Cory:
Unlock Your AI Assistant Now!
Abstract
Wire-based indices of coronary physiology are the gold standard for guiding revascularisation decisions in patients with coronary artery disease and angiographically intermediate coronary stenoses. FFRangio is a novel angiography-based technology for assessing the functional significance of epicardial coronary stenoses without pressure wires or hyperaemic stimulus. The primary objective of the Advancing Cath Lab Results with FFRangio Coronary Physiology Assessment trial (ALL-RISE; ClinicalTrials.gov: NCT05893498) is to compare clinical outcomes in patients with chronic coronary syndromes or non-ST-segment elevation acute coronary syndromes undergoing coronary angiography with ≥1 coronary lesion suitable for physiological assessment. Patients will be randomised to FFRangio-guided or to pressure wire-guided treatment. The primary endpoint is the occurrence of major adverse cardiovascular events (MACE) at 1 year (a composite of all-cause death, myocardial infarction, or unplanned clinically driven revascularisation), assessed for non-inferiority of FFRangio-based versus pressure wire-based guidance. If non-inferiority is met, reflex superiority guidance will be tested. Secondary endpoints include periprocedural and early complications up to 30 days, individual components of MACE at 1 year, patient-reported health status, procedural resource utilisation and healthcare-related costs, and operator-assessed usability of the FFRangio and pressure wire systems. With a sample size of 1,924 patients, the study has 82.7% power to assess non-inferiority with a non-inferiority margin of 3.5%. The ALL-RISE trial will provide prospective clinical outcomes data on the relative safety, efficacy, and cost-effectiveness of a workflow using FFRangio as compared with pressure wire-based approaches for coronary lesion assessment among patients being considered for percutaneous coronary intervention.
Pressure wire-based indices of coronary physiology are the gold standard for invasively guiding revascularisation decisions in patients with coronary artery disease and angiographically intermediate coronary stenoses12. Multiple studies have shown that fractional flow reserve (FFR; the ratio of the distal coronary pressure to the aortic pressure during maximal hyperaemia) is superior to coronary angiography alone for guiding revascularisation of angiographically intermediate lesions345678. Non-hyperaemic pressure ratios (NHPRs; e.g., instantaneous wave-free ratio [iFR], resting full-cycle ratio, and diastolic pressure ratio) have also been developed and validated in recent years9101112. Accordingly, both the American and European revascularisation guidelines recommend using pressure wire-based physiology to guide the treatment strategy in stable coronary lesions1314. However, despite multiple randomised clinical trials and guideline recommendations supporting its use, pressure wire-based physiological assessment continues to be underutilised in contemporary practice due to several factors, including additional procedural time, instrumentation of coronary vessels, and paucity of reimbursement1516.
Several angiography-based approaches for assessing the functional significance of coronary stenoses have recently been introduced and validated against pressure wire-based FFR11171819202122. However, some of these modalities require considerable manual interaction and a relatively long processing time for practical application in the cardiac catheterisation laboratory1117181920. The FFRangio System (CathWorks) is a novel technology that provides three-dimensional functional mapping of the coronary arteries using routine diagnostic angiograms. It employs a resistance-based model to calculate coronary flow, requires three angiograms to maximise diagnostic accuracy, and utilises artificial intelligence to minimise the manual steps required to perform an analysis.
In the prospective FAST-FFR validation study, FFRangio, a novel angiography-based functional assessment, was compared with pressure wire-derived FFR and demonstrated excellent concordance with both wire-based FFR results and their threshold-based interpretation23. Additional studies have confirmed the concordance between FFRangio and wire-based FFR, including the assessment of non-culprit lesions in non-ST-segment elevation acute coronary syndrome (NSTE-ACS)24. In data from 492 patients, the use of FFRangio to guide clinical decisions had comparable 1-year outcomes to those reported previously for wire-based FFR25.
However, there is a paucity of data evaluating clinical outcomes with FFRangio-guided treatment, particularly in direct comparison with the gold standard of pressure wire-based physiology. The primary objective of the ALL-RISE trial is to test whether FFRangio-guided treatment is non-inferior to pressure wire-guided treatment with respect to major adverse cardiovascular events (MACE) at 1 year in patients with coronary artery disease who are being evaluated for possible percutaneous coronary intervention (PCI). Secondary objectives include assessments of procedure time, contrast and resource utilisation, and the cost-effectiveness of FFRangio-guided treatment versus pressure wire-guided treatment.
Methods
Design of the ALL-RISE trial
The Advancing Cath Lab Results with FFRangio Coronary Physiology Assessment trial (ALL-RISE; ClinicalTrials.gov: NCT05893498) is a prospective, multicentre, 1:1 randomised, open-label trial with blinded event adjudication to test whether FFRangio-guided treatment is non-inferior to conventional pressure wire-guided treatment for preventing MACE in patients with coronary artery disease being evaluated for possible PCI (Figure 1).
The study is funded by CathWorks, Inc., and is being conducted at up to 60 sites globally (USA, Israel, Japan, Switzerland, and the United Kingdom), with a maximum of 200 patients randomised per site. At least 60% of patients will be enrolled in the USA. Independent analytic groups at the Cardiovascular Research Foundation (New York, NY, USA) will oversee a clinical events adjudication committee, a data safety monitoring board, an angiographic core laboratory, and a coronary physiology core laboratory.
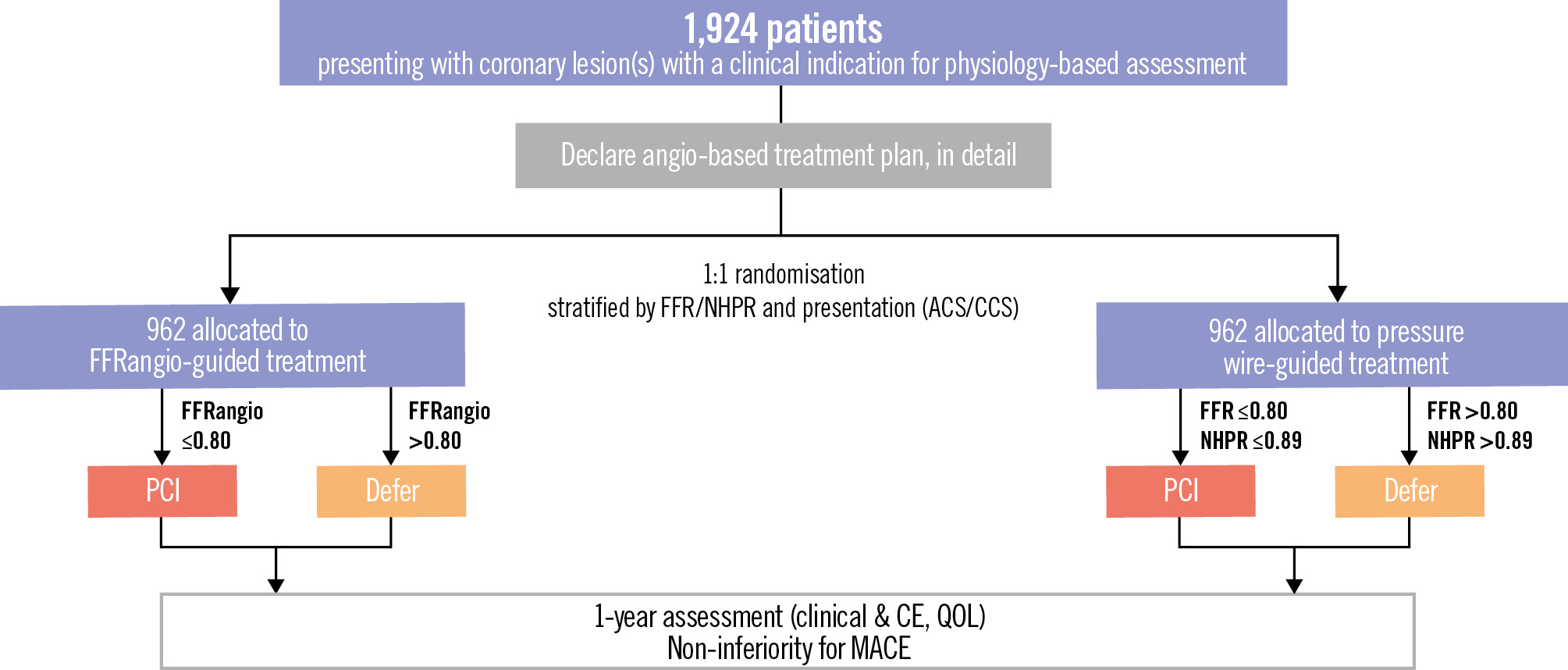
Figure 1. Study CONSORT diagram. ACS: acute coronary syndrome; CCS: chronic coronary syndrome; CE: clinical events; FFR: fractional flow reserve; FFRangio: angiography-derived FFR; MACE: major adverse cardiovascular events; NHPR: non-hyperaemic pressure ratio; QOL: quality of life
Study population
The study will enrol 1,924 patients with chronic coronary syndromes (CCS) or NSTE-ACS undergoing coronary angiography with at least 1 coronary lesion deemed appropriate for physiology-based assessment. Patients must meet all inclusion criteria and none of the exclusion criteria listed in Table 1 to be enrolled. Briefly, patients must be ≥18 years old and present with an accepted indication for PCI with 1 or more study lesions (angiographic visual diameter stenosis 50-90%) deemed appropriate for PCI and for both pressure wire and FFRangio physiological assessment. A study lesion is defined as the assessed coronary segment that includes a portion with a luminal diameter stenosis between 50% and 90% based on visual angiographic assessment. A study vessel is defined as the entire major assessed coronary vessel, including side branches.
Patients with prior coronary artery bypass grafting (CABG) with patent grafts to the study vessels and patients undergoing coronary physiology assessment as part of assessment for possible CABG (i.e., in whom CABG may be recommended based on the outcome of the physiology assessment) will not be eligible. Patients with severe left-sided valvular heart disease will also not be eligible for enrolment. Other exclusion criteria include study lesions in the left main coronary artery and Thrombolysis in Myocardial Infarction flow grade 2 or lower in a study vessel. For patients presenting with NSTE-ACS, clear culprit lesions are not eligible for inclusion, but non-culprit lesions may be considered as study lesions. Non-study lesions must be treated without complication prior to randomisation.
Table 1. Eligibility criteria.
| Inclusion criterion |
|---|
| Adult patients (≥18 years of age) with 1 or more study lesion(s) (diameter stenosis 50-90%) deemed appropriate for both pressure wire and FFRangio physiological assessment |
| Exclusion criteria |
| General exclusion criteria |
| Subject with STEMI within the previous 72 hours of study enrolment |
| Prior CABG with patent grafts to study vessel(s) |
| Patients undergoing coronary physiological assessment where one possible outcome is referral for CABG |
| Study vessel supplying a significant non-viable territory (e.g., prior transmural MI) |
| Severe left-sided valvular heart disease |
| Most recent documented LVEF ≤30% |
| Women who are pregnant or breastfeeding (women of childbearing potential are required to have a negative pregnancy test within 1 week of index procedure) |
| Patients with life expectancy <1 year as estimated by the treating physician |
| Subjects enrolled in other ongoing non-registry clinical studies that would impact the conduct or outcomes of this study (registries and long-term follow-up of other studies are allowed) |
| Subjects who have undergone angiography- or wire-based coronary physiological assessment for 1 or more potential study lesions within 30 days of enrolment |
| Angiographic exclusion criteria |
| Coronary angiograms not acquired per instructions as defined in the study protocol |
| Study lesion is the clear culprit for an NSTE-ACS |
| Angiographic evidence of procedural complication (e.g., acute stent thrombosis, flow-limiting dissection, perforation, slow/no reflow) prior to randomisation |
| TIMI 2 flow or lower in study vessel at time of enrolment |
| Study vessel is in a left coronary vessel with a separate left anterior descending and left circumflex ostia arising from the aorta (i.e., no left main coronary artery) |
| Study lesion involves left main coronary artery (stenosis ≥50%) |
| Study lesion is in an ectatic or aneurysmal coronary segment (defined as a lumen diameter 1.5 times the diameter of the reference vessel) |
| CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome; FFRangio: angiography-derived fractional flow reserve; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTE-ACS: non-ST-segment elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction |
Primary and secondary endpoints
The primary endpoint is the incidence of MACE at 1 year, defined as the composite of all-cause death, myocardial infarction (MI), or unplanned clinically driven revascularisation. For the principal analysis of the primary endpoint, spontaneous MI will be adjudicated according to the 4th Universal Definition of MI, and Type 4 MI will be adjudicated according to the Academic Research Consortium (ARC)-2 definition of periprocedural MI (Table 2).
Secondary endpoints include periprocedural complications and 30-day adverse events, individual components of MACE at 1 year, procedure duration and resource utilisation, patient-reported health status, healthcare-related costs, and usability of the FFRangio and pressure wire systems. Exploratory analyses will assess the relationship between post-PCI FFRangio results and the risk of adverse clinical outcomes.
Table 2. Definition of the primary endpoint.
| Death | Death events will be adjudicated by the CEC using Academic Research Consortium-2 definitions. |
|---|---|
| Cardiovascular death | Cardiovascular death is defined as death resulting from cardiovascular causes. The following categories may be collected: |
| Death caused by acute MI | |
| Death caused by sudden cardiac, including unwitnessed, death | |
| Death resulting from heart failure | |
| Death caused by stroke | |
| Death caused by cardiovascular procedures | |
| Death resulting from cardiovascular haemorrhage | |
| Death resulting from other cardiovascular causes | |
| Non-cardiovascular death | Non-cardiovascular death is defined as any death that is not thought to be the result of a cardiovascular cause. The following categories may be collected: |
| Death resulting from malignancy | |
| Death resulting from pulmonary causes | |
| Death caused by infection (including sepsis) | |
| Death resulting from gastrointestinal causes | |
| Death resulting from accident/trauma | |
| Death caused by other non-cardiovascular organ failure | |
| Death resulting from another non-cardiovascular cause | |
| Undetermined cause of death | Undetermined cause of death is defined as a death not attributable to any other category because of the absence of any relevant source documents. Such deaths will be classified as cardiovascular for endpoint determination. |
| Myocardial infarction | |
| Post-PCI (Type 4a) periprocedural MI | Periprocedural MI will be adjudicated as per Academic Research Consortium-2 definitions as follows: |
| Absolute rise in cardiac troponin (from baseline) ≥35 times upper reference limit (if creatine kinase MB is used, an absolute rise of ≥5 times the upper reference limit is required) | |
| Plus 1 (or more) of the following criteria: | |
| New significant Q waves or equivalent (≥40 ms in duration and ≥1 mm deep in voltage in 2 contiguous leads) | |
| Flow-limiting angiographic complications | |
| New “substantial” loss of myocardium on imaging | |
| Spontaneous MI (MI Type 1) |
Spontaneous MI (MI Type 1) will be defined based on the 4th Universal Definition of Myocardial Infarction. Spontaneous MI (Type 1) will be defined as the detection of a rise and/or fall of cardiac troponin values with at least 1 value above 99th upper reference limit and with at least 1 of the following: |
| Symptoms of acute myocardial ischaemia | |
| New ischaemic electrocardiogram changes | |
| Development of pathological Q waves | |
| Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with ischaemic aetiology | |
| Identification of a coronary thrombus by angiography including intracoronary imaging or by autopsy | |
| Spontaneous MI (MI Type 2) |
Spontaneous MI (MI Type 2) will be defined based on the 4th Universal Definition of Myocardial Infarction. Spontaneous MI (Type 2) will be defined as the detection of a rise and/or fall of cardiac troponin values with at least 1 value above 99th upper reference limit, and evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute coronary atherothrombosis, requiring at least 1 of the following: |
| Symptoms of acute myocardial ischaemia | |
| New ischaemic ECG changes | |
| Development of pathological Q waves | |
| Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with ischaemic aetiology | |
| Clinically indicated revascularisation | A revascularisation is clinically indicated if angiography at follow-up shows a percentage diameter stenosis ≥50% (by core lab QCA*) and if 1 of the following is present: |
| History of recurrent angina pectoris (or anginal equivalent symptoms), presumably related to the study vessel | |
| Objective signs of ischaemia at rest (ECG changes or biomarker changes) or during stress/exercise test (or equivalent) presumably related to the study vessel | |
| Abnormal results of any invasive physiological test | |
| Asymptomatic with ≥70% DS by core lab QCA or, if core lab QCA is not available, ≥90% DS by visual estimate (site reported) | |
| *The QCA core laboratory will be preferred for assessment of the clinically indicated revascularisation by the CEC. If QCA or angiograms are not available (e.g., due to imaging not being readable or angiogram permanently missing), then catheterisation core laboratory reports could be used for event adjudication of revascularisation. The CEC will determine whether revascularisation is clinically indicated or not for all types of revascularisation (study lesion, study vessel, and non-study vessel). CEC: clinical events committee; DS: diameter stenosis; ECG: electrocardiogram; MI: myocardial infarction; PCI: percutaneous coronary intervention; QCA: quantitative coronary analysis | |
Enrolment and randomisation
Patients who have signed an institutional review board/ethics committee-approved informed consent form and who have met all inclusion criteria and none of the exclusion criteria will be eligible for enrolment and randomisation. After obtaining the necessary angiograms, and prior to randomisation, the investigator will identify the vessels in which physiology is indicated (i.e., identify the study lesions which they plan to interrogate by wire-based physiology assessment if the patient is randomised to wire-based physiology), as well as which pressure wire-based physiological test will be performed (i.e., FFR or NHPR) if the patient is randomised to wire-based physiology. Prior to randomisation, the investigator will also declare, in detail, an angiography-based treatment plan for each such study lesion based on the angiographic information alone (i.e., whether they would perform or defer PCI) using a standardised case report form.
Block randomisation using permuted block sizes of 2 and 4 will be performed, with stratification by site, mode of pressure wire-based physiology test (FFR vs NHPR) and clinical presentation (NSTE-ACS vs CCS). Each patient will be randomised in a 1:1 fashion to either FFRangio or pressure wire-based coronary physiology assessment using an online tool (study database/electronic data capture). The subsequent treatment will be determined by the results of the assigned physiological test (Figure 1). Crossover to the alternative physiological guidance system will be considered a protocol deviation.
Study procedures
Diagnostic coronary angiography will be performed per the standard of care at each site but should adhere to the requirements for FFRangio assessment outlined in Supplementary Table 1 (technical requirements) and Supplementary Figure 1 (recommended angiographic projections). Intracoronary nitroglycerine is recommended but not required.
Pressure wire-based measurements
For subjects randomised to a pressure wire-based assessment, the acquisition of diagnostic images, the intended treatment plan, and the diagnostic FFR/NHPR measurements will be performed according to the standard of care at each site, in accordance with the guidelines below. An anticoagulant such as intravenous heparin will be administered, as will intracoronary nitroglycerine. If FFR is performed, use of adenosine will be preferred. In sites where adenosine is not available, administration of adenosine triphosphate (ATP) or papaverine will be permitted. FFR/NHPR measurements will follow the steps outlined in Supplementary Table 1.
FFRangio measurement
For subjects randomised to FFRangio-based assessment, the initial FFRangio measurement will be performed after acquisition of the routine diagnostic images and only after the intended treatment plan has been fully documented. If additional angiographic images are required to allow for FFRangio assessment, the number of additional angiograms used will be recorded. The process of assessing FFRangio is shown in Supplementary Table 2.
PCI procedure
Based on the results of either the wire-based physiological assessment or FFRangio, PCI will be performed on all haemodynamically significant lesions using established cutoff points (Figure 1)2326. PCI procedures will be performed according to standard techniques as determined by the primary operator. Staged procedures are permitted within 60 days in vessels not treated during the index procedure as per ARC-2 recommendations27. If no PCI procedure is indicated, the patient will be treated with optimal medical therapy alone at the discretion of the treating physician.
Post-PCI coronary angiography and FFRangio assessment
Two post-PCI angiograms performed at two of the original pre-PCI views will be acquired in all patients, irrespective of randomised treatment arm. Offline post-PCI FFRangio analysis will be performed using the 2 post-PCI angiograms and a third pre-PCI angiogram in which the treated lesion will be “ignored” to derive a post-PCI FFRangio measurement.
Follow-up
Postprocedural electrocardiograms and cardiac biomarkers (troponin T, if available, or biomarkers per local site standard of care) will be acquired only if there is a clinical suspicion of procedural complication or periprocedural MI. Follow-up visits will be performed at 30 days, 6 months, and 1 year after randomisation. Medication use and adverse events will be assessed at each visit. Both generic and disease-specific quality of life will be assessed at baseline, 30 days, and 1 year using the EuroQol 5-dimension 5-level (EQ-5D-5L) questionnaire, and the Seattle Angina Questionnaire-7 (SAQ-7) (Table 3).
Table 3. Schedule of activities.
| Study requirement | Baseline | Index procedure | 30±7 days | 6 months±14 days* | 1 year±30 days |
|---|---|---|---|---|---|
| Informed consent | X | ||||
| Demographics | X | ||||
| Eligibility criteria | X | ||||
| Medical history | X | ||||
| Clinical assessment | X† | X | X | ||
| Pregnancy test‡ | X | ||||
| Electrocardiogram§ | X | ||||
| SAQ-7 | X | X | X | ||
| EQ-5D-5L QOL assessment | X | X | X | ||
| Medications | X | X | X | X | X |
| Coronary angiography | X | ||||
| Procedural information | X | ||||
| Randomisation (FFRangio or wire-based FFR/NHPR) | X | ||||
| PCI procedure (if appropriate) | X|| | ||||
| Record of adverse events | X | X | X | X | |
| *Six-month assessment to be performed via phone consultation. †Clinical assessment includes cardiac biomarkers in acute coronary syndrome presentation. ‡Pregnancy test for women of childbearing potential. §For subjects presenting with NSTE-ACS. ||PCI can be staged. EQ-5D-5L: EuroQol 5-dimension 5-level; FFR: fractional flow reserve; FFRangio: angiography-derived FFR; NHPR: non-hyperaemic pressure ratio; NSTE-ACS: non-ST-segment elevation acute coronary syndrome; PCI: percutaneous coronary intervention; QOL: quality of life; SAQ: Seattle Angina Questionnaire | |||||
Statistical methods
The primary analysis will be performed based on the intention-to-treat (ITT) population.
Primary endpoint analysis
A Kaplan-Meier survival analysis will compare the 12-month cumulative incidence of MACE between FFRangio and pressure wire-based physiology. The Com-Nougue method will test the 1-sided non-inferiority hypothesis by evaluating whether the difference in event probabilities remains within the predefined non-inferiority margin. Based upon an estimated 12-month MACE rate of 7.5% in both study arms, using a 3.5% absolute non-inferiority margin and a 1-sided p-value<0.025, and assuming that 5% of patients will be lost to follow-up, a sample size of 1,924 evaluable patients is required to provide 82.7% power. Missing data will not be imputed in the primary analysis. If the primary endpoint analysis demonstrates non-inferiority of FFRangio, reflex superiority testing will also be performed (testing superiority of FFRangio over pressure wire-based assessment)28.
Sensitivity analyses of the primary endpoint will be performed on the ITT and per-protocol populations using multiple imputation.
Justification of the non-inferiority margin
Based on the available literature including clinical trials that have evaluated the use of coronary physiology to guide revascularisation, coronary stent trials, and other cardiovascular studies, the 1-year rate of the primary endpoint has been estimated to be 7.5% (Table 4). The prespecified non-inferiority margin is 3.5%, which represents <50% of the expected 1-year event rate of 7.5%, and was based on what the Steering Committee agreed was an acceptable upper bound for non-inferiority. This non-inferiority margin is similar to the non-inferiority margins used in the iFR-SWEDEHEART (3.2%)10 and DEFINE-FLAIR (3.4%)9 trials, which compared two invasive, wire-based physiology measures; and the FAVOR III Europe trial (3.4%), which compared non-invasive quantitative flow ratio (QFR) versus invasive FFR for guiding coronary revascularisation21.
Table 4. Clinical trials evaluating coronary physiology prior to revascularisation.
| Study | Citation | Comparators | N1 | N2 | 1-year MACE | Notes | |
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||||
| DEFINE-FLAIR | Davies et al9 | iFR vs FFR | 1,148 | 1,182 | 6.8 | 7 | All-cause death, non-fatal MI, unplanned revascularisation |
| FAME 3 | Fearon et al29 | FFR PCI vs CABG | 757 | 743 | 10.6 | 6.9* | Death, MI, stroke, repeat revascularisation, excluding CABG |
| FLOWER-MI | Puymirat et al30 | FFR vs angiography | 586 | 577 | 5.5 | 4.2* | All-cause death, non-fatal MI, unplanned hospitalisation for revascularisation, excluding angio-guided arm |
| FLAVOUR | Koo et al31 | FFR vs IVUS | 838 | 844 | 4.6 | 3.4* | Death, MI, revascularisation, excluding IVUS-guided arm |
| FAME 25 | De Bruyne et al5 | FFR PCI vs GDMT | 447 | 441 | 4.3 | 12.7* | Death, MI, urgent revascularisation, excluding medical therapy arm |
| FAME | Tonino et al4 | Angio-PCI vs FFR PCI | 496 | 509 | 18.3* | 13.2 | Death, MI, revascularisation, excluding angio-guided group |
| iFR SWEDEHEART | Götberg et al10 | iFR vs FFR | 1,019 | 1,018 | 6.7 | 6.1 | Death from any cause, non-fatal MI, unplanned revascularisation |
| COMPARE Acute | Smits et al32 | FFR vs angiography | 295 | 590 | 7.8 | 20.5* | STEMI post-infarct artery; MACCE; all-cause mortality, non-fatal MI, any revascularisation, cerebrovascular events (no cerebrovascular events in the complete arm, excluding infarct-only arm) |
| DEFER | Bech et al33 | Deferral of PTCA/PCI based on FFR vs performance | 91 | 144 | -* | -* | Excluded given no clear MACE endpoint |
| DANAMI-3-PRIMULTI | Engstrøm et al34 | FFR-guided complete revasc vs none after STEMI | 313 | 314 | 22* | 13 | All-cause mortality, non-fatal MI, IDR; excluding the no further revascularisation group |
| FAVOR III China | Xu et al35 | QFR vs angio-guided PCI | 1,912 | 1,913 | 8.8* | 5.8 | All-cause death, MI, IDR; excluding angio-guided patients |
| *These cells were not included in the weighted calculation due to alternative revascularisation or treatment modalities or a lack of physiological assessment prior to revascularisation or MACE endpoint adjudication. CABG: coronary artery bypass grafting; FFR: fractional flow reserve; GDMT: guideline-directed medical therapy; IDR: ischaemia-driven revascularisation; iFR: instantaneous wave-free ratio; IVUS; intravascular ultrasound; MACCE: major adverse cardiovascular and cerebrovascular events; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; QFR: quantitative flow ratio; revasc: revascularisation; STEMI: ST-segment elevation myocardial infarction | |||||||
Secondary endpoint and subgroup analyses
These analyses will be considered exploratory without adjustment for multiplicity. The primary and secondary endpoints will be compared across the subgroups listed in Supplementary Table 3.
Economic analyses
In addition to the main clinical study, data from the ALL-RISE trial will be used to perform an analysis of the economic benefit of FFRangio compared with wire-based assessments. Hospital costs will be assessed for all patients based on procedural and hospitalisation resource utilisation and standard US costs for each resource (including procedural time). Follow-up costs will be assessed for inpatient and outpatient cardiovascular care, including diagnostic testing, emergency room visits, hospitalisations, and additional coronary revascularisation procedures.
Given the non-inferiority design of the ALL-RISE trial, major differences in follow-up events or “downstream costs” between the two treatment groups are not expected. As such, the primary economic analysis will focus on index procedural costs and index hospitalisation costs and their differences. A secondary analysis will examine follow-up healthcare-related costs and total 1-year costs (including the index hospitalisation).
Study status and ongoing trials of other angio-based FFR systems
ALL-RISE completed recruitment in January 2025. The primary endpoint is at 1 year.
Several other angiography-derived coronary physiology indices are currently being evaluated in prospective, randomised clinical trials (Supplementary Table 4). Notably, the Functional Assessment by Virtual Online Reconstruction III – Europe (FAVOR III Europe) trial reported that QFR-guided PCI was inferior to FFR-guided PCI for the primary composite endpoint of all-cause death, MI, and unplanned revascularisation at 12 months.
Discussion
FFRangio uses a lumped resistance model instead of computational fluid dynamics, 3 angiograms instead of 1-2, and assesses the whole coronary tree with all its main branches, not just a single vessel or vessel segment. A comparison of current angio-based coronary technologies is presented in Supplementary Table 5. All of these technologies are different, with varying levels of diagnostic accuracy and reliability, and each one needs to be assessed on its own merits instead of grouping them all into a class effect. These findings have raised important questions regarding the clinical performance and reliability of angiography-based physiological assessment tools. In this context, the design of ALL-RISE, with prerandomisation designation of study lesions and detailed adjudication of angiographic lesions and clinical events, will offer important insights into the diagnostic and prognostic performance of FFRangio.
Limitations
Study investigators and teams will not be blinded to treatment assignment. However, after obtaining coronary angiograms, investigators must document a detailed treatment plan prior to randomisation (i.e., for each lesion, state whether they would treat or defer based on angiography alone). To mitigate the risk of bias in endpoint assessment, the clinical events committee will be blinded to treatment allocation, unless unblinding is necessary to determine device/procedure relatedness.
Both FFR and NHPR indices may be used in the control arm of ALL-RISE. While most studies suggest comparable performance, some indicate that NHPR may be less reliable than FFR. If a patient is randomised to pressure wire-based physiology and the operator doubts the result, they may remeasure using the alternative method. In cases of discordance, clinical judgment will guide which result to follow. Randomisation is stratified by the intended use of FFR or NHPR in the control arm, enabling FFRangio to be compared separately with each in exploratory analyses.
The components of the primary endpoint differ in clinical relevance and, likely, in their causal link to the intervention. Events unrelated to the diagnostic strategy may dilute any true differences between groups and increase the likelihood of meeting the non-inferiority margin. Therefore, considerable emphasis will be placed on interpreting the totality of the trial data, beyond the formal statistical test of non-inferiority.
Lastly, the high concordance between FFRangio and pressure wire-based FFR seen in FAST-FFR may limit the number of treatment decisions affected by randomisation, diluting observed effects and reducing power. However, if clinical outcomes after PCI are similar with both strategies, FFRangio may reasonably be considered non-inferior for guiding revascularisation.
Conclusions
ALL-RISE is a large-scale, prospective, randomised trial powered to test whether FFRangio-guided treatment leads to non-inferior rates of 1-year MACE when compared with conventional pressure wire-guided treatment in patients with coronary artery disease being evaluated for PCI. ALL-RISE will also assess the extent to which FFRangio-guided treatment affects short- and long-term resource utilisation and cost-effectiveness. With a goal of 1,924 patients randomised and followed up for 12 months, we expect that ALL-RISE will provide prospective clinical outcomes data on the relative safety, efficacy and cost-effectiveness of a workflow using FFRangio as compared with conventional wire-based approaches to coronary lesion assessment.
Funding
The ALL-RISE trial is sponsored by CathWorks, Inc.
Conflict of interest statement
B. Redfors reports consultant fees from Pfizer and Boehringer Ingelheim. M.V. Madhavan reports institutional educational grants to Columbia University from Boston Scientific. A.J. Kirtane reports institutional funding to Columbia University and/or the Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott, Amgen, CathWorks, Concept Medical, Philips, Recor Medical, Neurotronic, Biotronik, Chiesi, Bolt Medical, Magenta Medical, SoniVie, and Shockwave Medical; in addition to research grants, institutional funding includes fees paid to Columbia University and/or the Cardiovascular Research Foundation for consulting and/or speaking engagements in which A.J. Kirtane controlled the content; he has equity options in Bolt Medical and Airiver; and has received travel expenses/meals from Amgen, Medtronic, Biotronik, Boston Scientific, Abbott, CathWorks, Concept Medical, Novartis, Philips, Abiomed, Recor Medical, Chiesi, Zoll, Shockwave Medical, and Regeneron. W.F. Fearon reports institutional research support from Abbott, CathWorks, and Medtronic; consultant fees from Shockwave Medical; and has stock options with HeartFlow. R.Y. Yeh reports research grants from Abbott, Boston Scientific, and Medtronic; and serves as a consultant for Abbott, Boston Scientific, CathWorks, Edwards Lifesciences, Elixir Medical, Magenta Medical, Medtronic, and Shockwave Medical. D.J. Cohen reports institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Philips, CathWorks, and Corvia; and consulting income from Abbott, Boston Scientific, Edwards Lifesciences, Elixir Medical, and HeartBeam. R. Al-Lamee receives consultancy fees from Janssen Pharmaceuticals, Shockwave Medical, Abbott, CathWorks, Medtronic, and Philips; and has received speaker fees from Abbott, CathWorks, Philips, Medtronic, Servier, Omniprex, and Menarini. A. Jeremias reports consultant fees from Abbott, Philips, ACIST Medical Systems, Shockwave Medical, and Neovasc. G. Witberg has received consulting fees from CathWorks and Medinol. R.P. Sharma has received consulting fees, speaker fees, and honoraria from Edwards Lifesciences; has received consulting fees and has equity interest in egnite, Inc; and has received speaker fees and honoraria from Boston Scientific and Abbott. A. Kaki has received speaker honoraria, consultant fees, and research funding from Abiomed, Abbott, CSI, and Terumo. A. Froimovich is an employee at CathWorks. M.B. Leon reports institutional research support from Abbott, Boston Scientific, and Medtronic; and consulting equity from Triventures (founding investor in CathWorks). A. Popma has no relevant conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

