Cory:
Unlock Your AI Assistant Now!
Refractory angina is characterised by chronic chest pain due to reversible ischaemia which is unable to be controlled with optimal medical therapy or revascularisation1. Patients with refractory angina suffer severe limitations in daily activities, have a poor quality of life, and are a significant strain on healthcare systems due to their frequent rehospitalisations2. The coronary sinus (CS) Reducer (Shockwave Medical) creates a focal narrowing in the CS lumen in order to redistribute myocardial blood flow into the subendocardium of ischaemic myocardium. Randomised placebo-controlled trials such as COSIRA3 and ORBITA-COSMIC4 have shown significant improvements in frequency of anginal episodes and in quality of life in patients treated with the CS Reducer. The 6-month analysis of the REDUCER-I registry reported a 1.6% rate of major adverse cardiac events (MACE) for the primary safety endpoint and 69.8% of patients with improved Canadian Cardiovascular Society (CCS) class at 6 months for the primary effectiveness endpoint5.
We report here the one-year follow-up results of the non-randomised, real-world REDUCER-I study of patients with refractory angina treated with the CS Reducer. From 2016 to 2023, 400 patients were enrolled at 25 centres from 9 European countries, of whom 371 underwent implantation of the CS Reducer. All sites were required to follow local legal and regulatory requirements for ethics committee and institutional review board approvals. Endpoints included MACE (defined as a composite of cardiac death, major stroke, and myocardial infarction), CCS class, 6-minute walk tests (6MWT), Seattle Angina Questionnaire (SAQ), EQ visual analogue scale (EQ-VAS), and emergency department (ED) visits.
Of the 371 subjects implanted, there were 10 deaths, 9 withdrawals from the study, and 1 lost to follow-up prior to 12-month follow-up; there was 97% (339/351) compliance at 12-month follow-up. The cohort was 78.0% (312/400) male, 46.5% (185/398) had diabetes, 73.6% (293/398) had prior revascularisations, and 72.0% (280/389) were CCS class III/IV at presentation. The one-year Kaplan-Meier overall MACE rate was 7.5% (95% confidence interval [CI]: 5.2-10.8), driven primarily by the incidence of myocardial infarctions (5.6%, 95% CI: 3.6-8.5). There were 9 deaths within the first 365 days, one of which was due to an unknown cause. The other 8 were adjudicated as not being device- or procedure-related, with 6 of the 8 considered cardiac-related deaths.
At 12 months, the CCS class was significantly better compared to baseline (Figure 1A) (p<0.001) with 70.5% (227/322) of patients improving by ≥1 CCS class and 25.5% (82/322) improving by ≥2 CCS classes. The changes in CCS class were similar to what was reported in the 6-month analysis5 showing the durability of the CS Reducer’s unblinded treatment effect. 6MWT distance increased by a mean of 32.3±81.0 m at 12 months (p<0.0001), which is considered clinically significant for patients with coronary artery disease. Patients had significant improvements in quality of life at 12 months as assessed by SAQ scores (Figure 1B) and EQ-5D-5L tests (p<0.001). The improvements in the subcategories of anginal stability, anginal frequency, and overall quality of life were consistent with prior 6-month study results45, again showing the persistent effects of the Reducer.
Changes in the frequency of ED visits are important for patients with refractory angina who are often rehospitalised2. In the year before CS Reducer implantation, 33.1% (97/293) of the patients had 192 ED visits, amounting to 0.66±2.13 visits per patient. In the year after CS Reducer implantation, ED visits were significantly reduced with 13.3% (39/293) patients having a total of 66 visits, representing 0.23±1.01 visits per patient (Figure 1C) (p<0.001). Overall, 89.7% (87/97) of patients with at least one ED visit in the year prior to Reducer implantation had a decrease in the number of ED visits in the year following Reducer implantation.
Treatment for patients with refractory angina should emphasise improvements in quality of life, reduction in anginal symptoms, and reduction in hospitalisations15. The REDUCER-I study is one of the largest registries of clinically challenging real-world patients treated with the CS Reducer spanning multiple centres and countries. This one-year analysis demonstrated low MACE rates and significant improvements in functional capacity and CCS class. The reduction in angina severity and restoration of quality of life translated to significantly fewer ED visits for chest pain, which could potentially reduce the burden on healthcare resources. Furthermore, the sustained improvements from the interim three-year follow-up analysis suggest the durability of the treatment5.
The REDUCER-I study was limited in having both prospectively and retrospectively enrolled patients and was a single-arm unblinded registry. Whilst the results may reflect expected outcomes in clinical practice, the contribution of the placebo effect after CS Reducer implantation cannot be excluded. Data from ORBITA-COSMIC4 and the ongoing COSIRA II and REMEDY-PILOT (ClinicalTrials.gov: NCT05492110) randomised controlled trials will be able to assess the performance of the CS Reducer against a comparator arm and may also provide critical mechanistic insights regarding this device.
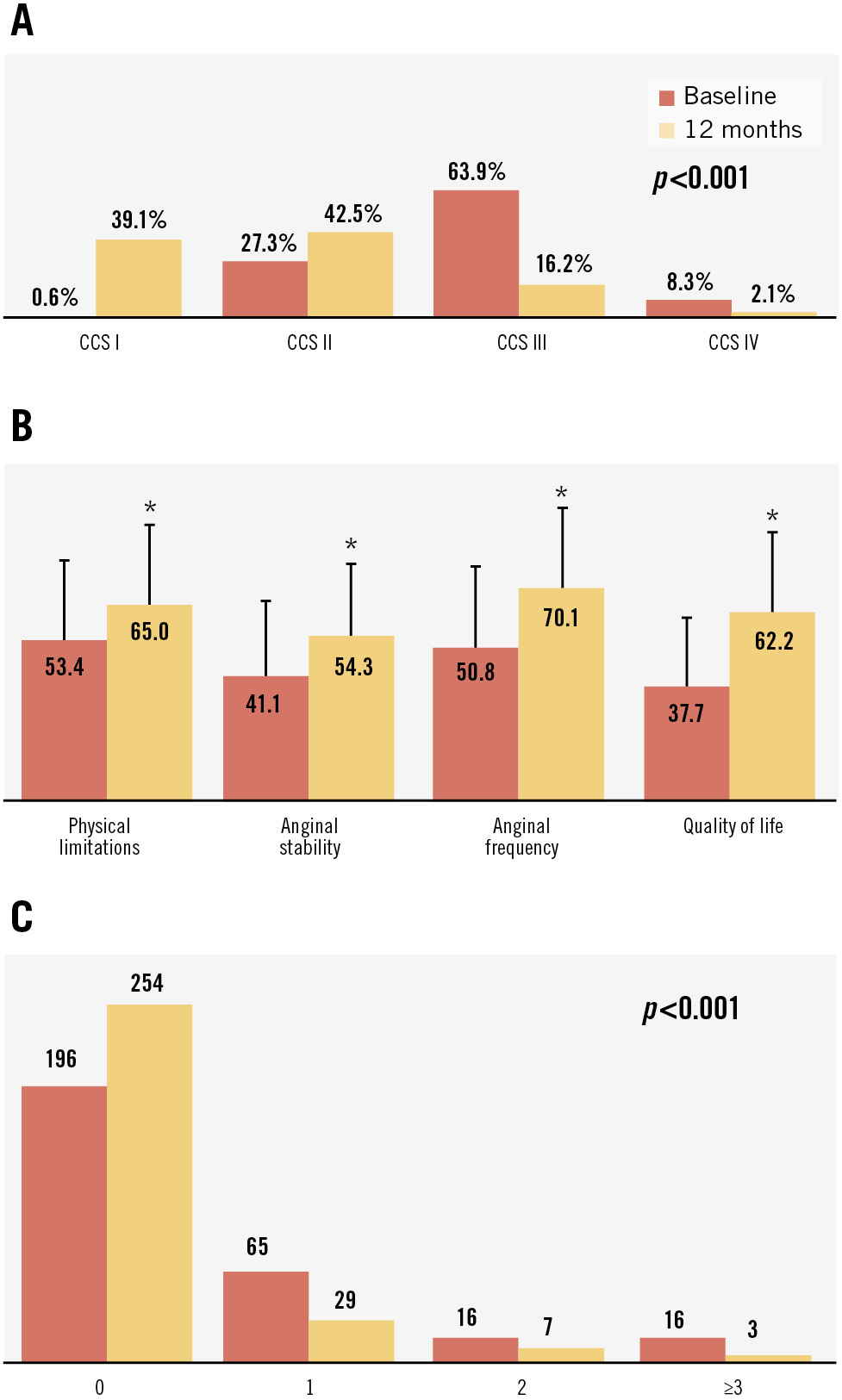
Figure 1. Positive outcomes after Reducer implantation. A) A significant shift in CCS class after 12 months; (B) higher SAQ scores (a higher score indicates improvement) at 12 months (*p<0.001); and (C) a reduction in the number of ED visits per patient in the year after Reducer (Shockwave Medical) implantation, with most patients not being rehospitalised. CCS: Canadian Cardiovascular Society; ED: emergency department; SAQ: Seattle Angina Questionnaire
Acknowledgements
The authors acknowledge the following from Shockwave Medical: Ryan Shields and Dorothy Baumer, for statistical support; Keisha Sandberg for clinical study support; and Ming-Jay Chow for assistance in the preparation of the manuscript.
Funding
The REDUCER-I study (ClinicalTrials.gov: NCT02710435) is sponsored by Shockwave Medical, Santa Clara, CA, USA although no specific funding was provided for this analysis.
Conflict of interest statement
S. Verheye and R. de Silva serve as proctors for and receive honoraria from Shockwave Medical. N.E.J. West is an employee of Shockwave Medical. S. Banai receives honoraria and is on the advisory board for Shockwave Medical. The other authors have no conflicts of interest to declare related to this manuscript.

