Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Final infarct size (FIS) and microvascular obstruction (MVO) are associated with heart failure hospitalisations and mortality in patients with acute myocardial infarction (AMI). Interventions beyond primary percutaneous coronary intervention (PCI) to reduce FIS are needed. Supersaturated oxygen (SSO2) therapy demonstrated reduced FIS in clinical trials.
Aims: We aimed to investigate whether routine use of SSO2 reduces FIS and MVO in clinical practice.
Methods: Intracoronary SSO2 was delivered for 60 minutes after successful primary PCI in patients with anterior AMI. Results from 20 SSO2 patients were compared with those from 20 similar non-SSO2 AMI patients. Multimodal imaging including single-photon emission computed tomography (SPECT), fibroblast activation protein inhibitor positron emission tomography (FAPI-PET), and cardiac magnetic resonance imaging (CMR) was performed to assess left ventricular function, FIS, area at risk, extent of myocardial fibroblast activation, myocardial salvage, and MVO.
Results: The two groups did not significantly differ regarding sex, age, weight, hypertension, dyslipidaemia, smoking and diabetes status, prehospital cardiac arrest, door-to-balloon time, Thrombolysis in Myocardial Infarction flow before and after PCI, or renal function (p>0.1 for each) and had comparable area at risk by CMR and FAPI-PET. SSO2 patients showed lower FIS determined by CMR (SSO2: 20% vs non-SSO2: 32%; p<0.001) and SPECT (SSO2: 16% vs non-SSO2: 29%; p=0.014). Myocardial salvage was higher in SSO2 patients (50% vs 28%; p=0.002). MVO occurred less often and was less extreme under SSO2 therapy.
Conclusions: SSO2 therapy was associated with significantly reduced microvascular obstruction and FIS in patients with anterior AMI in clinical routine practice.
Acute myocardial infarction (AMI) is one of the major contributors to cardiovascular morbidity and mortality. In the acute phase of ST-segment elevation myocardial infarction (STEMI), the time from first medical contact to primary percutaneous coronary intervention (PCI) is a strong predictor of an adverse outcome1. However, when surviving the initial prehospital and in-hospital phase, annual mortality following STEMI is around 1%/year and remains unaltered by intense lipid-lowering, antithrombotic or anti-inflammatory therapies2345678.
A meta-analysis of 10 randomised primary PCI trials including more than 2,500 STEMI patients showed that final infarct size (FIS) measured within 1 month of PCI by cardiac magnetic resonance imaging (CMR) or technetium-99m (99mTc) sestamibi single-photon emission computed tomography (SPECT) was strongly associated with all-cause mortality and hospitalisation for heart failure within 1 year9. This finding provided the rationale to use FIS as a surrogate parameter of adverse clinical outcome. Hence, several interventions aimed to reduce FIS following acute STEMI, because animal data suggest that almost half of FIS might be attributable to reperfusion rather than ischaemic injury1011.
Experimental data support the pathophysiological hypothesis that microvascular ischaemia caused by microvascular obstruction (MVO) contributes to myocardial tissue injury over a prolonged time during reperfusion. It is believed that supersaturated oxygen (SSO2) provides hyperoxaemic levels of dissolved oxygen, which are up to 10 times higher than normal, when it is infused in the infarct-related artery. This allows for high oxygen diffusion before flow is restored downstream. Increased oxygen delivery via the plasma to endothelial cells can prevent severe cell oedema, allow better perfusion, and correct persistent myocardial ischaemia, particularly in regions where erythrocyte flow is impaired by compromised microvascular luminal integrity12.
The first prospective, randomised trial of intracoronary hyperoxaemic reperfusion after primary PCI was published in 200713. While the overall trial was neutral in a population with all kinds of STEMI presenting within 24 hours of symptom onset, the subgroup of anterior AMI patients presenting within 6 hours showed a significant reduction of FIS, determined after 14 days. Subsequently, an additional study with the same procedural setup recruited exclusively anterior STEMI patients presenting within 6 hours from symptom onset to appropriately increase the number of such patients from both trials for statistical analysis. Here, FIS was reduced from 25.0% to 18.5% (p=0.02). The overall conclusion was that intracoronary SSO2 infusion into the infarct-related artery is feasible and safe, and it reduces infarct size14. To overcome the limitation of selective infusion catheters placed within the infarct-related coronary artery, the idea of applying SSO2 directly via a guiding catheter in the left main coronary artery evolved. In another 100 patients, CMR was performed at 4 and 30 days after SSO2 infusion for anterior STEMI. Infusion of SSO2 via the left main coronary artery was feasible and safe, resulting in a similar infarct size reduction as previously observed15. However, it remains unclear whether SSO2 therapy in clinical routine provides similar beneficial effects.
In the current study, we investigated the efficacy of a 60-minute intracoronary application of SSO2 immediately after PCI for the reduction of FIS (measured by CMR after 3-5 days) in acute anterior STEMI patients in a real-world setting and compared them with contemporary control patients receiving the same imaging following acute anterior STEMI without SSO2. To further exclude potential bias in the comparison, the area at risk (AAR) was assessed by CMR. The extent of myocardial fibroblast activation was derived by fibroblast activation protein inhibitor positron emission tomography (FAPI-PET) in all individual patients as a surrogate for AAR1617, and individual salvage indices were calculated based on the CMR-derived FIS and AAR (Central illustration).
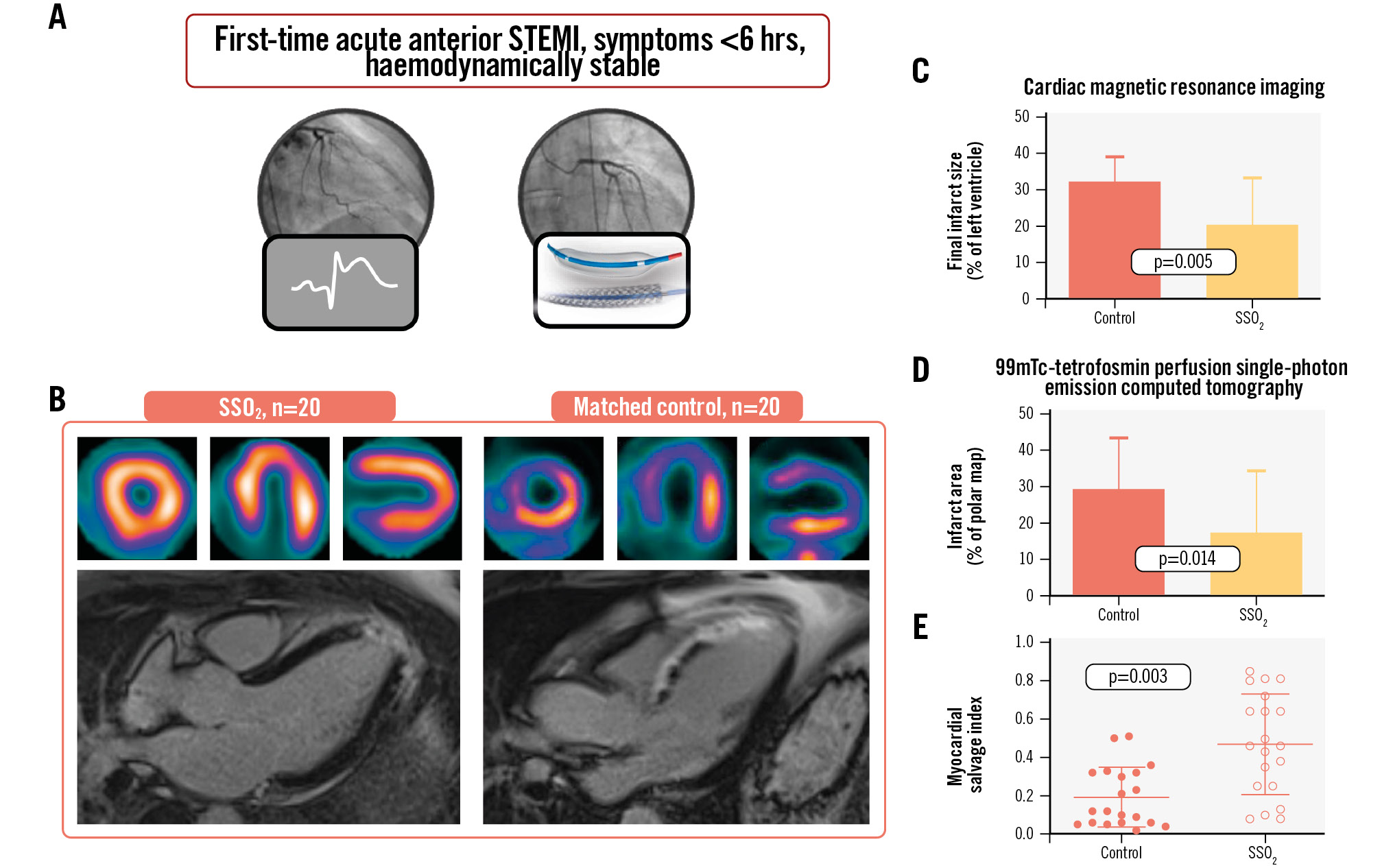
Central illustration. Impact of supersaturated oxygen therapy on infarct size. A) Patients with first-time acute anterior myocardial infarction presenting as haemodynamically stable within 6 hours from symptom onset received additive supersaturated oxygen (SSO2) therapy for 60 minutes and were compared to contemporary controls by cardiac magnetic resonance imaging and nuclear imaging using 99mTc-tetrofosmin perfusion single-photon emission computed tomography to assess the extent of infarct size and calculate myocardial salvage (B). C-E) Impact of additive SSO2 treatment on FIS. The impact of additive SSO2 therapy compared to controls on final infarct size (FIS) among patients with acute anterior myocardial infarction was determined by cardiac magnetic resonance imaging (CMR) (C) and 99mTc-tetrofosmin perfusion single-photon emission computed tomography (D). Values are means±SD. Myocardial salvage indices were individually calculated for all patients based on the CMR-derived area at risk and FIS (E). SD: standard deviation; STEMI: ST-segment elevation myocardial infarction
Methods
Study design and patient population
Patients presenting with first-time, haemodynamically stable acute anterior STEMI, with symptom onset within 6 hours before PCI, and successful revascularisation of a left anterior descending artery (LAD) culprit lesion were prospectively included in this analysis based on their approval status for SSO2 therapy. Patients with a history of previous myocardial infarction (MI) and/or revascularisation as well as those presenting in cardiogenic shock or remaining unconscious after cardiac arrest were, therefore, excluded. SSO2 therapy was available as part of clinical routine treatment during a period between March 2021 and January 2024 and was used by half of the primary PCI operators (SSO2 group, n=20). During the study period, 145 patients presented with acute anterior STEMI, of whom 37 experienced cardiac arrest or required mechanical circulatory support, another 32 experienced a second STEMI, 11 were haemodynamically unstable without assist devices, and 20 had symptom onset more than 6 hours prior to hospital admission, leaving 45 potentially eligible patients. A cohort of similar patients with acute anterior STEMI and the LAD as the location of the culprit lesion who received primary PCI and stent implantation without SSO2 therapy from the other operators served as controls (control group, n=20). We initially planned to use a nearest-neighbour matching approach. However, the limitations in approval status reduced most of the heterogeneity within the patient population by excluding non-STEMI, non-LAD-STEMI, shock, comatose post-arrest, presentations extending beyond 6 hours from symptom onset, and patients with pre-existing or incomplete revascularisation, but the number of potentially available controls were also limited. Hence, beyond the boundaries of the approval status, the cohort appeared to be comparable but was too small to apply formal matching by nearest-neighbour or propensity score matching. All patients were part of an ongoing larger imaging study in STEMI patients approved by the local ethics committee (#9553_BO_K_2021). Patients gave written informed consent prior to imaging.
Patient treatment
STEMI patients were directly taken to the cath lab and underwent immediate coronary catheterisation upon arrival. All patients were loaded with 500 mg acetylsalicylic acid and received an intravenous (i.v.) bolus of unfractionated heparin (5,000 IU) as early as possible, and at the latest in the cath lab. PCI was performed by an experienced interventional cardiologist. Additional doses of heparin were administered at the discretion of the interventional cardiologist depending on the duration of the procedure. Immediately after revascularisation, a prasugrel loading dose (60 mg) was administered on the table in the cath lab.
SSO2 administration
For patients receiving intracoronary SSO2 therapy, activated clotting times were kept above 250 seconds. In case of partial pressure of oxygen in arterial blood (PaO2) below 80 mmHg, patients received supplementary oxygen prior to SSO2 therapy, as previously described in more detail18. In brief, potentially eligible patients received PCI via femoral access. After successful PCI, the femoral arterial 6 Fr sheath was exchanged for a dedicated 7 Fr sheath (Merit Medical), and a 5 Fr TherOx delivery catheter (ZOLL) was placed in the ostium of the left main coronary artery. The sideport of the arterial 7 Fr sheath was connected to the drawing line of the TherOx cartridge, the system was primed, and hyperoxaemic blood was returned to the patient via the 5 Fr infusion catheter directly into the left main. After drawing from the arterial sheath, blood was oxygenated to hyperbaric levels (760-1,000 mmHg PaO2) and delivered to the left coronary system via the delivery catheter (100 mL/min) for a total of 60 minutes. Afterwards, post-infusion angiography was performed, and haemostasis was achieved using an 8 Fr Angio-Seal vascular closure device (Terumo).
Blood sampling
Blood samples including arterial blood gases and estimated glomerular filtration rate were obtained as part of clinical routine.
Cardiac magnetic resonance imaging
At 4.1±2.3 days after AMI, CMR studies were performed using a standard 1.5T scanner (MAGNETOM Avanto [Siemens Healthineers]). A predefined post-STEMI CMR protocol was used in all patients. Analyses included left ventricular (LV) ejection fraction (LVEF), LV mass, extent of myocardial oedema, late gadolinium enhancement, and microvascular obstruction. Cine images were acquired using a steady-state free precession (True FISP [Siemens Healthineers]) sequence. Myocardial oedema was assessed by T2-weighted CMR using a turbo inversion recovery magnitude (TIRM) sequence. Phase-sensitive inversion recovery (PSIR) sequences were obtained 10-15 minutes after the intravenous application of 0.15 mmol/kg gadolinium-based contrast agent (gadobutrol). FIS was defined by late gadolinium enhancement (measured as a percentage of the total LV myocardial mass), AAR was defined by myocardial oedema (% of LV myocardial mass), and MVO was defined as hypoenhanced necrotic myocardium within the hyperenhanced infarct zone. Other modalities (such as FAPI-PET) were used additionally to estimate the AAR since CMR uses oedema to calculate the AAR, and a trend for lower AARs following SSO2 treatment has previously been observed in initial porcine animal models and interpreted as oedema reduction by the intervention itself19. The myocardial salvage index (MSI) was calculated as the proportion of non-necrotic myocardium inside the oedematous myocardium, whereby an MSI of 1 indicated that the whole AAR would be infarcted. CMR analysis was carried out using cvi42 software (Circle Cardiovascular Imaging).
Radionuclide imaging
At 5.0±2.4 days after AMI, resting myocardial perfusion SPECT was obtained to determine the success of reperfusion using 99mTc-tetrofosmin and a dedicated cardiac camera (GE Discovery NM 530C [GE Healthcare]). Infarct size was determined using commercially available software for polar map generation (4DM [INVIA]) and a local normal database for comparison.
FAPI-targeted PET was conducted at 6.5±2.6 days after AMI, using the specific ligand, [68Ga]-FAPI-46, which was synthesised in-house under good manufacturing practice as previously described1720 and used clinically according to §13.2b of the German Pharmaceuticals Act to determine myocardial injury. Static PET images were acquired for 20 minutes using a Biograph mCT 128 PET/computed tomography (CT) system (Siemens Healthineers), beginning 60 minutes after intravenous injection of [68Ga]-FAPI-46. Low-dose CT scans were used for attenuation correction. Images were iteratively reconstructed, using time-of-flight and point-spread function information (True X [Siemens Healthineers]). Peak and mean standardised uptake values (SUV) were obtained for infarct, remote myocardium, blood pool (left atrium) and other organs (liver, spleen, bone marrow, lungs) using volumes of interest (VOI) of 1 cm³ and commercial software (syngo.via, version V10B [Siemens Healthineers]). Cardiac FAPI volume was assessed using an isocontour VOI including all voxels above a threshold of the mean SUV of the blood pool plus 2 standard deviations (SD), as previously described17.
Clinical follow-up
Patients were followed up for the period of their hospital stay with respect to their clinical status. Bleeding events were assessed by the Thrombolysis in Myocardial Infarction (TIMI) bleeding classification, as previously reported21. Further follow-up at 6 months was scheduled, including CMR.
Statistical analysis
This non-randomised investigation had no formal power analysis. Statistical analyses were performed using SPSS, version 29 (IBM) and GraphPad Prism, version 10.2.2 (GraphPad Software). Continuous variables are reported as mean±SD or median, (interquartile range [IQR]), depending on the presence or absence of a normal distribution, and categorical variables as counts and percentages. Continuous data were evaluated for normality of distribution with the Shapiro-Wilk test. The 2-sided t-test was used for parametric variables, whereas the Mann-Whitney U test was used for non-parametric comparisons. Associations between categorical variables were tested using the chi-square (x2) test. A 2-sided p-value<0.05 was considered statistically significant.
Results
Patient characteristics
The observed population consisted of 40 haemodynamically stable acute anterior STEMI patients treated with percutaneous intervention for STEMI within 6 hours of symptom onset and presenting a full dataset of imaging and laboratory parameters. Of these 40 patients, 20 received intracoronary infusion of SSO2 for 59±3 minutes following successful PCI. A total of 19 patients received the therapy for the full 60 minutes; the first patient received SSO2 therapy for 47 minutes. All patients tolerated the additive therapy in the cath lab without adverse events or discomfort. All patients received unfractionated heparin (70 IU/kg) and acetylsalicylic acid (500 mg i.v.) prior to PCI as well as prasugrel (60 mg orally) immediately after PCI in the cath lab. Patient characteristics are summarised in Table 1.
As the analysis investigates the effect of SSO2 in non-randomised patients, we compared the estimated area of endangered (ischaemic) myocardium (AAR) using two different imaging techniques: myocardial oedema by CMR, and the volume of the injured area by FAPI-PET. With the two imaging modalities, there were no significant differences between groups (CMR: 40.3±11.0% vs 46.3±4.8%; p=0.061; FAPI-PET MI volume: 106±60 cm3 vs 128±55 cm3; p=0.2666).
Table 1. Baseline demographics.
| Patient characteristics | Control (n=20) | SSO2 (n=20) | p-value |
|---|---|---|---|
| Age, years | 58±11 | 57±12 | 0.825 |
| Male sex | 21 (95) | 16 (80) | 0.151 |
| Body mass index, kg/m2 | 28.4±4.5 | 27.6±3.8 | 0.581 |
| Cardiovascular risk factors | |||
| Diabetes | 6 (30) | 3 (15) | 0.256 |
| Hyperlipidaemia | 9 (45) | 11 (55) | 0.527 |
| Smoker | 15 (75) | 15 (75) | 1.000 |
| Family history of coronary artery disease | 6 (30) | 7 (35) | 0.736 |
| Hypertension | 11 (55) | 10 (50) | 0.752 |
| Number of CVRFs | 3 (1-3) | 2 (1-2) | 0.376 |
| Systolic blood pressure, mmHg | 125±20 | 131±19 | 0.350 |
| Laboratory results (admission) | |||
| Arterial lactate, mmol/L | 2.0±0.8 | 1.8±0.8 | 0.289 |
| eGFR, mL/min/1.73 m² | 86±19 | 89±18 | 0.799 |
| PCI | 20 (100) | 20 (100) | 1.000 |
| Stents, n | 1.5±0.9 | 2.1±1.1 | 0.103 |
| Stent length, mm | 29±17 | 45±33 | 0.046 |
| Door-to-wire time, min | 40±19 | 36±19 | 0.611 |
| Door-to-balloon time, min | 42±19 | 40±19 | 0.897 |
| Symptom-to-door time, min | 128±44 | 117±99 | 0.617 |
| Symptom-to-balloon time, min | 168±40 | 154±95 | 0.574 |
| Patients with total ischaemic time <3 hours | 14 (70) | 17 (85) | 0.278 |
| Location of culprit lesion | 1.000 | ||
| LAD, proximal | 15 (75) | 15 (75) | |
| LAD, mid | 5 (25) | 5 (25) | |
| LAD, distal | 0 (0) | 0 (0) | |
| TIMI flow, initial | 0.416 | ||
| 3 | 0 (0) | 1 (5) | |
| 2 | 5 (25) | 7 (35) | |
| 0/1 | 15 (75) | 12 (50) | |
| TIMI flow, final | 0.643 | ||
| 3 | 17 (85) | 18 (90) | |
| 2 | 3 (15) | 2 (10) | |
| 0/1 | 0 (0) | 0 (0) | |
| Values are number (%) of observations, median (interquartile range), or mean±standard deviation. CVRF: cardiovascular risk factor; eGFR: estimated glomerular filtration rate; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; SSO2: supersaturated oxygen; TIMI: Thrombolysis in Myocardial Infarction | |||
Infarct size and myocardial salvage
Similar to the AAR, FIS was determined by two different imaging techniques: gadolinium-late enhancement in CMR and necrotic infarcted area by SPECT. FIS determined by CMR was reduced from 32.3±6.9% in controls to 20.4±13.1% in SSO2-treated patients (p=0.005), and FIS determined by 99mTc-tetrofosmin perfusion SPECT was reduced from 29.2±14.4% in controls to 17.4±17.2% in SSO2-treated patients (p=0.014) (Central illustration C, Central illustration D).
As CMR provided both AAR and FIS, an individual MSI was calculated. MSI determined by CMR increased from 19±16% in controls to 47±27% in SSO2-treated patients (p=0.003) (Central illustration E). Representative images for radionuclide imaging of perfusion and activated fibroblasts are shown in Figure 1.
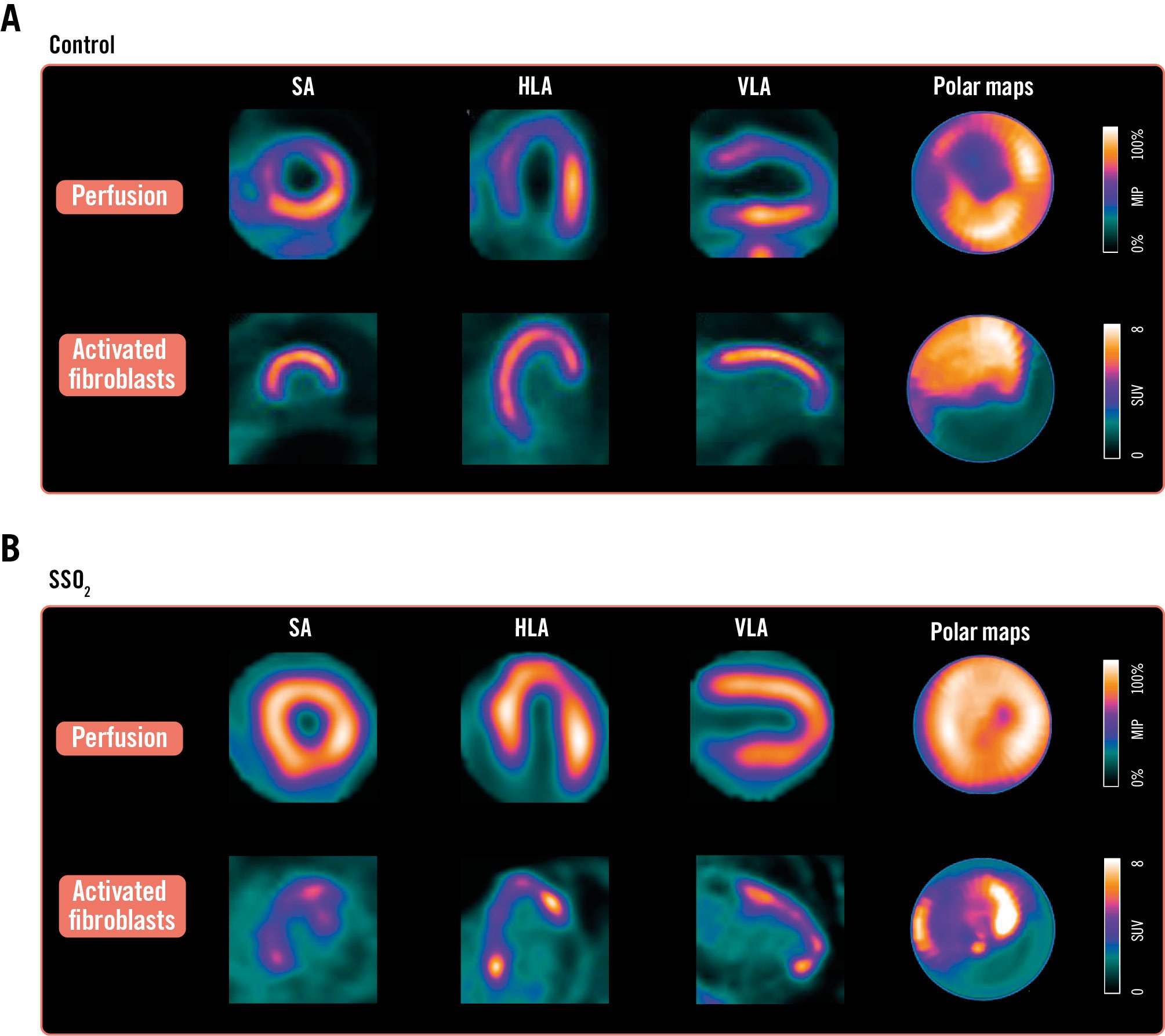
Figure 1. Perfusion and activated fibroblast imaging with and without SSO2 treatment. Individual images for 99mTc-tetrofosmin perfusion SPECT (top rows of A and B) and activated fibroblasts identified in and adjacent to the hypoperfused area (bottom rows of A and B) in a patient with conventional primary percutaneous coronary intervention without (A) and with (B) an additive 60 minutes of treatment with supersaturated oxygen (SSO2) after successful revascularisation. HLA: horizontal long axis; SA: short axis; SPECT: single-photon emission computed tomography; VLA: vertical long axis
Microvascular obstruction in CMR
As MVO is a critical contributor to FIS and major adverse cardiovascular events after acute MI, its occurrence was analysed. MVO determined by CMR was reduced from a median of 3.02% (IQR 1.18-7.56%) in controls to 0.08% (IQR 0.00-1.62%) in SSO2-treated patients (p=0.003) (Figure 2A). While the rate of any MVO decreased from 90% in controls to 58% in SSO2-treated patients, the decrease of substantial MVO exceeding 1.55% of LV mass, as was previously determined to be prognostically important22, decreased from 75% to 26%. Furthermore, there was an interaction between the extent of MVO and the increase in myocardial salvage in favour of SSO2 therapy (Figure 2B).
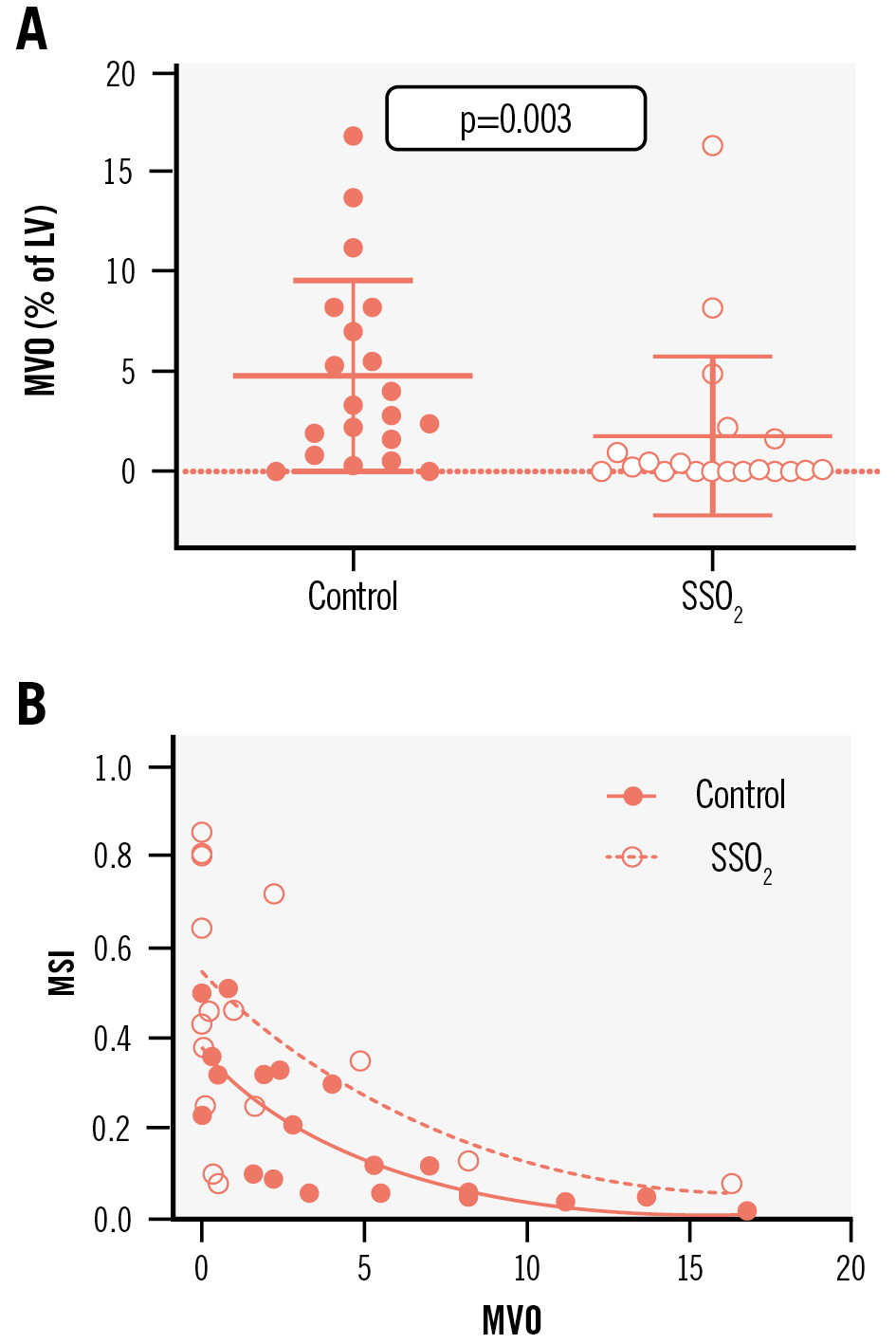
Figure 2. Impact of additive SSO2 therapy compared to controls on MVO among patients with acute anterior myocardial infarction was determined by CMR imaging (A). MSI were individually calculated for all patients based on the CMR-derived area at risk and FIS, and these were correlated with the MVO from the same patient (B). CMR: cardiac magnetic resonance; FIS: final infarct size; LV: left ventricle; MSI: myocardial salvage indices; MVO: microvascular obstruction; SSO2: supersaturated oxygen
LV function at baseline and 6-month follow-up
LVEF determined by routine CMR at day 4.1±2.3 after AMI and PCI tended to be higher in patients treated with SSO2 compared to controls (SSO2: 52.0±8.7% vs control: 44.3±7.9%, p=0.050) (Figure 3A). With regard to potential long-term improvement, both groups improved in terms of LVEF over the 6 months following PCI, reaching statistical significance (SSO2: 58.2±5.6% vs control: 49.9±6.2%; p=0.009) (Figure 3B).
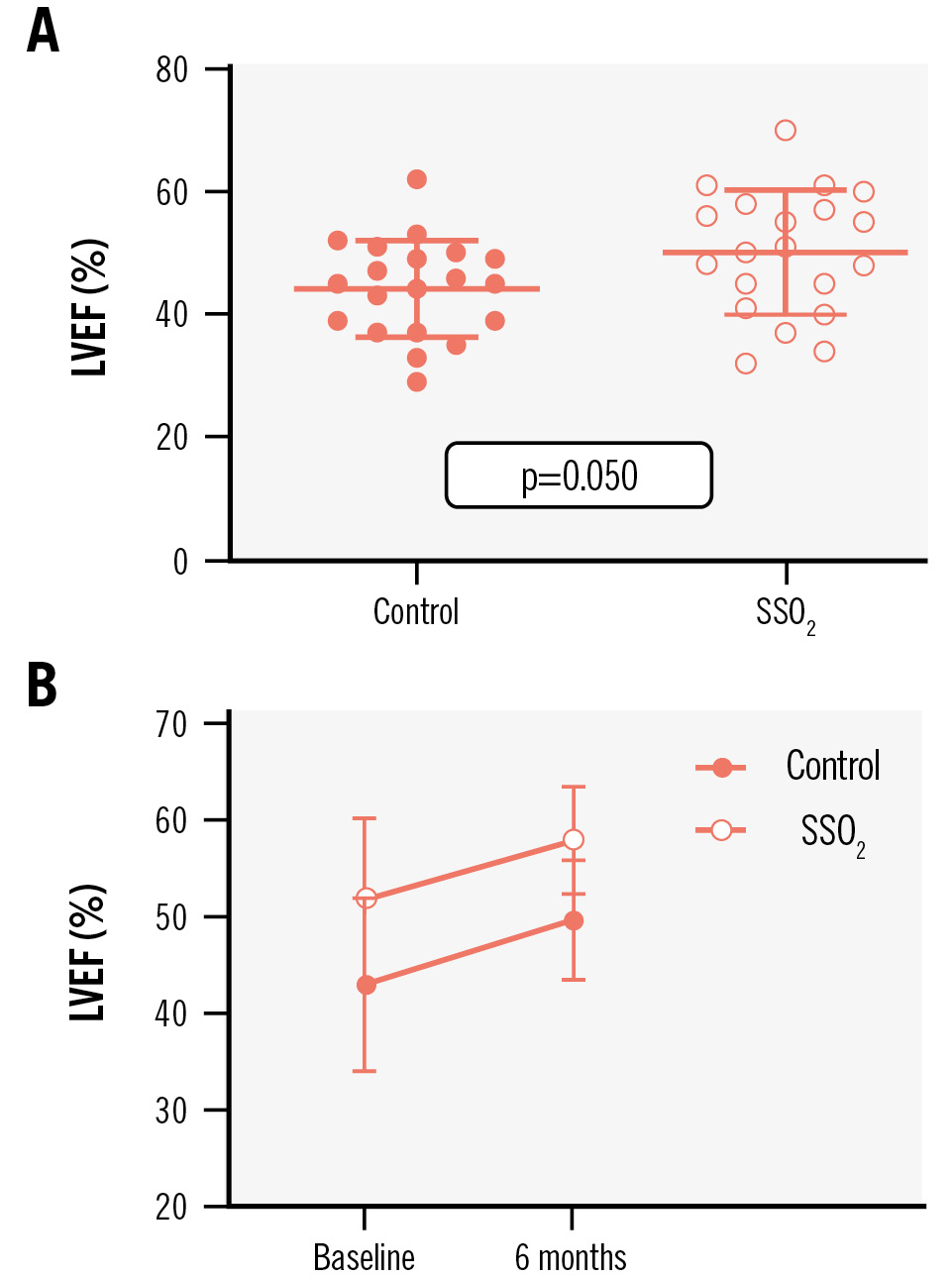
Figure 3. Impact of additive SSO2 treatment on LVEF. The impact of additive treatment with supersaturated oxygen (SSO2) compared to controls on left ventricular ejection fraction (LVEF) among patients with acute anterior myocardial infarction was determined by cardiac magnetic resonance imaging prior to discharge during the index hospitalisation (A) and at 6-month follow-up (B).
Discussion
In this prospective observational study, we show a significant reduction in final infarct size following routine clinical application of intracoronary SSO2 therapy in first-time acute anterior STEMI patients. Individual salvage of endangered myocardium was significantly greater in patients treated with SSO2 than in patients not receiving SSO2 treatment. The results are strengthened by the utilisation of multiple independent imaging techniques, which all demonstrated significant myocardial salvage (Table 2).
After the successful implementation of STEMI fast-track programmes for primary PCI, ischaemic times dropped dramatically. With more successful and quicker revascularisation, ischaemia in isolation now has a lower contribution than previously to overall infarct size, while the relevance and relative contribution of reperfusion injury is increasing232425. Hence, several interventional approaches have emerged, targeting the reduction of reperfusion injury through SSO2131415, LV unloading26, therapeutic2728 and intracoronary2930 hypothermia as well as coronary sinus counterpulsation243132; these are the most advanced approaches in clinical studies. As described above, SSO2 therapy reduced infarct size in clinical trials and is already approved for routine clinical practice131418. LV unloading with a microaxial flow pump is currently being investigated after a pilot trial showed the feasibility and safety of the procedure but failed to demonstrate a relevant reduction in infarct size26. A large trial on systemic hypothermia in AMI has been stopped because of safety concerns after a pilot trial did not show a reduction in infarct size2728. Another trial using selective intracoronary hypothermia has been conducted, and did not decrease infarct size33. A larger trial on coronary sinus counterpulsation was prematurely halted with no positive effects on infarct size32. In summary, SSO2 is currently the only approved therapy that has thus far provided evidence of a significant reduction of infarct size from adequately powered trials.
More than half a century ago, hyperbaric oxygenation in large animals provided evidence that enhanced oxygen bioavailability can reduce FIS and lower mortality in dog and pig models of experimental MI3435. These findings encouraged physicians in the 1960s to treat acute MI patients with hyperbaric oxygenation36. By doubling the atmospheric pressure, they increased the amount of oxygen dissolved in plasma 14-fold. After adjusting for disease severity between STEMI patients receiving or not receiving hyperbaric oxygen therapy in a randomised controlled trial, a significant mortality reduction from 22.9% to 16.5% was documented37.
In order to increase oxygen availability within the myocardium, the TherOx system has been developed, which allows the intracoronary infusion of patient blood enriched by aqueous oxygen38. To bring this technology to routine clinical practice, three relevant clinical trials have already been conducted. The first trial was AMIHOT I13. Intracoronary infusion of SSO2 was applied for 90 minutes through an intracoronary catheter in 269 patients with all kinds of acute STEMI undergoing primary PCI within 24 hours of symptom onset. The overall trial did not show a benefit regarding infarct size reduction but detected that anterior STEMI patients presenting within 6 hours after symptom onset may be the potential patient cohort to benefit13. Hence, AMIHOT II increased the number of patients with anterior STEMI presenting within 6 hours from symptom onset, using a Bayesian borrowing method to integrate the results from both trials in similar patients and showing a significant FIS reduction14. Now, the feasibility and safety of infusing SSO2 directly into the left main coronary artery over 60 minutes using a standard 5 Fr catheter has been demonstrated15.
In our investigation, we show, first, that a resulting infarct size of about 30% of the LV in contemporarily state-of-the-art-treated patients with large anterior STEMI is relevant, i.e., in a range frequently associated with increased mortality and heart failure hospitalisation9. A major contributor to this large infarct size in patients being treated by effective and fast STEMI programmes might rather be reperfusion injury occurring immediately after revascularisation233940. The potential role of SSO2 therapy in limiting reperfusion injury and reducing myocardial infarct size has been reviewed elsewhere121824. The major obstacle to an additive treatment in routine clinical practice is that it requires additional time in the cath lab in order to reduce FIS and MVO, neither of which can be detected during coronary intervention, but which are both related to midterm outcome922. While the aim is to reduce heart failure hospitalisation and mortality over the years after PCI, the cath lab team does not have direct feedback about having done something positive in this scenario, as compared to opening an occluded coronary artery, improving coronary blood flow, reducing ST-segment elevation, or limiting the extent of ventricular arrhythmias, e.g., when the acute PCI happens during an on-call duty.
When the effort is made to examine FIS and MVO by different imaging techniques during the acute admission, both are found to be lower in SSO2-treated patients compared to contemporary controls. This is in accordance with the proposed pathophysiological mechanisms: SSO2 reduces MVO and improves myocardial salvage. The beneficial effects result in less myocardial damage, as demonstrated by the much lower creatinine kinase release and higher myocardial salvage, and finally, an improved LV ejection fraction early on and after 6 months. Based on previous meta-analyses, the observed reductions of MVO by 67% and of FIS by 37% have the potential to translate into a meaningful reduction of hard outcomes determined by mortality and heart failure hospitalisation922.
Table 2. Imaging data.
| Patient characteristics | Control (n=20) | SSO2 (n=20) | p-value |
|---|---|---|---|
| Cardiac magnetic resonance imaging | |||
| Final infarct size, as a % of LV mass | 32.2±6.9 | 20.4±13.1 | 0.005 |
| LV end-diastolic volume, mL | 172±35 | 167±47 | 0.700 |
| LV end-systolic volume, mL | 97±28 | 85±40 | 0.279 |
| LV ejection fraction, % | 44.3±7.9 | 50.2±10.4 | 0.050 |
| 99mTc-tetrofosmin perfusion single-photon emission computed tomography | |||
| Infarct area, as a % of polar map | 29.2±14.4 | 17.4±17.21 | 0.014 |
| Fibroblast activation protein inhibitor positron emission tomography | |||
| Myocardial infarction volume, cm3 | 128±55 | 106±60 | 0.267 |
Limitations
There are several limitations to our analysis. Even though data were collected prospectively, we do not have a randomised comparison. The present investigation was insufficiently powered, and thus the results should be considered hypothesis-generating, especially given the modest sample size. However, infarct size reduction by SSO2 therapy has already been proven in adequately sized randomised controlled trials. Our cohort was meant to position the intervention in the real-world STEMI situation, where it showed a similar infarct size despite even larger endangered myocardial territories. The cohort and design of the study do not allow the assessment of harder clinical endpoints.
Conclusions
We show that the administration of intracoronary SSO2 after successful PCI in acute anterior STEMI patients is effective and safe in clinical routine. In our study, the utilisation of multiple independent imaging techniques after SSO2 application all demonstrated significant myocardial salvage, as observed by a reduction in final infarct size. This may potentially contribute to better LV function and fewer heart failure events.
Impact on daily practice
Reperfusion injury significantly contributes to infarct size, which is associated with morbidity and mortality, and supersaturated oxygen (SSO2) therapy has been shown to reduce infarct size in clinical trials. Real-world infarctions tend to threaten larger territories, and the observed infarct size reduction in our study was ~30% lower than in standard-of-care revascularisation by primary percutaneous coronary intervention alone. Nevertheless, SSO2 therapy can be easily implemented in everyday clinical practice.
Acknowledgements
We thank the nursing staff of the cath lab and the cardiology intensive care unit for supporting this study.
Funding
No specific funding was received for this study. The consumables and TherOx console were provided by ZOLL free of charge. The imaging analysis was supported by the Deutsche Forschungsgemeinschaft (DFG, Clinical Research Unit KFO 311 to J. Bauersachs and F.M. Bengel as well as the Clinician Scientist Programme PRACTIS to J. Diekmann), the Leducq Foundation (Transatlantic Network “Immunofib”, to F.M. Bengel and J. Diekmann), and “REBIRTH - Research Center for Translational Regenerative Medicine” (State of Lower Saxony; to J. Bauersachs and F.M. Bengel).
Conflict of interest statement
A. Schäfer received lecture fees and honoraria from Abiomed, Amgen, AstraZeneca, Daiichi Sankyo, Boehringer Ingelheim, BMS, Novartis, Pfizer, and ZOLL; as well as research support from Abiomed and Daiichi Sankyo. J. Bauersachs received lecture fees and honoraria from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, and Corvia; as well as research support from ZOLL, CVRx, and Abiomed. D. Berliner received honoraria for lectures/consulting from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, and Pfizer. The other authors have no conflicts of interest to declare.

