Cory:
Unlock Your AI Assistant Now!
Over recent years there has been a revolution in the applications of coronary angiography in the assessment of coronary artery pathology. Computerised methodologies have been introduced that are able to process coronary artery models reconstructed from angiographic images and accurately evaluate lesion haemodynamic severity, plaque deformability, and peripheral vascular resistance. Despite the inherent limitations of angiography to quantify lumen dimensions and measure coronary flow, cumulative evidence supports the validity of this concept. Methodologies developed to compute the fractional flow reserve (FFR) are now indicated by European guidelines in treatment planning: radial wall strain, which measures the changes in lumen dimensions during the cardiac cycle in order to assess the mechanical properties of plaque, has been found able to detect vulnerable lesions1, while solutions that were proposed to measure microvascular resistance from coronary angiography (Figure 1) seem able to provide useful prognostic information and predict microvascular obstruction (MVO) and infarct size post-revascularisation in patients with an acute coronary syndrome234.
In this issue of EuroIntervention, Zhang et al examined for the first time the efficacy of the AngioPlus system (Pulse Medical), which relies on the segmentation of a single angiographic projection and the computation of flow division in coronary bifurcations using Murray’s Law in order to measure microvascular resistance in patients with ST-elevation myocardial infarction (STEMI) undergoing successful percutaneous coronary intervention (PCI)5. The authors processed the angiographic data of the patients recruited in the EARLY-MYO-CMR study (n=435) who had cardiac magnetic resonance (CMR) imaging within two weeks post-revascularisation to measure the angiographic microcirculatory resistance (AMR). The best cutoff was identified for predicting microvascular disease on CMR (defined as the presence of MVO or intramyocardial haemorrhage), and then the prognostic value of this cutoff (26.6 mmHg*s/dm) was tested in a cohort of 2,261 patients admitted with STEMI.
The authors found that patients with a high AMR had a higher incidence of cardiovascular events (30.9% vs 21.5%; p<0.001), at 44-month follow-up. The higher event rate was due to increased cardiac mortality (6.9% vs 4.1%; p<0.001) and hospitalisation for heart failure (19.6% vs 10.5%; p<0.001) – with a large proportion of events in the high AMR cohort occurring at the index admission, as shown in the Kaplan-Meier curves – while there was no difference between groups in the incidence of myocardial infarction and target lesion revascularisation. The findings of this report are in line with the results of studies that used a flow wire or three-dimensional quantitative coronary angiography to assess microvascular resistance of the culprit vessel post-PCI in patients with STEMI26.
The authors must be congratulated for conducting this analysis that constitutes the largest in patients with STEMI. Their findings underscore the value of the AngioPlus system to assess microvascular injury using a single angiographic projection, a feature that is anticipated to facilitate its use in research. However, it has to be stressed that this approach also has limitations. The use of a single projection is likely to underestimate the length of the reconstructed segment and the blood flow velocity and, as a consequence, to overestimate the AMR. Moreover, blood flow velocity estimations vary in different angiographic projections; therefore, the use of a single projection to measure blood flow velocity is likely to affect the reproducibility of the AMR computation7. Other limitations of the present study include the absence of information with regard to the medications that may have been administered at the end of the procedure to reduce non-reflow and the fact that the authors did not clarify whether the aortic pressure measurements were available at the time of the acquisition of the angiographic runs used for the AMR computation. Moreover, the authors approximated hyperaemic blood velocity from the angiographic runs acquired under resting conditions using a formula that has limited accuracy in patients with STEMI8. These limitations may account for the different AMR cutoff that was found in this study to predict events compared to the index of microvascular resistance (IMR) cutoffs reported in the literature (>27.6 mmHg*s/dm vs 40 mmHg*s)6. Finally, unlike other studies, the authors did not quantify the MVO and intramyocardial haemorrhage and therefore did not examine the association between AMR and the extent of myocardial injury3.
Despite these methodological limitations, AMR provided useful prognostic information and had an acceptable performance in detecting myocardial injury (area under the curve: 0.721). In patients with STEMI, microvascular dysfunction following revascularisation has been attributed to distal embolisation and to reperfusion injury, is an established predictor of worse outcomes, and constitutes a target for therapy. Several pharmacotherapies have been introduced over recent years that are currently undergoing clinical evaluation, and mechanical therapies have been considered to improve microvascular function and reduce infarct size9. It is therefore important to accurately identify high-risk individuals with microvascular dysfunction while they are on the catheterisation table so as to deliver focal therapies that will reduce microvascular injury and improve prognosis. Angio-derived indices of IMR have the potential to become the method of choice for assessing the extent and severity of microvascular dysfunction as, unlike invasive IMR, they do not require further instrumentation of the culprit vessel with an FFR wire and pharmacological induction of hyperaemia. Prospective studies are currently testing the diagnostic performance of angio-IMR solutions and are expected to provide additional evidence that will support their application in clinical practice10.
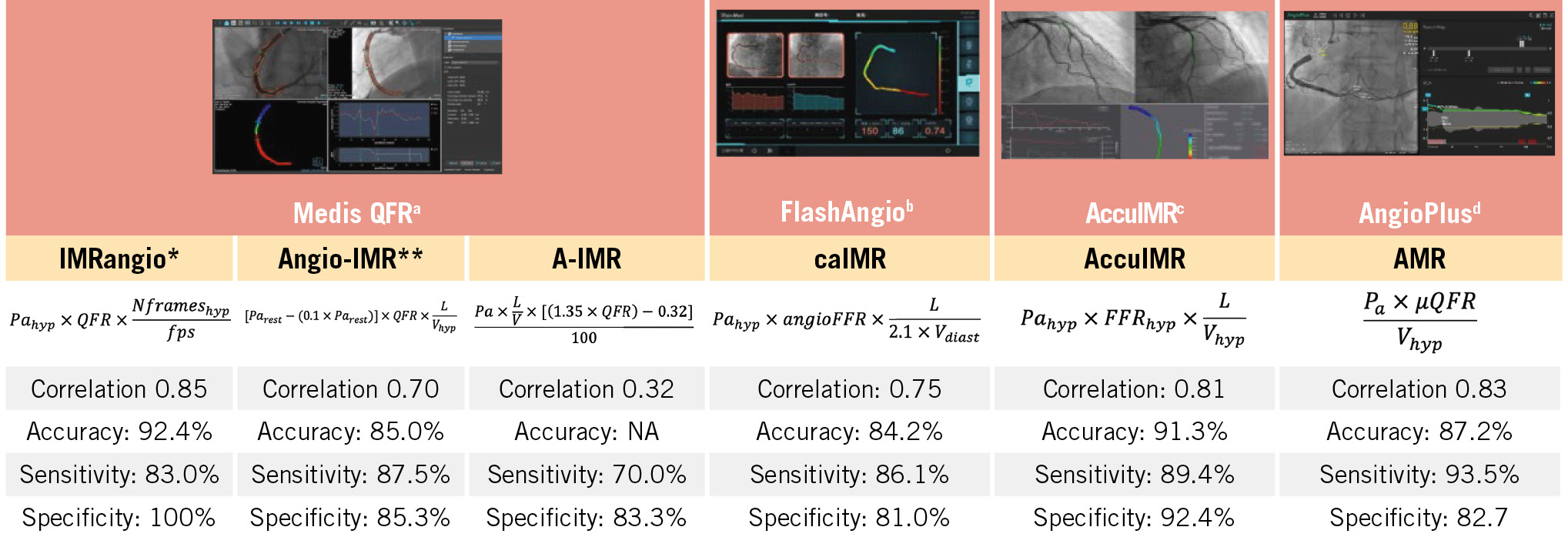
Figure 1. Computerised methodologies introduced for the computation of the IMR from coronary angiographic images. The agreement of these approaches with the flow wire IMR estimations and their performance to detect an IMR >25 U in the first validation studies is also provided. aBy Medis Medical Imaging Systems; bby RainMed Medical Group; cby ArteryFlow Technology; dby Pulse Medical. *The validation results reported for IMRangio refer to a wire-based IMR cutoff of >40 U. **The validation results reported for angio-IMR refer to a wire-based IMR cutoff of >23 U. Angio: angiography; diast: diastole; FFR: fractional flow reserve; fps: frames per second; hyp: hyperaemia; IMR: index of microcirculatory resistance; L: length; NA: not available; Pa: aortic pressure; QFR: quantitative flow ratio; V: velocity
Conflict of interest statement
H. M. Garcia-Garcia has received funding from Medtronic, Biotronic, Abbott, Neovasc, Corflow, Philips, Chiesi, Boston Scientific, Cordis, Medis Medical Imaging Systems, Elixir, Terumo, and MedHub. The other authors have no conflict of interests to declare.

