Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The biolimus A9-coated polymer-free stent was not non-inferior for target lesion failure (TLF) when compared with an ultrathin-strut biodegradable-polymer sirolimus-eluting stent within 1 year in the Randomized Comparison of a Polymer-Free Biolimus-eluting BIOFREEDOM Stent With a Biodegradable-Polymer Sirolimus-eluting ORSIRO Stent in Patients Treated With Percutaneous Coronary Intervention (SORT OUT IX) trial.
Aims: We aimed to assess the 5-year outcomes of the drug-coated polymer-free biolimus-eluting stent versus an ultrathin-strut biodegradable-polymer sirolimus-eluting stent.
Methods: The SORT OUT IX trial was a registry-based, randomised, all-comer population trial. The primary endpoint, TLF, was defined as the composite of cardiac death, myocardial infarction (MI) not related to any segment other than the target lesion, or target lesion revascularisation (TLR) within 1 year. Follow-up was extended to 5 years.
Results: A total of 3,151 patients were randomly assigned to receive either the polymer-free biolimus-coated stent (1,572 patients [1,966 lesions]) or the ultrathin-strut biodegradable-polymer sirolimus-eluting stent (1,579 patients [1,985 lesions]). Only 0.3% of participants were lost to follow-up, and all of those were due to emigration. At 5 years, TLF was 14.1% in the polymer-free biolimus-coated stent group and 12.0% in the ultrathin-strut biodegradable-polymer sirolimus-eluting stent group (rate ratio [RR] 1.19, 95% confidence interval [CI]: 0.97-1.44). Cardiac death, MI and definite stent thrombosis did not differ between the two groups. Clinically driven TLR was 7.6% versus 5.0% (RR 1.56, 95% CI: 1.17-2.07) and was higher in the polymer-free biolimus-coated stent group at 5 years, attributable to a higher risk of TLR within the first year.
Conclusions: Five-year TLF did not differ significantly between the drug-coated biolimus-eluting stent group and the ultrathin-strut biodegradable-polymer stent group, but the risk of TLR was higher in the drug-coated biolimus-eluting stent group.
The durable polymer used in first- and second-generation drug-eluting stents (DES) has been suspected to be a potential trigger for vessel wall inflammation and late adverse outcomes after percutaneous coronary intervention (PCI)1. Hence, DES with biodegradable polymers have been developed to reduce this risk2345. The biodegradable polymers in DES may not have a class effect, as polymer degradation times, drug release profiles, inflammatory potential, and stent strut thickness can differ. The randomised controlled SORT OUT VII trial5 showed non-inferiority of the ultrathin-strut biodegradable-polymer sirolimus-eluting stent as compared to the biodegradable-polymer biolimus-eluting stent for target lesion failure (TLF) at 1-year and 5-year follow-up6. Definite stent thrombosis was less frequent within the first year in the ultrathin-strut biodegradable-polymer sirolimus-eluting stent group, but this difference was not maintained after 5 years, and no significant difference was found between 1 and 5 years. In the BIOSCIENCE trial27, the ultrathin-strut biodegradable-polymer sirolimus-eluting stent had a similar TLF rate as the durable-polymer everolimus-eluting stent after 5 years. In contrast, in an ST-segment elevation myocardial infarction (MI) population, the ultrathin-strut biodegradable-polymer sirolimus-eluting stent was superior to the durable-polymer everolimus-eluting stent with respect to TLF after 5 years8.
Potential limitations of polymers may also be solved by polymer-free drug-coated stents91011, which have been compared with bare metal stents (BMS) in patients with high bleeding risk12. In the LEADERS FREE trial12, the polymer-free drug-coated biolimus-eluting stent was superior to a BMS in patients with high bleeding risk with respect to safety and efficacy endpoints after 2 years13.
In the Randomized Comparison of a Polymer-Free Biolimus-eluting BIOFREEDOM Stent With a Biodegradable-Polymer Sirolimus-eluting ORSIRO Stent in Patients Treated With Percutaneous Coronary Intervention (SORT OUT IX) trial14, the polymer-free biolimus A9-coated stent did not meet criteria for non-inferiority compared with the ultrathin-strut biodegradable-polymer sirolimus-eluting stent for major adverse cardiovascular events at 12 months. The follow-up was extended to 5 years, by which time the biodegradable-polymer coating had completely degraded in the biodegradable-polymer stent, and the stent surfaces of both stents were similar to those of BMS.
Methods
Patients and study design
SORT OUT IX was a randomised, multicentre, all-comer, single-blind, two-arm, non-inferiority trial comparing the stainless steel biolimus A9-coated BioFreedom stent (Biosensors) to the biodegradable-polymer sirolimus-eluting Orsiro stent (Biotronik) for the treatment of coronary artery lesions. Details of study conduct, data management and clinically driven event detection have been described previously14. In brief, patients were eligible if they were 18 years or older and had an indication for treatment with a drug-eluting stent. Patients with an allergy to aspirin, clopidogrel, ticagrelor, prasugrel, sirolimus, or biolimus; inability to provide written informed consent; or life expectancy of less than 1 year were not eligible for enrolment. Written informed consent for trial participation was obtained before randomisation. The study complied with the Declaration of Helsinki and was approved by the Regional Committees on Health Research Ethics for Southern Denmark (S-20150132) and the Danish Data Protection Agency (15/47707). The trial was registered at ClinicalTrials.gov: NCT02623140.
Study stents
The biolimus A9-coated BioFreedom stent has a strut thickness of 120 μm. The stent platform is composed of stainless steel and is a polymer-free and carrier-free drug-coated stent. The abluminal surface of the stent has a microstructured surface that contains the biolimus A9. The stent releases umirolimus (also known as biolimus A9), a highly lipophilic sirolimus analogue (15.6 μg/mm2), into the vessel wall over a period of 1 month.
The sirolimus-eluting Orsiro stent has a strut thickness of 60 μm for stents with a nominal diameter of ≤3.0 mm and 80 μm for the two larger sizes. The sirolimus-eluting Orsiro stent surface is fully coated with a layer of amorphous, hydrogen-rich silicon carbide (aSiC:H), which acts as a diffusion barrier (PROBIO [Biotronik]) and seals the bare metal surface, reducing ion release. The asymmetric biodegradable-polymer compound, a carrier material for the delivery and release of sirolimus, is a high-molecular-weight poly L-lactic acid (PLLA). The polymer is fully degraded within 12-24 months. The drug load is 1.4 μg per mm2, and the drug is released over a period of 12-14 weeks.
Outcome measures
The primary endpoint, TLF, was defined as the composite of cardiac death, target lesion myocardial infarction (not related to any lesion other than the study lesion) or target lesion revascularisation (TLR) with PCI or coronary artery bypass grafting within 12 months, assessed at 12 months and reported previously14. Follow-up at 5 years was registry based. Follow-up visits were not scheduled but were clinically driven, based on patients seeking care from the healthcare system for self-reported angina or equivalent symptoms. Secondary endpoints in the study comprised the individual components of the primary endpoint (i.e., cardiac death, MI and TLR), all-cause death (cardiac and non-cardiac), target vessel revascularisation, and stent thrombosis according to the Academic Research Consortium definition15, and a patient-related composite endpoint (all death, all MI, or any revascularisation). All definitions have been described previously14. An independent event committee reviewed all endpoints and source documents to adjudicate causes of death, reasons for hospital admission, and diagnosis of MI. Two dedicated PCI operators at each participating centre independently reviewed cine films for the event committee to classify stent thrombosis, TLR and target vessel revascularisation (with either PCI or coronary artery bypass grafting). The independent event committee was blinded to study stent type assignment during the adjudication process (Supplementary Appendix 1). This methodology has been used in previous SORT OUT studies51617.
Clinical event detection
At 5-year follow-up, data on mortality, coronary angiography, repeat PCI, coronary artery bypass grafting and hospital admission were obtained from the following national Danish administrative and healthcare registries: the Civil Registration System18; the Western Denmark Heart Registry19; and the Danish National Registry of Patients20. The Danish National Registry of Patients maintains records on all hospitalisations in Denmark. Universal tax-supported healthcare is provided by the Danish National Health Service, guaranteeing residents free access to general practitioners and hospitals. The Danish Civil Registration System has kept electronic records on sex, birth date, residence, emigration date, and vital status changes since 196818, with daily updates; the 10-digit civil registration number assigned at birth and used in all registries allows accurate record linkage. Loss to follow-up was minimised in the study, as vital status data for our study participants were provided by the Civil Registration System.
Statistical analysis
Distributions of continuous variables between the two study arms were compared using the 2-sample t-test (or the Cochran test for cases of unequal variance) or the Mann-Whitney U test, depending on whether the data followed a normal distribution. Distributions of categorical variables were compared using the χ² test. For analyses of all endpoints, follow-up continued until the date of an endpoint event or death, or 60 months after stent implantation or emigration, whichever came first. Time-to-event curves (depicted as cumulative incidence) were constructed, accounting for the competing risk of death (in cases where death was not included in the outcome). Patients who received the ultrathin-strut biodegradable-polymer sirolimus-eluting stent were used as the reference group overall and for subgroup analyses. Rate ratios (RR) were calculated for TLF at 5-year follow-up. The intention-to-treat principle was used in all analyses. A 2-sided p-value of <0.05 was considered statistically significant. Analyses were conducted using SAS software, version 9.4 (SAS Institute).
Results
A total of 3,151 patients were included in the study between 14 December 2015 and 21 April 2017. The patients were randomly assigned to receive either the polymer-free biolimus-coated stent (1,572 patients [1,966 lesions]) or the ultrathin-strut biodegradable-polymer sirolimus-eluting stent (1,579 patients [1,985 lesions]). Nine patients (biolimus-coated stent: 2; sirolimus-eluting stent: 7) were lost to follow-up, all due to emigration (0.3%) (Figure 1). Selected baseline and lesion characteristics are presented in Table 1.
At 5-year follow-up, the primary endpoint, TLF, was 14.1% in the polymer-free biolimus-coated stent group and 12.0% in the ultrathin-strut biodegradable-polymer sirolimus-eluting stent group (RR 1.19, 95% confidence interval [CI]: 0.97-1.44) (Central illustration, Table 2, Figure 2). TLF did not differ significantly between the polymer-free biolimus-coated stent group and the ultrathin-strut biodegradable-polymer sirolimus-eluting stent group within the first year or between 1 to 5 years (Table 2, Figure 3). Cardiac death, non-cardiac death, MI, and definite stent thrombosis did not differ significantly between the two groups at 5 years. The risk of clinically driven TLR (7.6% vs 5.0%; RR 1.56, 95% CI: 1.17-2.07) was higher in the polymer-free biolimus-coated stent group compared with the ultrathin-strut biodegradable-polymer sirolimus-eluting stent group at 5 years, attributable to a higher risk of TLR within the first year (3.5% vs 1.3%; RR 2.76, 95% CI: 1.65-4.62). From 1 to 5 years, TLR did not differ significantly between the two stent types (4.4% vs 3.9%; RR 1.13, 95% CI: 0.80-1.62) (Table 2, Figure 2, Figure 3). The findings for TLF were consistent across prespecified subgroups (Figure 4). When timepoints were divided into 0-2 years and 2-5 years (corresponding to the time at which the polymer had degraded on the ultrathin-strut biodegradable-polymer sirolimus-eluting stent), there were no significant differences in the outcomes (Supplementary Table 1) except for a higher rate of TLR in the polymer-free biolimus-coated stent within the first 2 years and after 5 years, whereas the rates of TLR did not differ between the groups from 2 to 5 years. The patient-related composite endpoint (all death, all MI, or any revascularisation) at 5 years (30.6% vs 28.8%; RR 1.06, 95% CI: 0.93-1.22) did not differ significantly between the two groups (Table 2).
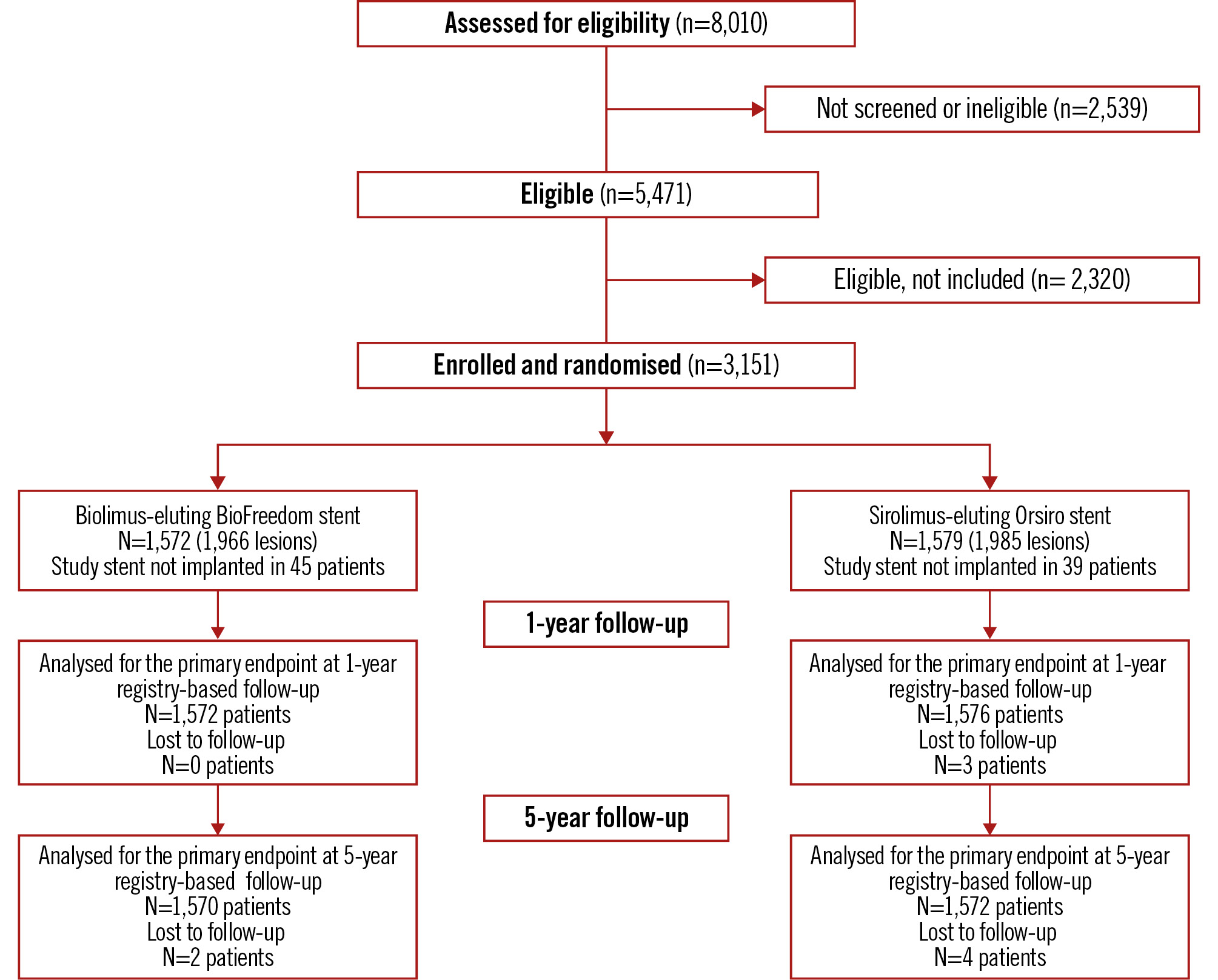
Figure 1. CONSORT flow diagram.
Table 1. Baseline characteristics and summarised lesion and procedural information.
| BioFreedom stent (N=1,572) | Orsiro stent (N=1,579) | p-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 66.4±10.7 | 66.1±11.1 | 0.35 |
| Male sex | 1,219 (77.5) | 1,221 (77.3) | 0.88 |
| Diabetes mellitus | 304 (19.3) | 303 (19.2) | 0.92 |
| Hypertension | 893 (59.0) | 850 (56.0) | 0.21 |
| Hypercholesterolaemia | 830 (55.0) | 777 (51.5) | 0.16 |
| Current smoker | 443 (29.8) | 437 (29.3) | 0.78 |
| Body mass index, kg/m2 | 27.8±7.5 | 27.6±8.0 | 0.47 |
| Previous myocardial infarction | 224 (14.7) | 234 (15.2) | 0.53 |
| Previous percutaneous coronary intervention | 322 (20.9) | 311 (20.1) | 0.59 |
| Previous coronary artery bypass grafting | 130 (8.4) | 108 (7.0) | 0.13 |
| Clinical characteristics | |||
| Indication for percutaneous coronary intervention | 0.61 | ||
| ST-segment elevation myocardial infarction | 367 (23.3) | 397 (25.1) | |
| Non-ST-segment elevation myocardial infarction or unstable angina | 454 (28.9) | 454 (28.8) | |
| Stable angina | 671 (42.7) | 644 (40.8) | |
| Other | 80 (5.1) | 84 (5.3) | |
| Lesion characteristics | |||
| Number of lesions | 1,966 | 1,985 | |
| Target lesions per patient | 0.57 | ||
| 1 | 1,209 (76.9) | 1,196 (75.7) | |
| 2 | 282 (17.9) | 311 (19.7) | |
| 3 | 67 (4.3) | 59 (3.7) | |
| >3 | 14 (0.9) | 13 (0.8) | |
| No. per patient | 1.3±0.6 | 1.3±0.6 | 0.70 |
| Procedural information | |||
| No. of stents | |||
| Per patient | 1.6±1.0 | 1.6±0.9 | 0.28 |
| Per lesion | 1.3±0.6 | 1.2±0.6 | 0.13 |
| Total stent length, mm | |||
| Per patient | 31.1±21.9 | 30.6±19.8 | 0.48 |
| Per lesion | 24.7±16.0 | 24.3±13.6 | 0.32 |
| Reference vessel size, mm | 3.4±0.6 | 3.4±0.6 | 0.51 |
| Differences between stents were tested by χ2 statistics for categorical variables presented as numbers (n.) and proportions (%), by the t-test for continuous variables presented as mean±standard deviation (SD) and with Cochran-Cox approximation in case of unequal variances. | |||
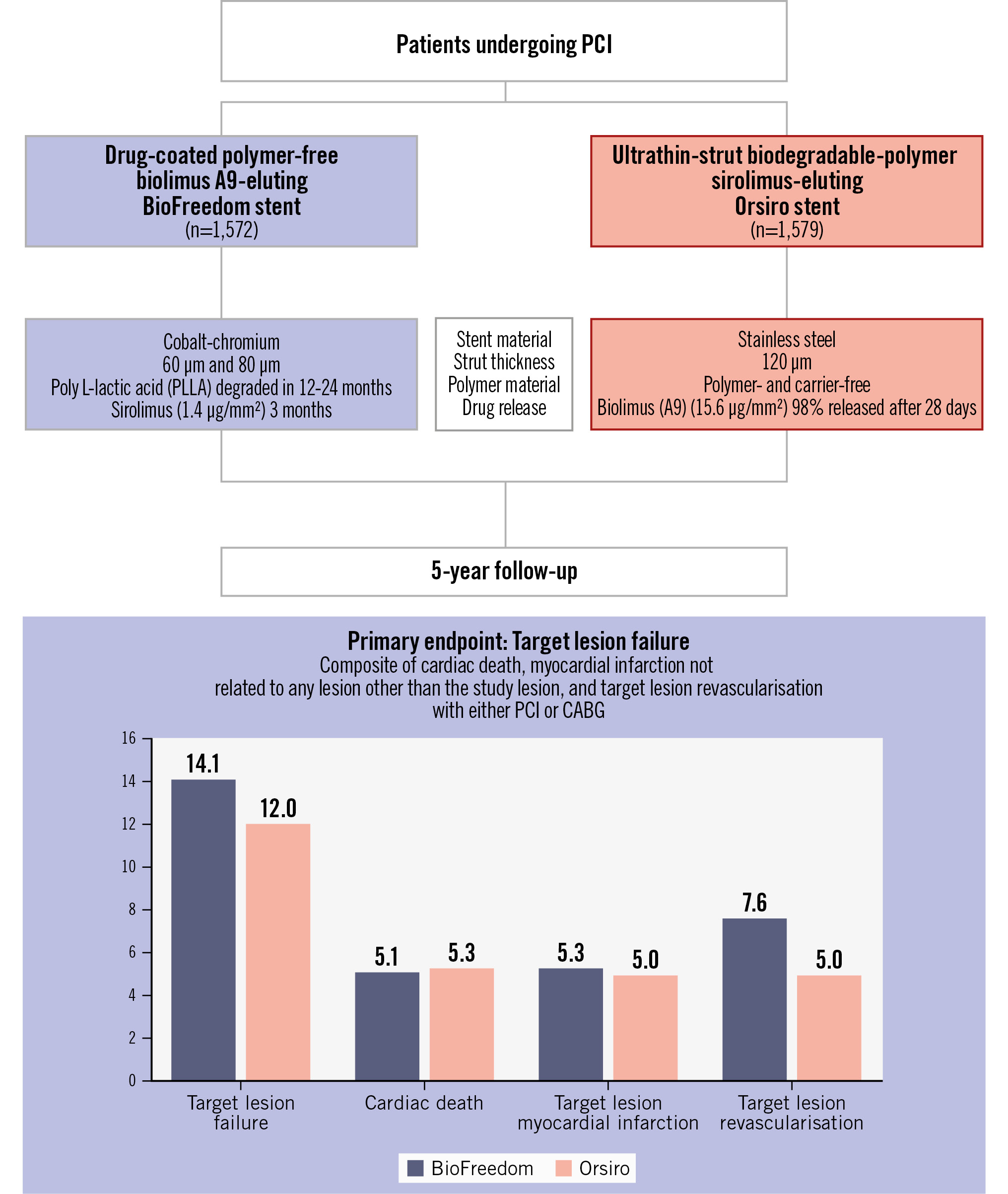
Central illustration. The SORT OUT IX trial. BioFreedom stent by Biosensors; Orsiro stent by Biotronik. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention
Table 2. Five-year clinical outcomes.
| Outcome | BioFreedom | Orsiro | RR (95% CI) | p-value |
|---|---|---|---|---|
| Target lesion failure* | 221 (14.1) | 190 (12.0) | 1.19 (0.97-1.44) | 0.088 |
| 0-1Y | 79 (5.0) | 59 (3.7) | 1.34 (0.96-1.89) | 0.089 |
| 1-5Y | 142 (9.6) | 131 (8.8) | 1.11 (0.88-1.41) | 0.388 |
| Death | ||||
| All-cause death | 186 (11.8) | 178 (11.3) | 1.04 (0.85-1.28) | 0.674 |
| 0-1Y | 31 (2.0) | 43 (2.7) | 0.72 (0.45-1.14) | 0.161 |
| 1-5Y | 155 (10.1) | 135 (8.8) | 1.15 (0.91-1.45) | 0.235 |
| Cardiac death | 80 (5.1) | 84 (5.3) | 0.95 (0.70-1.29) | 0.755 |
| 0-1Y | 16 (1.0) | 29 (1.8) | 0.55 (0.30-1.01) | 0.056 |
| 1-5Y | 64 (4.2) | 55 (3.6) | 1.16 (0.81-1.67) | 0.406 |
| Non-cardiac death | 106 (6.7) | 94 (6.0) | 1.13 (0.86-1.49) | 0.394 |
| 0-1Y | 15 (1.0) | 14 (0.9) | 1.07 (0.52-2.21) | 0.860 |
| 1-5Y | 91 (5.9) | 80 (5.2) | 1.14 (0.84-1.54) | 0.395 |
| Target lesion myocardial infarction | 84 (5.3) | 79 (5.0) | 1.06 (0.78-1.45) | 0.696 |
| 0-1Y | 26 (1.7) | 26 (1.6) | 1.00 (0.58-1.72) | 0.989 |
| 1-5Y | 58 (3.8) | 53 (3.5) | 1.10 (0.75-1.59) | 0.631 |
| Myocardial infarction | 126 (8.0) | 124 (7.9) | 1.02 (0.79-1.30) | 0.900 |
| 0-1Y | 37 (2.4) | 40 (2.5) | 0.92 (0.59-1.44) | 0.722 |
| 1-5Y | 89 (5.9) | 84 (5.6) | 1.06 (0.79-1.43) | 0.699 |
| Stent thrombosis | ||||
| Definite stent thrombosis | 23 (1.5) | 28 (1.8) | 0.82 (0.47-1.43) | 0.481 |
| 0-1Y | 11 (0.7) | 11 (0.7) | 1.00 (0.43-2.30) | 0.992 |
| 1-5Y | 12 (0.8) | 17 (1.1) | 0.70 (0.34-1.48) | 0.354 |
| Definite or probable stent thrombosis | 28 (1.8) | 35 (2.2) | 0.80 (0.48-1.32) | 0.377 |
| 0-1Y | 16 (1.0) | 18 (1.1) | 0.88 (0.45-1.74) | 0.723 |
| 1-5Y | 12 (0.8) | 17 (1.1) | 0.70 (0.34-1.48) | 0.354 |
| Target lesion revascularisation | 120 (7.6) | 79 (5.0) | 1.56 (1.17-2.07) | 0.002 |
| 0-1Y | 55 (3.5) | 20 (1.3) | 2.76 (1.65-4.62) | <0.001 |
| 1-5Y | 65 (4.4) | 59 (3.9) | 1.13 (0.80-1.62) | 0.484 |
| Target vessel revascularisation | 161 (10.2) | 135 (8.5) | 1.21 (0.96-1.52) | 0.115 |
| 0-1Y | 76 (4.8) | 56 (3.5) | 1.35 (0.95-1.91) | 0.090 |
| 1-5Y | 85 (5.8) | 79 (5.3) | 1.09 (0.80-1.49) | 0.573 |
| Patient-related endpoint | 481 (30.6) | 454 (28.8) | 1.06 (0.93-1.22) | 0.365 |
| 0-1Y | 211 (13.4) | 215 (13.6) | 0.97 (0.80-1.18) | 0.774 |
| 1-5Y | 270 (19.8) | 239 (17.6) | 1.14 (0.96-1.36) | 0.142 |
| Values are n (%). Cumulative incidence of a particular event in the given period was calculated with death as the competing risk. Differences in incidence rate ratios were tested using χ2 statistics. *Primary endpoint: target lesion failure – composite of cardiac death, myocardial infarction not related to any lesion other than the target lesion, and target lesion revascularisation with either PCI or CABG. The patient-related endpoint included all death, all myocardial infarctions or any revascularisation. CABG: coronary artery bypass grafting; CI: confidence interval; PCI: percutaneous coronary intervention; RR: rate ratio | ||||
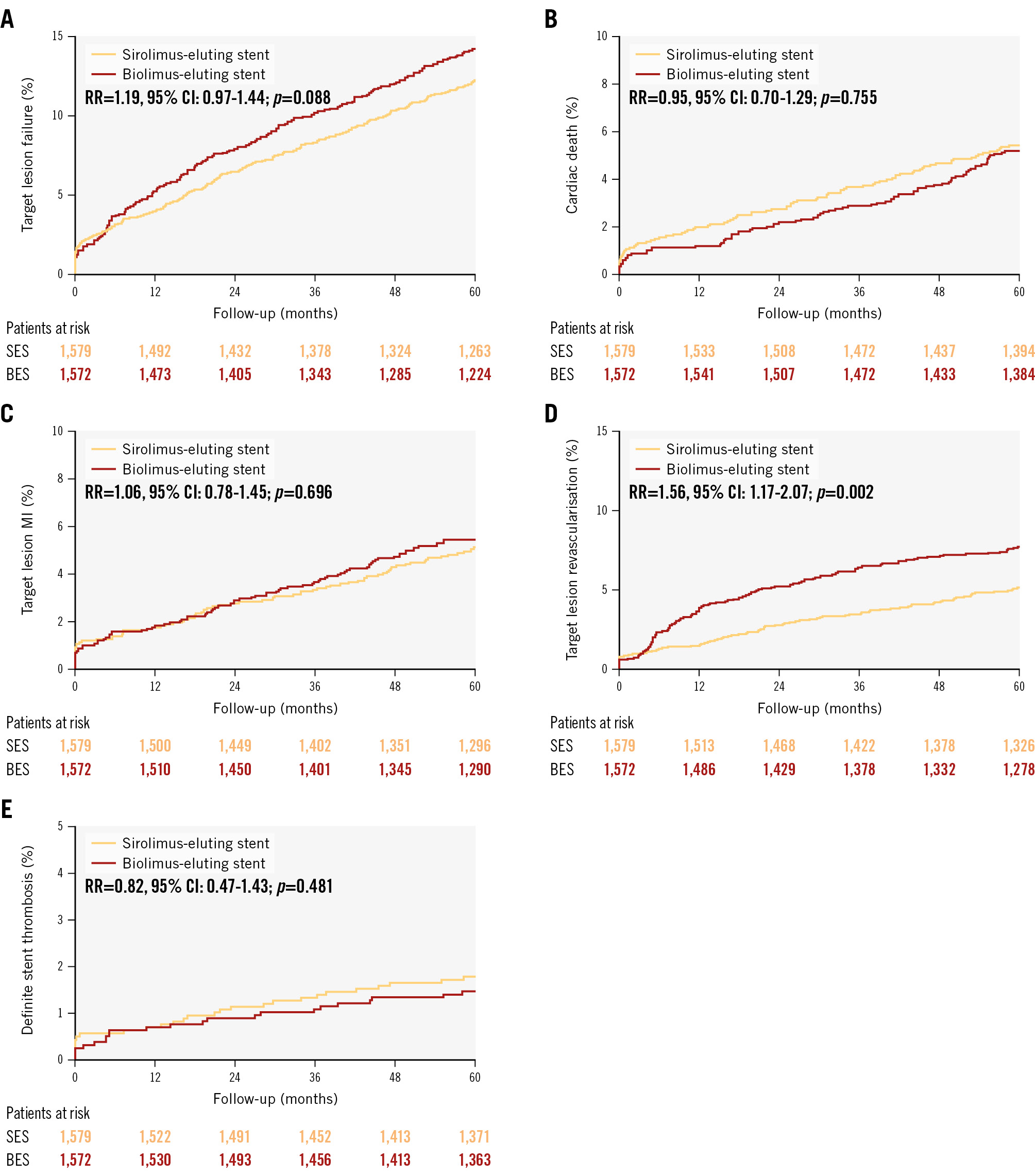
Figure 2. Cumulative incidences for target lesion failure and individual components of the primary endpoint, and definite stent thrombosis at 5 years. A) Target lesion failure; (B) cardiac death; (C) target lesion-related myocardial infarction; (D) target lesion revascularisation; (E) definite stent thrombosis. BioFreedom stent by Biosensors; Orsiro stent by Biotronik. BES: biolimus-eluting stent; CI: confidence interval; MI: myocardial infarction; RR: rate ratio; SES: sirolimus-eluting stent
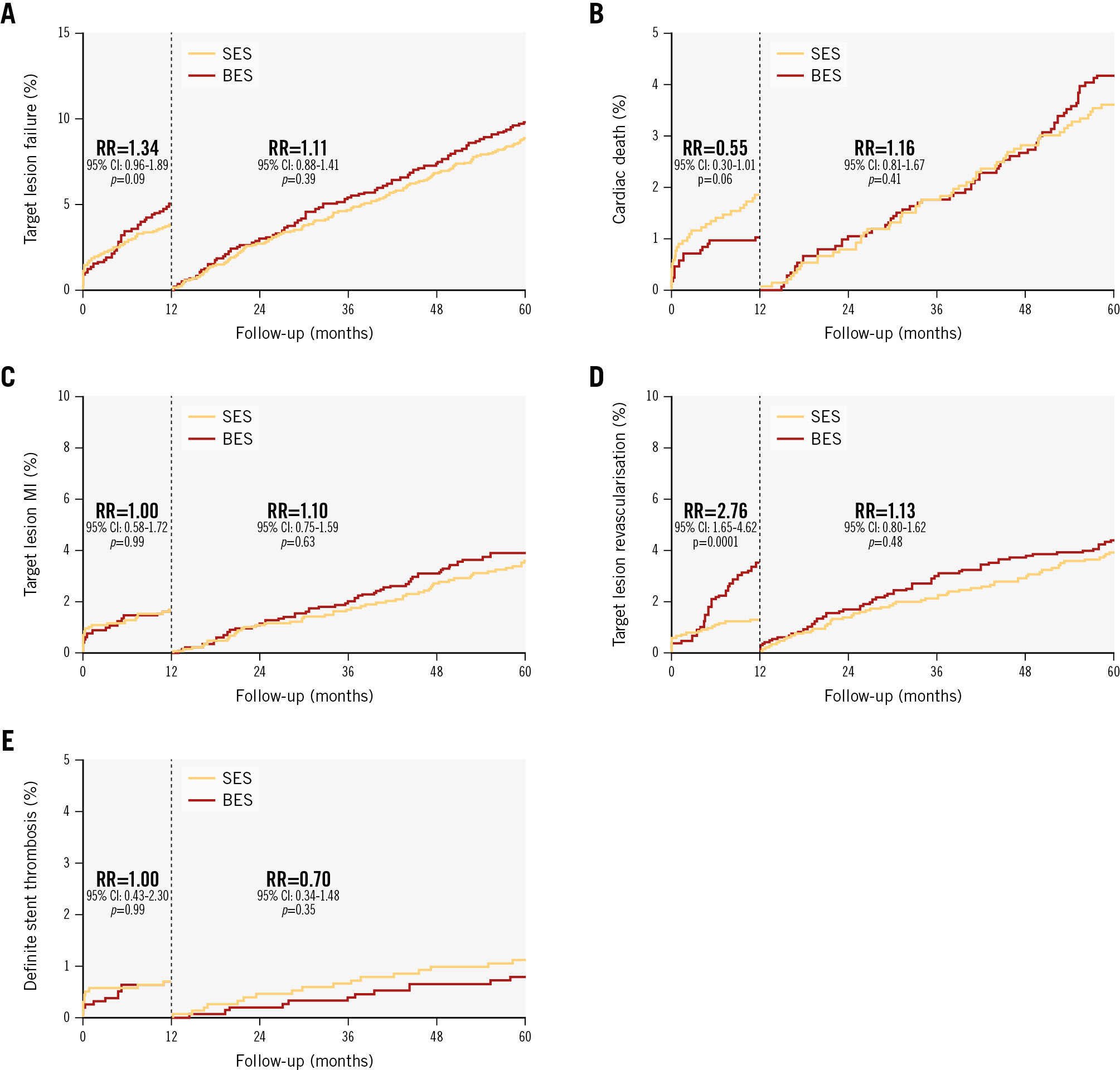
Figure 3. Cumulative incidences for target lesion failure and individual components of the primary endpoint, and definite stent thrombosis with a landmark set at 1 year. A) Target lesion failure; (B) cardiac death; (C) target lesion-related myocardial infarction; (D) target lesion revascularisation; (E) definite stent thrombosis. BES: biolimus-eluting stent; CI: confidence interval; MI: myocardial infarction; RR: rate ratio; SES: sirolimus-eluting stent
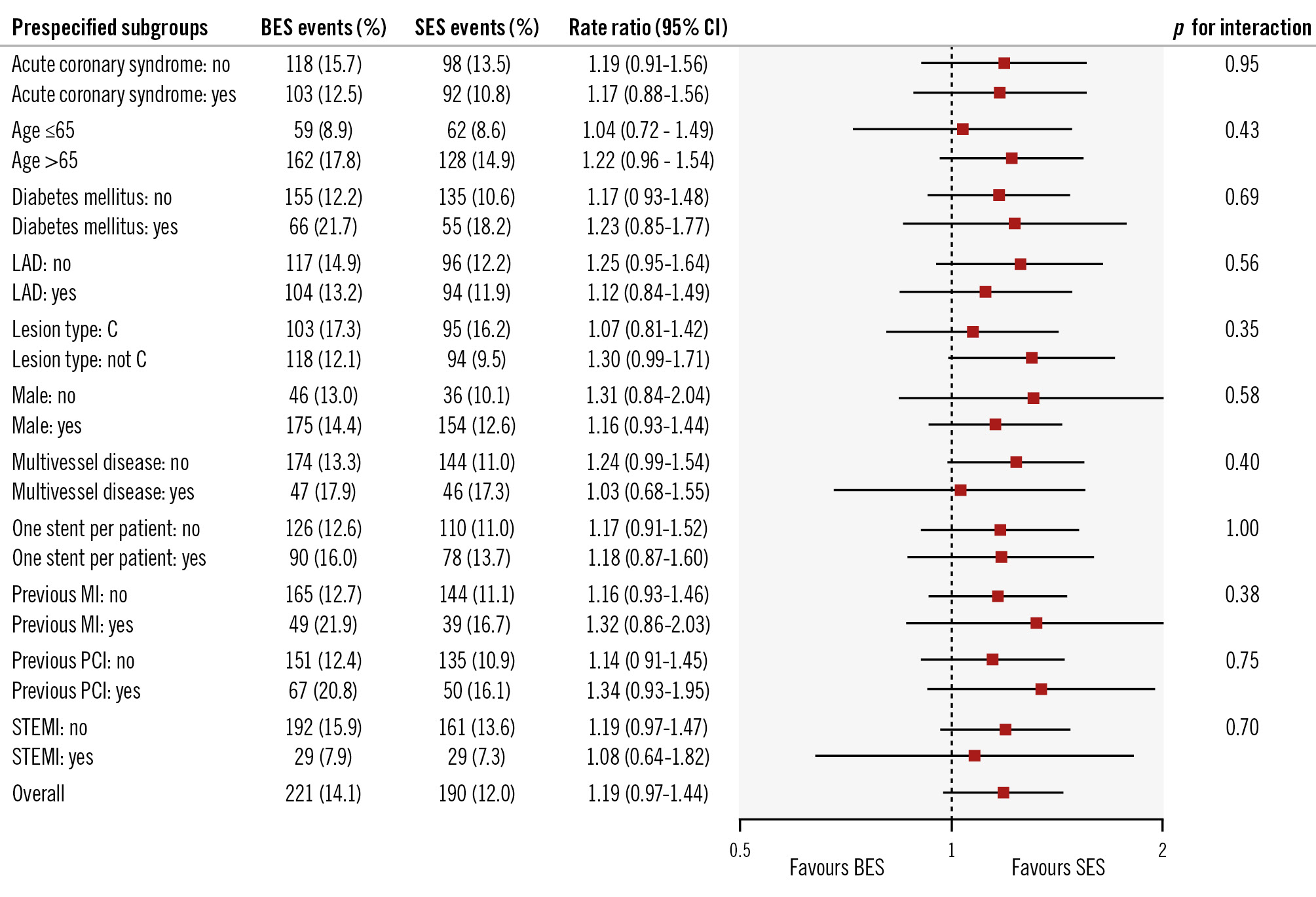
Figure 4. Prespecified subgroup analysis for the primary endpoint at 5-year follow-up. P-values in the Forest plot are all two-sided for interaction. BES: biolimus-eluting stent; CI: confidence interval; LAD: left anterior descending artery; MI: myocardial infarction; PCI: percutaneous coronary intervention; SES: sirolimus-eluting stent; STEMI: ST-segment elevation myocardial infarction
Discussion
In this 5-year follow-up study of the SORT OUT IX trial, TLF did not differ significantly between the biolimus A9-coated BioFreedom stent and the ultrathin-strut, biodegradable-polymer, sirolimus-eluting Orsiro stent. The safety profile, with rates of cardiac mortality, MI, and stent thrombosis, did not differ significantly between the two groups, whereas the rate of TLR was higher in the biolimus A9-coated BioFreedom stent group, attributable to a higher risk of TLR within the first year. The present study is the first study with long-term clinical follow-up after treatment with a drug-coated stent in an all-comer patient population and the first head-to-head randomised comparison with long-term follow-up between a drug-coated stent and a modern ultrathin-strut, biodegradable-polymer stent.
The first-generation sirolimus-eluting and paclitaxel-eluting DES have shown an increased risk of stent thrombosis, especially late and very late stent thrombosis21, which has been suggested to be related to a potential inflammatory response triggered by the presence of polymer remnants after drug release is complete22. Stents with biodegradable polymers for drug release, along with continuous progress in stent platforms towards thinner struts, lower turbulence and thrombogenicity after implantation23, have been developed to overcome these limitations.
Neither clinical trials724 nor meta-analyses25 with long-term follow-up have shown a clinical benefit of biodegradable polymers. As the assumed benefit of a biodegradable polymer is to remove the polymer-triggered inflammatory stimulation associated with durable-polymer DES, this benefit would be expected to impact long-term follow-up after the degradation time of the polymer. In the BIOSCIENCE7 trial, the ultrathin-strut, biodegradable-polymer, sirolimus-eluting stent was non-inferior to the durable-polymer, everolimus-eluting stent for TLF at 1 year, and after 5 years, the ultrathin-strut, biodegradable-polymer, sirolimus-eluting stent and the durable-polymer, everolimus-eluting stent showed similar 5âyear outcomes (20.2% vs 18.8%). In the BIO-RESORT26 trial, both of the very thin-strut, biodegradable-polymer stents (everolimus-eluting or sirolimus-eluting) were non-inferior to the durable-polymer, zotarolimus-eluting stent at 12 months in an all-comers population. After 5 years, both the very thin-strut, biodegradable-polymer stents (TLF 12.7%) and the durable-polymer, zotarolimus-eluting stent (TLF 14.1%) showed similar outcomes with regard to TLF24; these were comparable to the rates of TLF at 5 years in the present SORT OUT IX trial (biolimus A9-coated BioFreedom stent: 14.1% and the ultrathin-strut, biodegradable-polymer, sirolimus-eluting Orsiro stent: 12.0%). Neither definite stent thrombosis within 1 year nor definite stent thrombosis after 1 year differed significantly between the biodegradable-polymer stents and the durable-polymer stents. Both the BIOSCIENCE7 and the BIO-RESORT24 trials had similar rates of definite stent thrombosis as the present SORT OUT IX trial.
As an alternative to biodegradable-polymer stents, a polymer-free elution technology has also been developed91011, although it has been associated with lesser efficacy in inhibiting intima hyperplasia within the stent. The polymer-free and carrier-free, biolimus A9-coated BioFreedom stent used in SORT OUT IX contains a highly lipophilic sirolimus analogue that releases the biolimus A9 into the vessel wall over a period of 1 month. Both the polymer-free BioFreedom stent and the polymer-free, amphilimus-eluting stent have been used in clinical settings and have documented favourable and comparable safety and efficacy profiles in all-comer patients undergoing PCI9101127. In the ReCre8 Trial11, the polymer-free, amphilimus-eluting stent was non-inferior to the zotarolimus-eluting stent up to 3 years.
In the LEADERS FREE trial, the first-year benefit of the biolimus-coated BioFreedom stent over BMS in high bleeding risk patients was maintained at 2 years13. There is limited long-term follow-up of the biolimus-coated BioFreedom stent, but in the first-in-human evaluation9 of the stent, clinical event rates were similar to the paclitaxel-eluting stent after 5 years, and there was no stent thrombosis. The LEADERS FREE high bleeding risk population had higher event rates than those recorded in SORT OUT IX, but there was no catch up from years 1 to 2, and only one case of definite stent thrombosis was seen from years 1 to 2. In SORT OUT IX, TLR was higher in the biolimus-coated BioFreedom stent group within the first year, as the TLR curves diverged after 4 months to 1 year, whereas TLR did not differ significantly from years 1 to 5. The overall results demonstrated similar event rates from years 1 to 5, covering a time period beyond the degradation time of the polymer, during which both stent platforms are BMS. The biolimus-coated BioFreedom stent has thicker stent struts (120 μm compared with 60-80 μm for the sirolimus-eluting Orsiro stent) and a faster drug release (1 month vs 3 months). Both strut thickness and drug release time may have contributed to the higher TLR rate observed with the biolimus-coated BioFreedom stent in SORT OUT IX, as the period beyond the degradation did not influence TLR. Also, stent platform properties, polymer properties (polymer vs no polymer) and drug properties may have influenced TLR. Long-term follow-up after DES implantation remains important despite newer stent technologies with thinner stent struts and biodegradable polymers. The traditional 1-year primary endpoint assessment might be insufficient to predict 5-year clinical outcomes in patients treated with coronary drug-eluting stent implantation. A newer drug-coated stent with thinner struts (84-88 μm) on a cobalt-chromium platform, with similar drug dose and release kinetics as the BioFreedom stent, has been developed: the BioFreedom Ultra (Biosensors). This newer stent, BioFreedom Ultra, was non-inferior to the BioFreedom stent used in SORT OUT IX with regard to late lumen loss, and TLR did not differ between these two stents28. However, it has not been tested in an all-comer study with clinical endpoints.
Limitations
In line with previous SORT OUT trials1617, the SORT OUT IX trial relied on registry-based outcome ascertainment without telephone or clinical follow-up. Although the Danish healthcare databases capture events of sufficient severity for patients to seek medical attention, we cannot exclude that these records might underestimate event rates compared with telephone or clinical follow-up by dedicated trial staff. Information about medical therapy at 5-year follow-up was not available.
Conclusions
In the SORT OUT IX trial, the polymer-free, biolimus A9-coated BioFreedom stent did not meet criteria for non-inferiority for TLF at 12 months compared with the ultrathin-strut, biodegradable-polymer, sirolimus-eluting Orsiro stent in an all-comers population. However, 5-year TLF did not differ significantly between the drug-coated, biolimus-eluting stent and the ultrathin-strut, biodegradable-polymer stent. A higher risk of TLR, attributable to a higher risk of TLR within the first year, was seen with the drug-coated biolimus-eluting stent.
Impact on daily practice
In the SORT OUT IX trial, the first study to compare the biolimus A9-coated stent to a modern drug-eluting stent in a population-based all-comers setting, the biolimus A9-coated polymer-free stent was not non-inferior for target lesion failure (TLF) when compared with the ultrathin-strut biodegradable-polymer sirolimus-eluting stent within 1 year. At 5-year follow-up, SORT OUT IX demonstrated similar TLF between the drug-coated biolimus-eluting stent and the ultrathin-strut biodegradable-polymer stent. A higher risk of target lesion revascularisation, attributable to a higher risk of TLF, within the first year was seen with the drug-coated biolimus-eluting stent. The overall results demonstrated similar event rates from 1-5 years, covering a time period beyond the degradation time of the polymer, when both stent platforms are bare metal stents.
Funding
This study was an investigator-initiated study from the SORT OUT organisation and was supported with equal, unrestricted grants from Biosensors (Morges Switzerland) and Biotronik (Bülach, Switzerland). The two companies did not have access to the study database and had no involvement in the study design, data collection, data analysis, or interpretation of results. Furthermore, the companies did not have the opportunity to review the manuscript.
Conflict of interest statement
L.O. Jensen has received research grants from Biotronik, Biosensors, and OrbusNeich, to her institution. E.H. Christiansen received institutional grants from Asahi Intecc, Philips, OrbusNeich, and Abbott. J. Ellert-Gregersen is on an advisory board for Boston Scientific. T. Engstrøm is on an advisory board for Novo Nordisk and Abbott; and he has received speaker fees from Abbott, Novo Nordisk, and Boston Scientific. J.F. Lassen has participated on a data safety monitoring board for B. Braun; has received travel grant from Terumo; and speaker fees from Medtronic and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

