during the initial phase was thoroughly debated, considering its relevance to procedural success and potential complications.
A pragmatic approach was then adopted to decide on the inclusion of each criterion in the new classification system. A voting process was implemented wherein each board member could vote “yes” or “no” for each proposed criterion. A criterion was included in the final classification system only if it received affirmative votes from more than 50% of the board members. This method ensured that the final classification system was representative of a consensus among experts. The voting results led to the formulation of the current ELAAC classification system, which categorises LAA anatomies based on their complexity and presumed associated procedural risks.
ELAAC classification system
The proposed “ELAAC” classification system is based on five key LAA parameters: Entrance/ostium, Landing zone, overall Anatomy, Axis/orientation and Contraction.
Each key parameter contains one or several key characteristics, which are described below with their potential implications during LAAC.
Entrance/ostium
The LAA ostium is traditionally the plane where the appendage opens into the left atrium. Here, we define the LAA ostium by a virtual line connecting the inferior LAA margin, proximal to the left circumflex (LCx) artery and above the mitral valve annulus, to the superior LAA margin, with the ostial plane being perpendicular to the central axis of the LAA neck. On a three-dimensional (3D) multiplanar reconstruction axial view, the LAA ostium may or may not include the left upper pulmonary vein (LUPV) ridge. Importantly, the LAA working depth, the pulmonary ridge (PR) distance and the presence of an early sharp bend must be assessed from the predefined LAA ostium (Figure 1).
The classification system identifies three key characteristics: the presence of a large ostium diameter, a long pulmonary vein ridge and a low ostium position (Figure 2).
1) A large ostium is characterised by a diameter >35 mm. Implication: the presence of a large ostium poses significant challenges for device sizing and positioning given the available range of device sizes. Devices that are undersized may fail to achieve proper LAA seal, resulting in PDL, which is associated with an increased risk of thromboembolism1112. In addition, the selection of an inadequately oversized device at the landing zone to properly cover the large ostial area may cause excessive tissue stress and can increase the risk of pericardial effusion.
2) A long pulmonary ridge is defined as extending more than 10 mm from the ostium plane. Implication: failure to cover the pulmonary ridge will result in the creation of a residual area and a neocavity in the proximal LAA between the upper edge of the closure device and the ridge, favouring flow turbulence, low shear stress and blood stasis, thereby increasing the risk of DRT formation1314. The implant depth can be assessed by measuring the distance between the pulmonary ridge and the upper edge of the occluder device. Of note, increasing implantation depths are associated with higher DRT rates1516. The presence of a long pulmonary ridge can also result in an “unclear/not well-defined” LAA ostium delineation, where operators must rather rely on the LCx position as the main anatomical landmark to secure device positioning.
3) A low ostium is defined as an inferior LAA margin positioned less than 5 mm from the mitral valve annulus. Implication: this anatomical variation may result in potential device interference with surrounding structures (mainly the mitral valve/annulus) and difficulties in achieving optimal device alignment. It is frequently associated with misalignment of the delivery sheath with the LAA, leading to impaired coaxiality between the device and the orientation of the LAA.
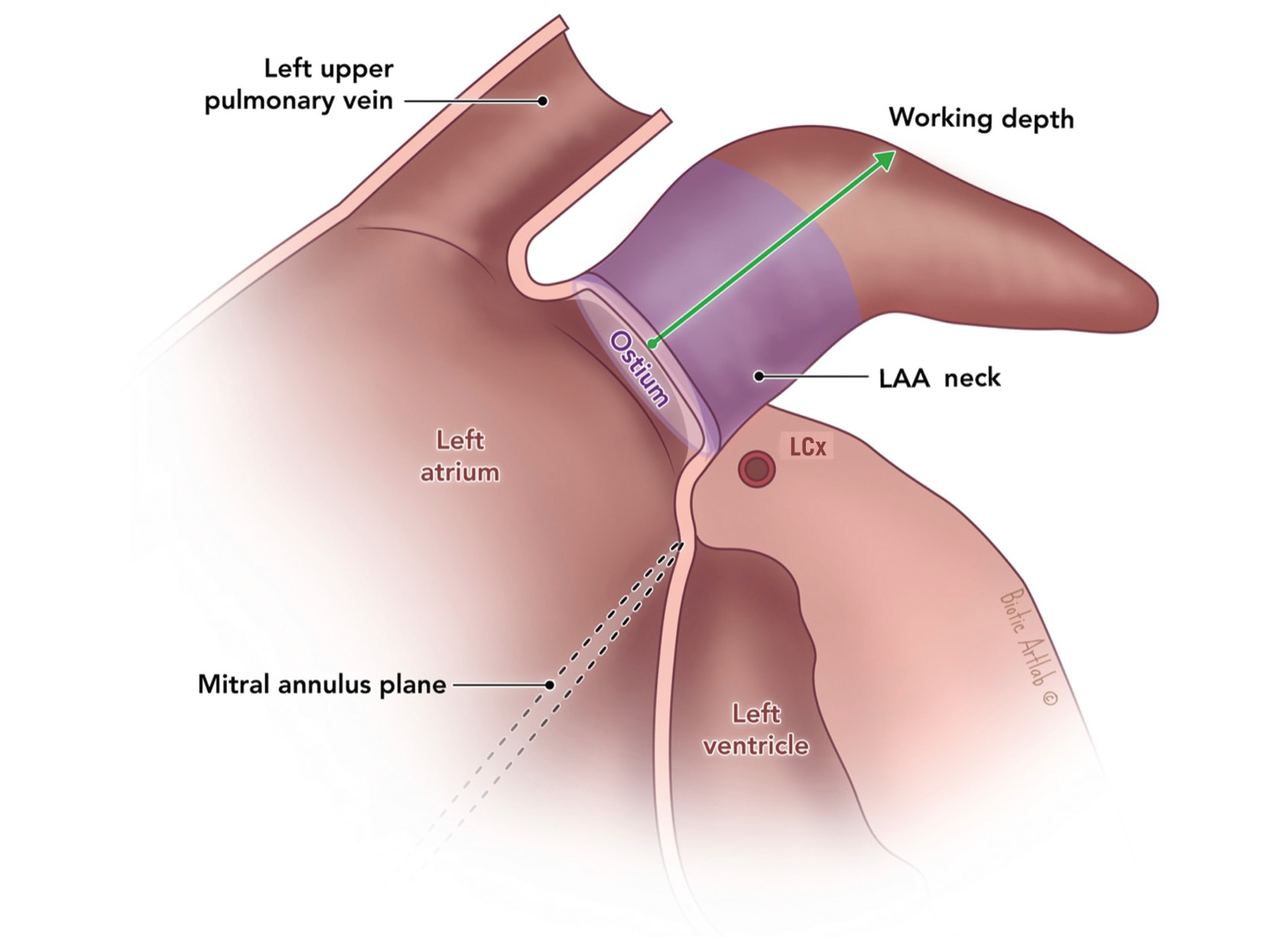
Figure 1. Left atrial appendage – anatomy. Reproduced with permission from Biotic Artlab. LAA: left atrial appendage; LCx: left circumflex
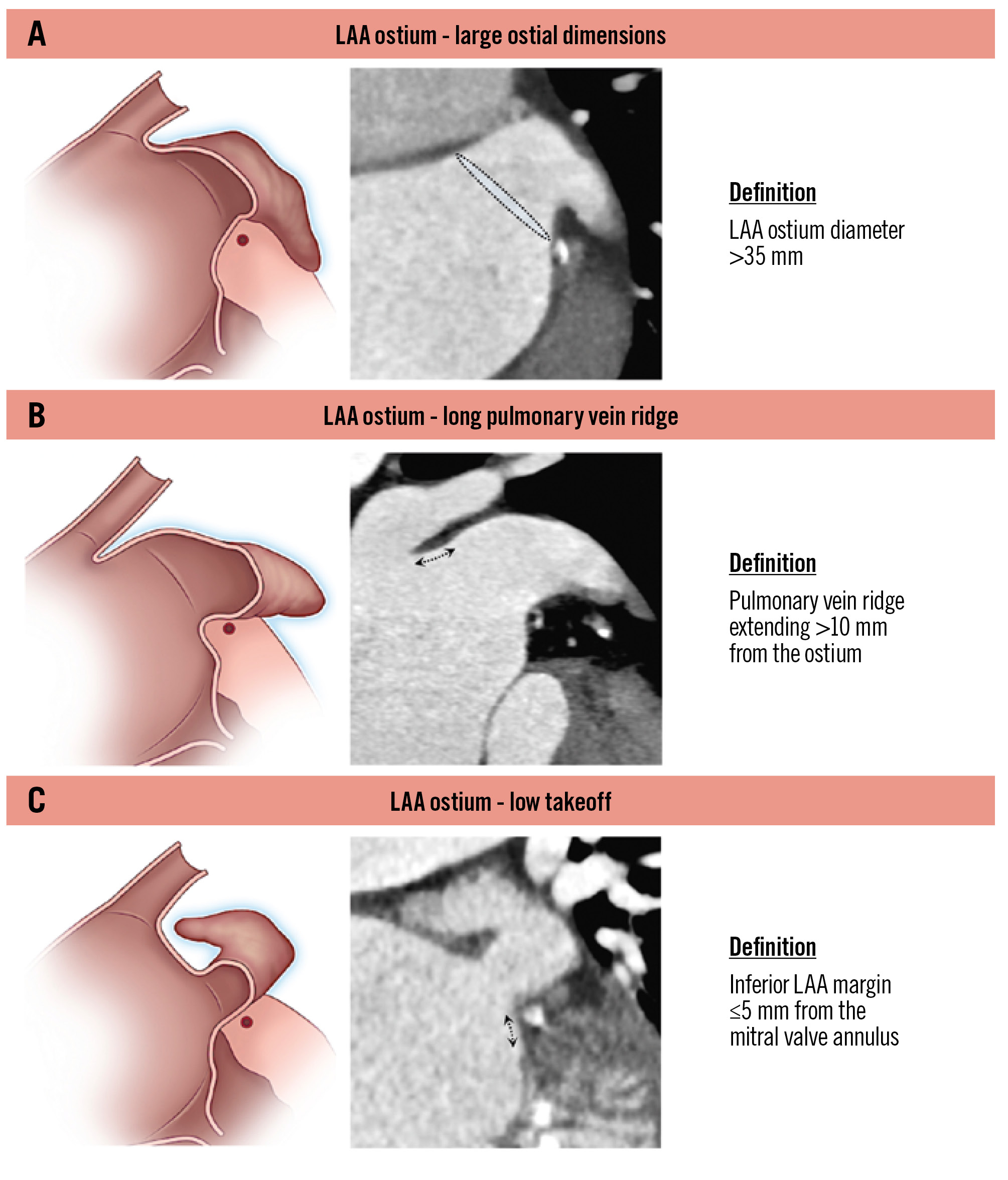
Figure 2. Left atrial appendage – ostial parameters. Definitions related to the LAA ostium: (A) large ostial dimensions; (B) long pulmonary vein ridge; (C) low takeoff. LAA: left atrial appendage
Landing zone
The landing zone within the LAA is where the occlusion device is intended to anchor.
The classification system identifies four key landing zone characteristics: the presence of a large diameter, high eccentricity, a proximal lobe, and proximal bifurcation/protuberance (Figure 3).
1) A large landing zone is characterised by a diameter >30 mm.
2) High eccentricity is defined as an eccentricity index (maximum diameter/minimum diameter) ≥1.5. Implication: both criteria can complicate achieving an effective LAA seal. Of note, high eccentricity indicates significant asymmetry, with a large difference between maximum and minimum landing zone diameters, which implicates challenges for device sizing and for the device to conform to the landing zone shape17.
3) A proximal lobe is defined as an outpouching of the LAA within the landing zone of at least 1 cm in both width and depth. Implication: a failure to cover the proximal lobe will result in incomplete closure. The presence of this anatomical parameter might favour the use of a disc-and-lobe device over a plug-based device. When using a plug-based device, an effort should be made to cover the proximal lobes by targeting a more proximal implantation, but only if it is feasible without increasing the risk of device embolisation.
4) Proximal bifurcation/protuberance is defined as any LAA prominence (large pectinate, internal septum) protruding more than 5 mm into the landing zone. Implication: these features may decrease the effective available area for the device to deploy and can result in improper device engagement, leading to stability issues and potential embolisation. The presence of a proximal bifurcation can result in the presence of two equivalent main LAA lobes, which can be challenging or even impossible to occlude with a single-component/plug-based occluder.
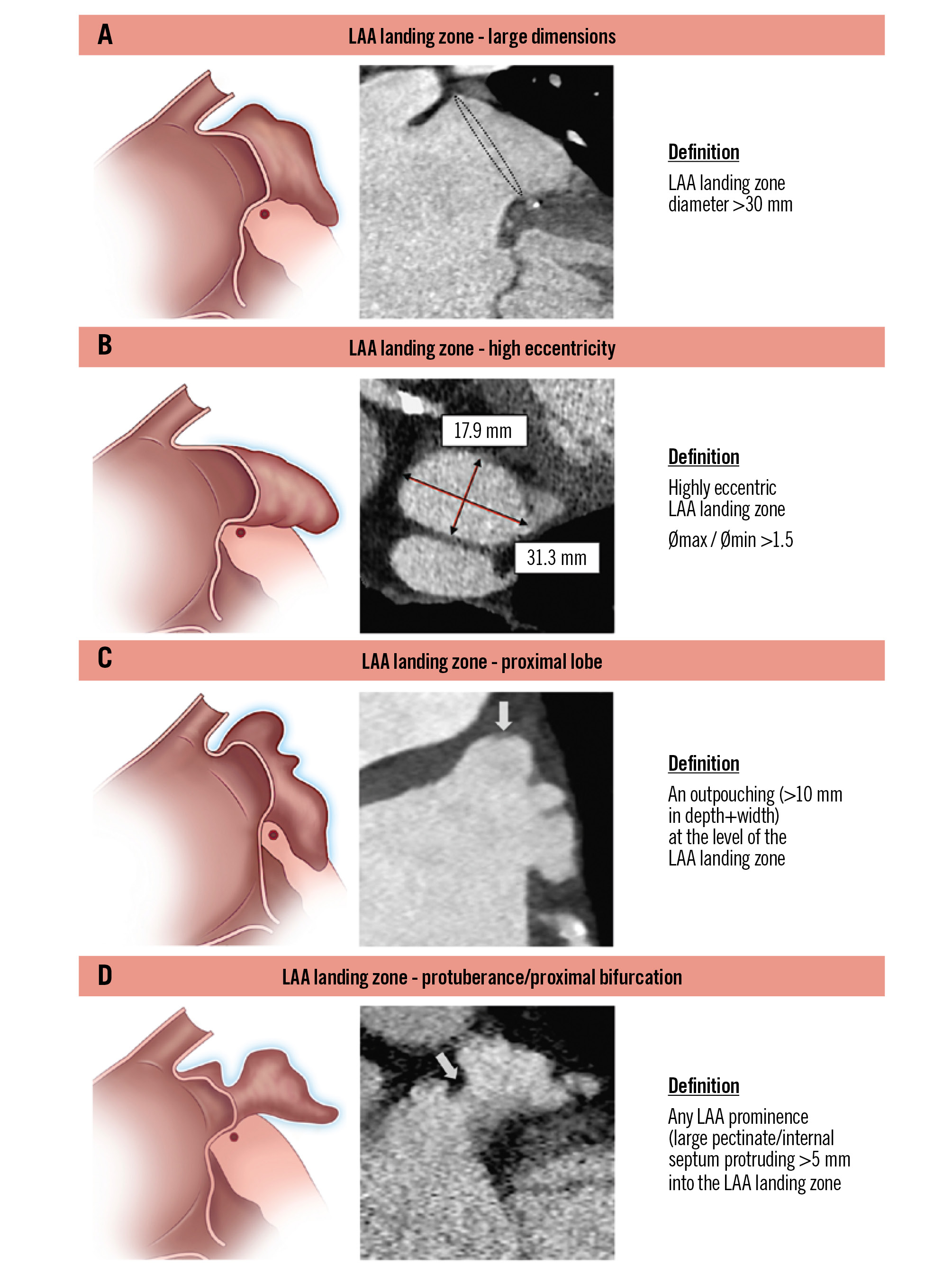
Figure 3. Left atrial appendage – landing zone parameters. Definitions related to the LAA landing zone: (A) large dimensions; (B) high eccentricity; (C) proximal lobe; (D) protuberance/proximal bifurcation. LAA: left atrial appendage
Anatomy
The classification system identifies three key anatomical characteristics of the LAA: the presence of an early sharp bend, a short depth and a funnel-shaped LAA (Figure 4).
1) An early sharp bend (either anterior or posterior) is identified when the bend occurs within 10 mm of the LAA ostium and with an angle >60° within the main lobe.
2) A short depth is defined as a distance of less than 15 mm from the ostial plane to the back wall of the LAA. Care must be taken to avoid including accessory small LAA lobes that are unable to accommodate the expansion of current LAAC devices.
3) A funnel-shaped anatomy is characterised by an ostium-to-landing zone tapering diameter of more than 30%.
Implication: an early sharp bend can result in “off-axis” device implantation and subsequent suboptimal device apposition against the LAA wall, leading to PDL18. Both short depth and funnel-shaped anatomies increase the difficulty of securing the device properly within the landing zone, increasing the risk of device embolisation. Moreover, in a funnel-shaped LAA, the radial force of devices is pronounced in the more distal part of the LAA, which may contribute to a higher risk of erosion. We recommend the use of devices with a low profile for short depths. The presence of a funnel-shaped anatomy might favour the use of a plug-based device in order to secure stable device anchoring.
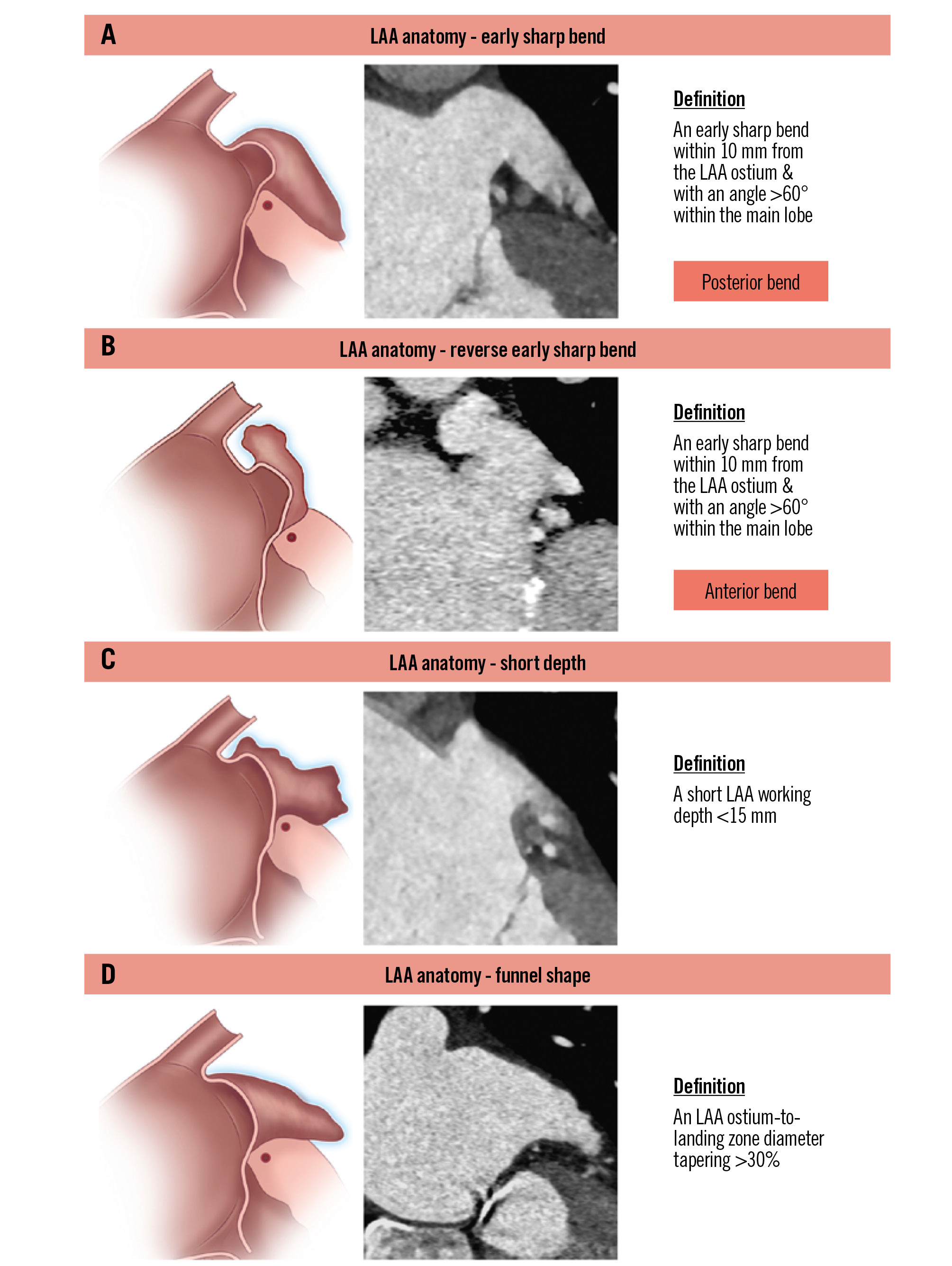
Figure 4. Left atrial appendage – anatomical parameters. Definitions related to LAA anatomy: (A) early sharp bend; (B) reverse early sharp bend; (C) short depth; (D) funnel shape. LAA: left atrial appendage
Axis of the LAA
The classification system considers two key characteristics: pronounced anterior and posterior deviations, defined as forward or backward tilt of the appendage axis relative to the heart’s longitudinal axis (Figure 5). Implication: both pronounced anterior and posterior deviations can complicate the procedural approach and device/catheter manipulation within the LAA19.
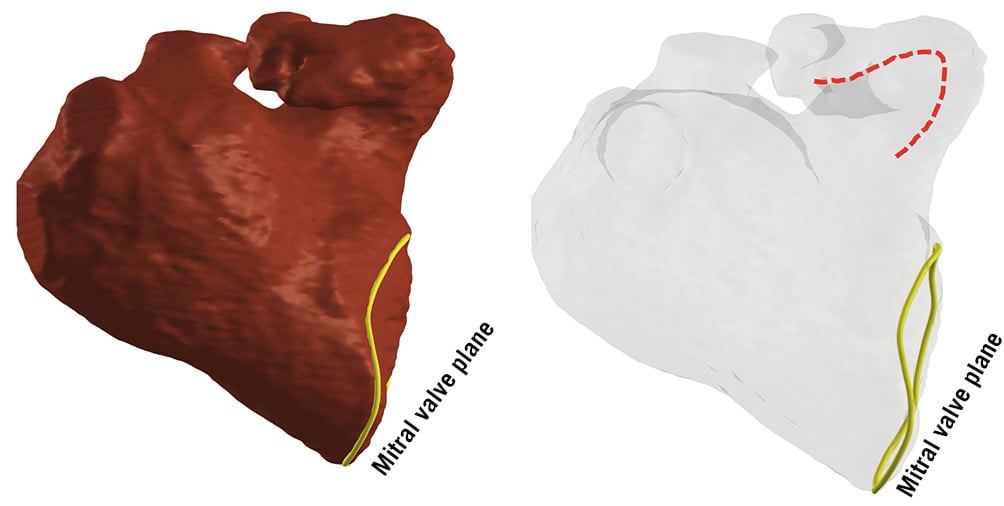
Figure 5. Three-dimensional rendering illustration of an LAA with pronounced anterior deviation. Dotted red line represents forward tilt of the LAA axis. LAA: left atrial appendage
Contraction
A hypercontractile LAA is characterised by LAA diameter changes greater than 50% during the cardiac cycle. Implication: a hypercontractile LAA can lead to significant dynamic changes in LAA dimensions, complicating device placement and increasing the risk of embolisation due to vigorous LAA movements. It is important to avoid device undersizing and ensure good anchoring to prevent instability from the dynamic changes.
ELAAC complexity assessment
Two complementary modalities for the classification system were further refined based on additional feedback from the first ELAACC meeting (Central illustration):
1) A scoring system that assigns one point for each of the twelve key characteristics. The total score will range from 0 to a maximum of 12, and categorises LAAC procedures from simple to increasing complexity, providing a quantitative assessment that aids in preprocedural planning and risk management.
2) A colour-coded system that uses colour gradations to represent the complexity of LAAC procedures, based on the total number of key characteristics. It provides a visual and intuitive guide that can quickly communicate key information to the implanting team.
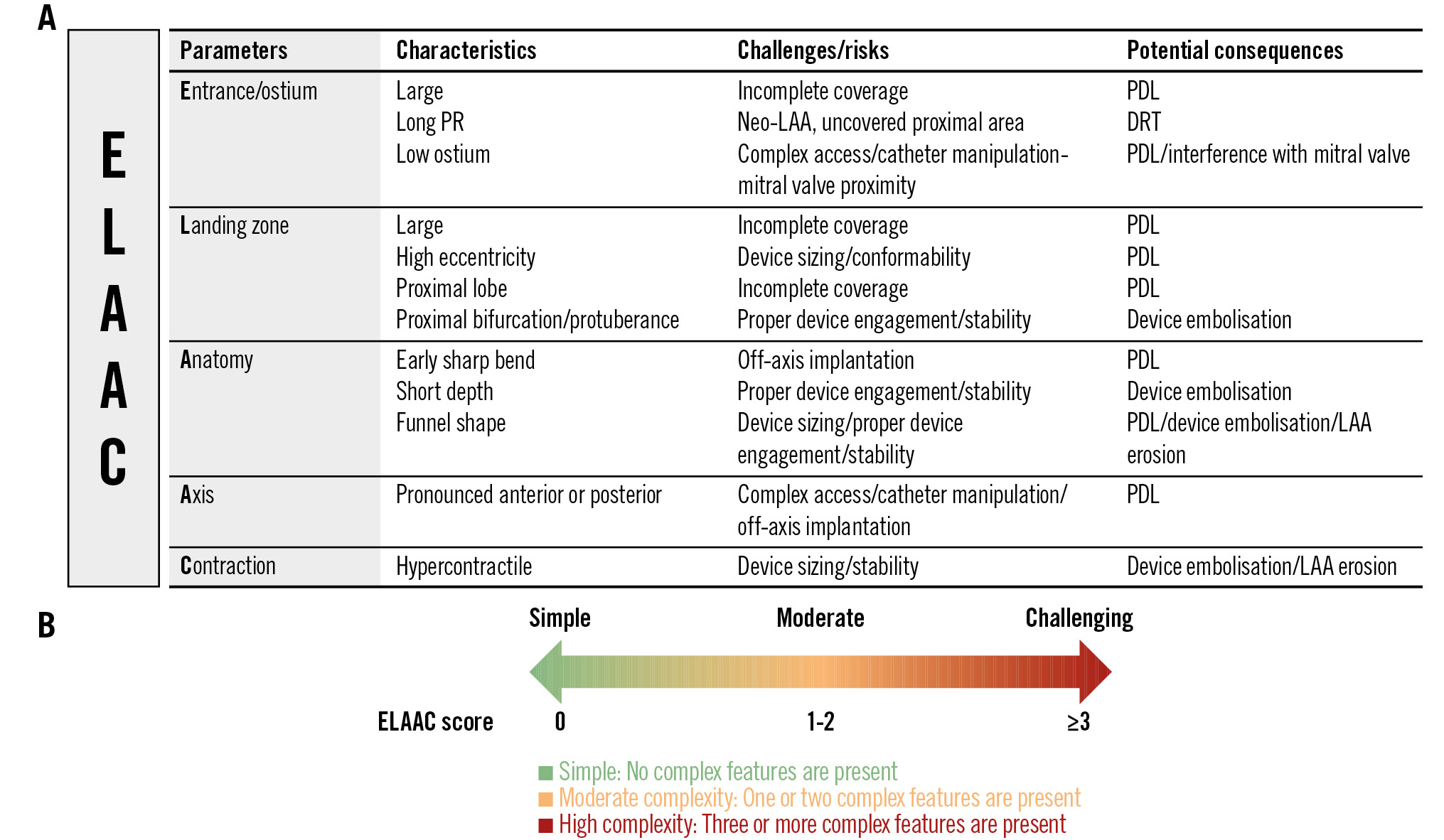
Central illustration. ELAAC classification. The scoring system assigns one point for each of the twelve key characteristics (A). The total score ranges from 0 to a maximum of 12, and categorises LAAC procedures from simple to increasing complexity, providing a quantitative assessment that aids in preprocedural planning and risk management. Next to the scoring system, a colour-coded system uses colour gradations to represent the complexity of LAAC procedures, based on the total number of key characteristics (B). DRT: device-related thrombus; ELAAC: Entrance/ostium, Landing zone, overall Anatomy, Axis/orientation and Contraction; LAA: left atrial appendage; PDL: peridevice leak; PR: pulmonary ridge
Distribution of ELAAC parameters and scores
To provide an initial overview of the epidemiology of ELAAC parameters in a real-world setting, we conducted a retrospective analysis of the last 200 patients that underwent LAAC at two high-volume centres (each centre included 100 patients). Cardiac computed tomography (CT) with multiplanar reconstructions and 3D volume rendering were used to analyse anatomical parameters, while functional hypercontractile LAA was assessed using procedural echocardiography or angiography. All cases were reviewed by a highly experienced LAAC implanter from each centre (O. De Backer and J.E. Nielsen-Kudsk), both of whom have extensive training in LAA imaging interpretation. The frequency distribution of ELAAC parameters is presented in Figure 6. The most common parameters included an early sharp bend (25.5%), pronounced anterior/posterior axis (19.5%) and long PR (13.0%). Conversely, less frequent parameters included proximal lobe (6.5%) and low ostium position (5%).
The ELAAC score, derived from the classification parameters, ranges from 0 (simple anatomy) to a maximum of 5 (highly complex anatomy). The distribution of scores is shown in Figure 7.
This distribution reveals that most LAAC cases fall within the simple-to-moderate category (scores 0-2), representing 78.5% of cases. However, 21.5% of cases scored ≥3, indicating challenging anatomies that may require advanced techniques or planning to ensure procedural success.
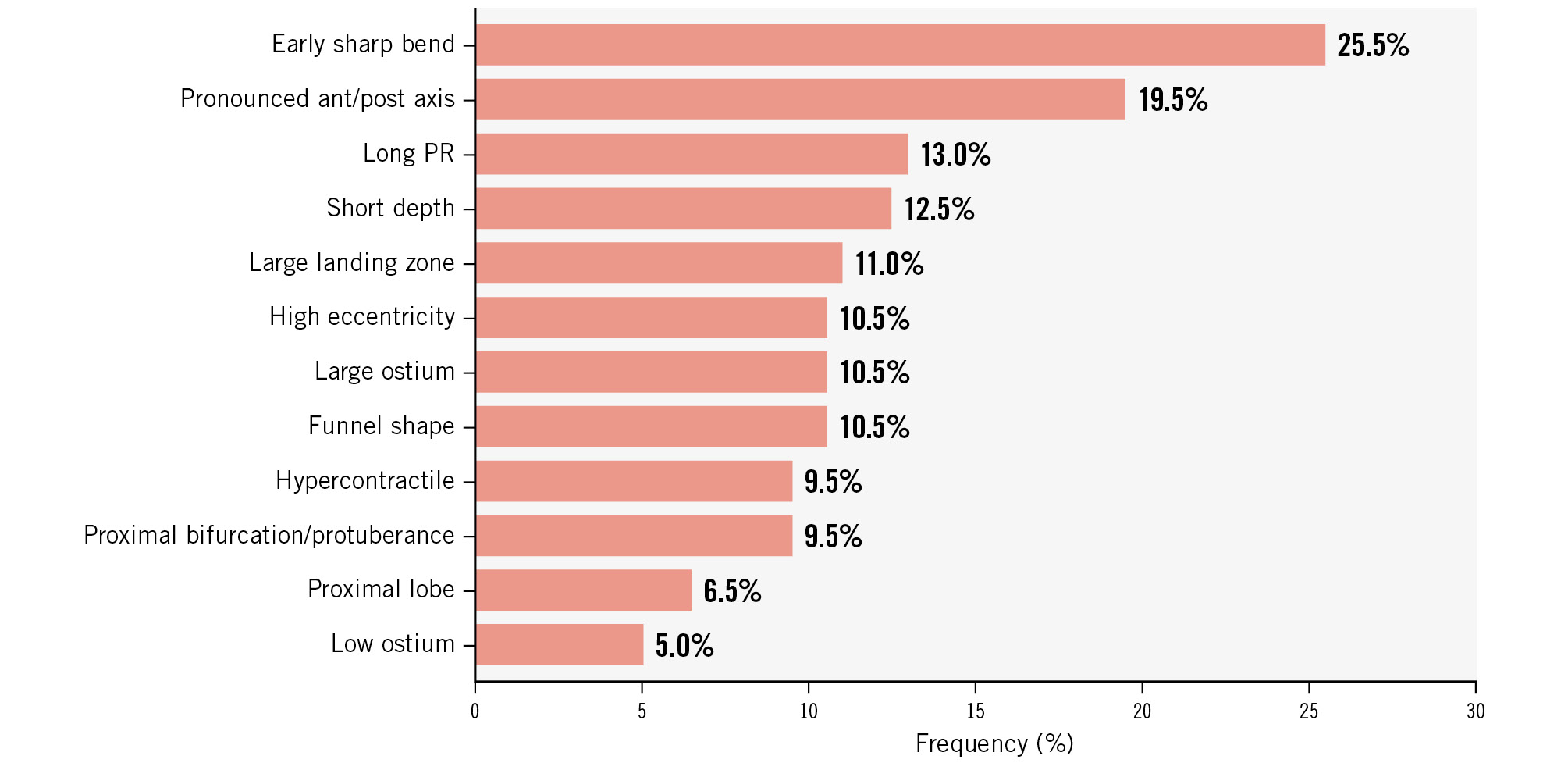
Figure 6. Frequency distribution of ELAAC parameters. Retrospective analysis of the last 200 patients from two high-volume centres (each centre included its last 100 patients). ant: anterior; ELAAC: Entrance/ostium, Landing zone, overall Anatomy, Axis/orientation and Contraction; post: posterior; PR: pulmonary ridge
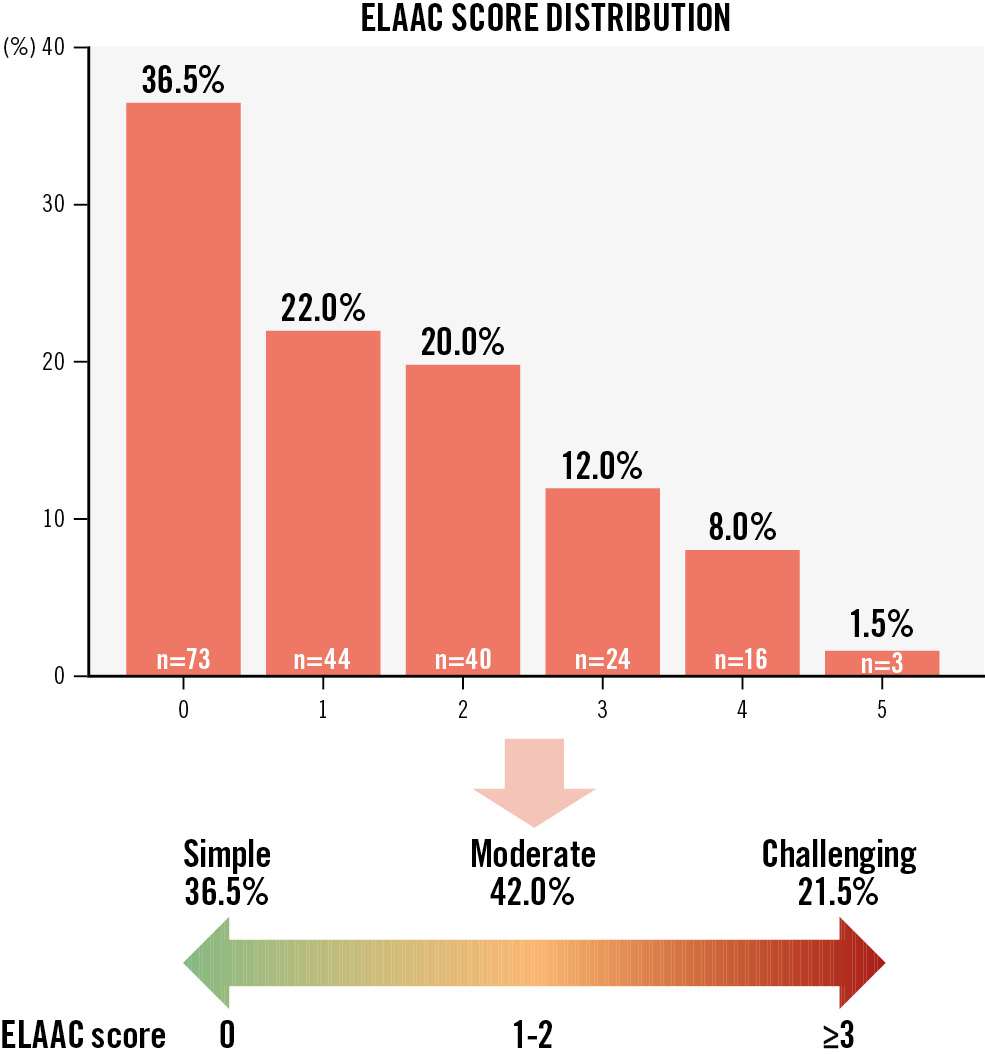
Figure 7. Frequency distribution of ELAAC scores and ELAAC categories into simple, moderate and challenging anatomies. ELAAC: Entrance/ostium, Landing zone, overall Anatomy, Axis/orientation and Contraction
Discussion
The left atrial appendage exhibits considerable anatomical variability, which can significantly impact the success of LAAC procedures. Current approaches usually rely on a combination of imaging techniques and operator experience to navigate these variations202122.
The proposal for a new implanter classification for LAAC underscores the necessity of a more comprehensive framework that goes beyond mere visual descriptions of the LAA shape.
As compared to previous LAA anatomical classifications, the novelty of the current ELAAC classification lies in its more structured and procedure-oriented approach.
By incorporating easy and well-defined key LAA anatomical/functional parameters, this classification aims to provide a standardised method for assessing LAAC complexity, with the potential to improve procedure efficiency and reduce complication rates.
By identifying high-risk anatomical and functional features preprocedurally, operators can indeed tailor their procedural approach and device selection to mitigate these risks.
For instance, recognising a large diameter or high eccentricity in the landing zone will prompt optimal device sizing and/or the selection of devices with enhanced sealing capabilities, thereby reducing the occurrence of significant residual leaks. The combination of several high-risk parameters within the same patient may even preclude optimal LAA sealing, which can be anticipated upfront. Another significant implication of this classification system lies in its potential to standardise training and education. By providing a structured framework for assessing procedural LAA complexity, this system can be integrated into training programmes to enhance the proficiency of clinicians performing LAAC, which can lead to more consistent outcomes and higher procedural success rates across different institutions23.
The integration of advanced imaging techniques is essential for enhancing the precision, reproducibility, and generalisability of the proposed ELAAC classification system. Modalities such as cardiac CT with multiplanar reconstruction and 3D volume-rendered imaging can provide critical insights into challenging anatomical features, aiding in accurate device sizing and procedural planning. Three-dimensional transoesophageal echocardiography (TOE), with its dynamic and real-time imaging capabilities, allows the assessment of functional characteristics like hypercontractile LAA. Computational simulations enable virtual device deployment, helping clinicians anticipate complications such as PDL or DRT24. In case of suspicion of a complex LAA on TOE, a cardiac CT should be considered.
At the first ELAACC meeting in Barcelona in February 2024, there was a decision to initiate a large-scale ELAACC Research Registry of at least 5,000 patients with the aim of assessing real-life patient management, and procedural and clinical outcomes for LAAC in Europe. The parameters of the ELAAC classification system have been integrated into this registry. Therefore, the ELAACC Research Registry will serve as an important clinical tool for validation of the proposed ELAAC classification.
Limitations
The present classification is based on expert consensus, but a prospective validation is lacking to date. Future research should focus on validating this classification system with clinical outcomes to refine its predictive accuracy. Large-scale, multicentre studies will provide valuable data on the effectiveness of this classification system in predicting procedural complexity and complications. Additionally, the integration of advanced imaging techniques into the classification criteria could further enhance its precision and reproducibility. The parameters included in the proposed classification may affect the procedural complexity differently depending on the device used. Finally, an important area for future research is the development of occluder devices tailored to the anatomical and functional features identified by this classification system.
Conclusions
The proposed ELAAC implanter classification system provides a structured approach to assess the anatomical and functional challenges of LAAC.
By understanding these parameters and their quantifiable thresholds, clinicians can better prepare for and potentially mitigate the procedural risks associated with varying LAA anatomies. Future research should focus on validating this classification with clinical outcomes to refine its predictive accuracy and improve the safety of LAAC procedures.
Conflict of interest statement
S. Berti is a proctor for Abbott and Edwards Lifesciences. R. Galea has received speaker honorarium from Boston Scientific. O. De Backer reports institutional research grants and consulting fees from Abbott, Boston Scientific, and Eclipse Medical. L. Räber reports research grants to the institution from Abbott, Biotronik, Boston Scientific, HeartFlow, Sanofi, Regeneron, Medis Medical Imaging Systems, and Bangerter‐Rhyner Stiftung; and speaker or consultation fees from Abbott, Amgen, AstraZeneca, Canon, Gentuity, Novo Nordisk, Medtronic, Occlutech, and Sanofi, outside the submitted work. J.E. Nielsen-Kudsk is a consultant for Boston Scientific; and a consultant/proctor for Abbott. A. Aminian is a proctor and consultant for Abbott and Boston Scientific. I. Cruz-Gonzalez is a proctor and consultant for Abbott, Boston Scientific, and Lifetech. X. Freixa is a proctor and consultant for Abbott and Lifetech. X. Iriart is a proctor and consultant for Abbott and Boston Scientific; and reports speaker honoraria and travel expenses from Philips. N.C. Wunderlich reports speaker honoraria and travel expenses from Boston Scientific, Abbott, Lifetech, GE HealthCare, Philips, and Siemens Healthineers. P. Garot reports advisor/speaker fees from Abbott, Biosensors, Boston Scientific, and GE HealthCare; he reports serving as Medical Director and being a shareholder at CERC (Cardiovascular European Research Center), a contract research organisation dedicated to cardiovascular diseases.

