Cory:
Unlock Your AI Assistant Now!
Abstract
Coronary obstruction is a rare but serious complication during transcatheter aortic valve implantation (TAVI), particularly in valve-in-valve procedures, which is associated with high mortality. This review explores various leaflet modification techniques designed to mitigate this risk. Among the most studied methods is Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA), which uses transcatheter electrosurgery to lacerate the aortic valve leaflet and maintain coronary perfusion. Other promising techniques include ShortCut, a mechanical leaflet splitting device, and Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle (UNICORN), which utilises radiofrequency energy to achieve leaflet laceration. These approaches differ in their procedural complexity, equipment requirements, and clinical outcomes. Each technique’s efficacy in preventing coronary obstruction is discussed, alongside the potential complications and the procedural challenges encountered in both native aortic valve and valve-in-valve settings. This review also highlights the importance of careful patient selection, advanced imaging techniques, and the need for further research to optimise these strategies.
Transcatheter aortic valve (TAV) implantation (TAVI) is an established treatment for symptomatic severe aortic stenosis (AS)1. In recent years, younger patients with a life expectancy likely surpassing the effective lifespan of the implanted valve have been treated2. Furthermore, the number of valve-in-valve procedures being performed each year is rapidly increasing3. Hence, clinicians will be challenged to treat an increasing number of failed transcatheter and surgical aortic bioprosthetic valves in the future.
Coronary obstruction (CO) is an uncommon (0.6-0.8%) complication of native aortic valve TAVI that is associated with a high 30-day mortality risk (40-50%)45. Moreover, the risk of CO during valve-in-valve procedures is fourfold higher, particularly when the bioprosthesis is stentless (e.g., Freestyle [Medtronic]) or has externally mounted leaflets (e.g., Mitroflow [LivaNova], Trifecta [Abbott])6. The true risk of CO may even be higher than that reported in published studies, as patients with the highest risk of coronary obstruction would likely have been referred for surgery or managed conservatively, and the incidence of delayed CO is hard to quantify accurately.
Obstruction of the coronary ostia occurs most frequently because of the deflection of a calcified leaflet towards the coronary ostium, preventing coronary perfusion. Leaflet deflection may also seal off the sinus of Valsalva, leading to sinus sequestration. The timing of CO can be either acute (intraprocedural) or delayed (post-procedure). Risk factors for CO are well described and include low cusp height, low coronary height, short virtual valve-to-coronary (VTC) distance (<4 mm), and bulky calcified leaflets (leaflet calcium volume >600 mm3)7. However, risk prediction is imperfect, and computed tomography (CT) analysis is sensitive but non-specific in identifying the true risk of TAVI-induced CO8910.
Against this background, clinicians have sought to develop adjunctive procedures to help mitigate CO risk during TAVI. Coronary protection with the placement of a “chimney stent” that extends into the aorta beyond the valve, thereby deflecting the leaflet, is the preferred strategy for most operators as it is technically more straightforward and reproducible. However, frequent periprocedural complications, the risk of stent deformation and thrombosis, the need for extended antiplatelet therapy, and challenging coronary reaccess have led to chimney stenting being deemed a potentially suboptimal treatment strategy101112. Aortic valve leaflet modification techniques have been developed to treat the underlying cause of CO and aim to produce better long-term outcomes.
This review aims to address a critical gap in existing literature by providing an in-depth discussion on procedural nuances, material requirements, and scenarios where leaflet modification techniques are effective or limited. Novel insights into emerging technologies and their potential to overcome current limitations are also explored.
Techniques
Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA)
BASILICA is a transcatheter electrosurgical procedure that aims to maintain coronary perfusion after TAVI by lacerating the native or bioprosthetic leaflet directly in front of the coronary artery at risk of occlusion (Central illustration, Moving image 1). It is the most comprehensively studied leaflet modification technique to date. Once the target leaflet is identified on transoesophageal echocardiography (TOE), the base of the leaflet is traversed using an electrified Astato XS 20 wire (Asahi Intecc) inside a telescoping catheter system (145 cm PiggyBack Wire Converter microcatheter [Teleflex], 6 Fr Amplatz left guide catheter [Cook Medical]) (Figure 1A). Once the leaflet has been traversed, the microcatheter component of the telescope system is withdrawn, and the Astato wire is exposed proximally outside the body. A 1 mm wire section is then denuded (non-circumferential) just distal to the withdrawn microcatheter. This section is then kinked to form a “flying V”, with the denuded section on the inner curve. This section of the wire will be used to lacerate the leaflet. Next, the distal end of the Astato wire is snared using an Amplatz GooseNeck snare (Medtronic) inside a 6 Fr Multipurpose-1 guide catheter (Merit Medical) in the left ventricular outflow tract (LVOT) (Figure 1B). Once snared, the wire is externalised to position the “flying V” at the leaflet’s base (Figure 1C). The wire and catheter are clamped in place, and a pigtail is placed in the left ventricle via the main vascular access to facilitate timely valve deployment if haemodynamic compromise occurs. The wire is electrified using “pure cut” at 50 to 70 watts, with gentle tension applied to both ends of the wire. A 5% dextrose solution is flushed through the aortic and LVOT guide catheters to displace blood within and provide electrical insulation. Leaflet laceration is confirmed on TOE. In cases where double leaflet BASILICA is required (“doppio”), a second large vascular access is needed (14-18 Fr). Both aortic catheters are introduced through the same large sheath. The “flying V” is set up for each leaflet as described above. The first leaflet is lacerated, and that catheter is removed before a pigtail catheter is placed in the ventricle in preparation for valve deployment. Once the pigtail is in place, the second leaflet is lacerated, and all BASILICA-related catheters are removed before the valve is deployed.
The BASILICA technique was examined in the BASILICA investigation device exemption (IDE) trial10. This prospective, multicentre study included 30 patients at high or prohibitive surgical risk (17 bioprosthetic, 13 native aortic valves) and deemed high CO risk. A total of 23 patients underwent solo BASILICA, and 7 patients underwent doppio. Patients with severely calcified masses on the target leaflet were excluded. Successful BASILICA traversal and laceration were achieved in 33 of the 37 leaflets treated (95%), and procedural success (leaflet laceration, successful equipment removal, freedom from CO, and absence of a need for intervention in the lab) was achieved in 93% of cases. In the 2 cases where the wire did not traverse the leaflet, there was confluent heavy calcification at the nadir of the leaflet. A transient haemodynamic compromise occurred in 7% of cases, which resolved quickly after valve deployment. The primary safety endpoint was reached in 70% of patients, driven by a 20% incidence of major vascular complications related to TAVI rather than the BASILICA procedure. Finally, three patients (10%) had a stroke (1 disabling). At 1-year follow-up for this cohort, there were no additional deaths (10% 1-year mortality), and no further strokes were reported.
In the multicentre international BASILICA registry (n=214, 25 centres, 72.8% bioprosthetic valves, 60% treated with balloon-expandable valves, 78.5% solo BASILICA), traversal and laceration of all intended leaflets were achieved in 94.4% of patients13. Physicians at each centre determined the risk of CO. Procedural success (leaflet traversal and laceration without mortality, CO, or emergency intervention) was achieved in 86.9% of cases. Crude outcomes were similar between solo and doppio cases and between native and bioprosthetic cases. Reassuringly, the rate of major vascular complications was significantly lower (3.8%) compared to the BASILICA IDE trial. CO did occur in 10 cases (6 partial, 4 complete). Cases with partial CO were treated with orthotopic stenting (stent placed through the TAVI valve frame rather than the chimney stent). Of the complete CO cases, 1 patient had acute left main occlusion resulting in cardiogenic shock that necessitated cardiopulmonary bypass. Orthotopic stenting was ultimately performed in this case, and the patient fully recovered. Two cases had CO secondary to the sealing skirt of the Evolut R valve (Medtronic) blocking the left main coronary artery. In each case, the valve was snared and pulled back into the aorta, with a second valve deployed without CO. One of these patients developed cardiogenic shock and ultimately died after the procedure.
BASILICA has several benefits. It can be taught and disseminated if supported by an engaged proctorship programme. It does not require expensive equipment, and the tools are low profile and nimble. Some limitations warrant consideration. Firstly, high-quality pre-TAVI CT imaging is essential to ensure the leaflet’s base is free of confluent calcium to facilitate wire traversal. Secondly, the catheter and wire must be carefully aligned to ensure the leaflet is lacerated directly in front of the coronary ostium. If not aligned, the benefit of BASILICA is reduced, and there is a risk of at least partial CO. Thirdly, BASILICA alone may have limited efficacy in certain TAV-in-TAV scenarios as there may be inadequate leaflet splay due to index TAVI leaflets being pinned against their frame by the new TAV device14. Benchtop testing has found that newer-generation valves (SAPIEN 3 [Edwards Lifesciences] and Evolut R) demonstrate poorer splay than older-generation devices (SAPIEN XT [Edwards Lifesciences], Lotus [Boston Scientific])14. Also, patients undergoing TAVI may still experience CO despite a successful leaflet laceration if the valve skirt occludes flow or if there is severe commissural misalignment with the valve post landing in front of the coronary ostium. Finally, BASILICA is technically challenging and time intensive for lower-volume operators.
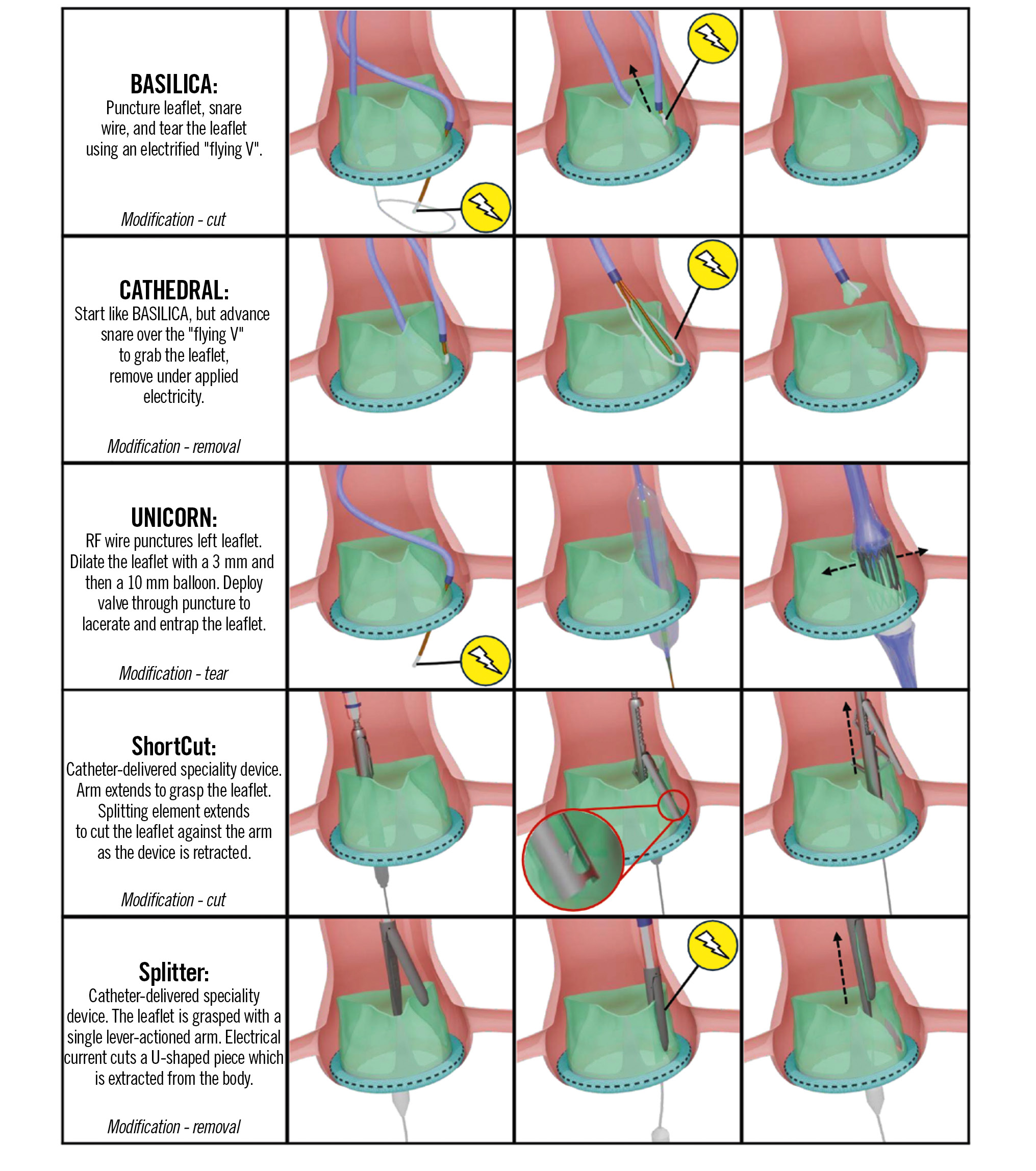
Central illustration. Comparison of leaflet modification techniques for preventing coronary obstruction during TAVI. Device illustrations are artistic approximations. BASILICA: Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; CATHEDRAL: CATHeter Electrosurgical Debulking and RemovAL; RF: radiofrequency; TAVI: transcatheter aortic valve implantation; UNICORN: Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle
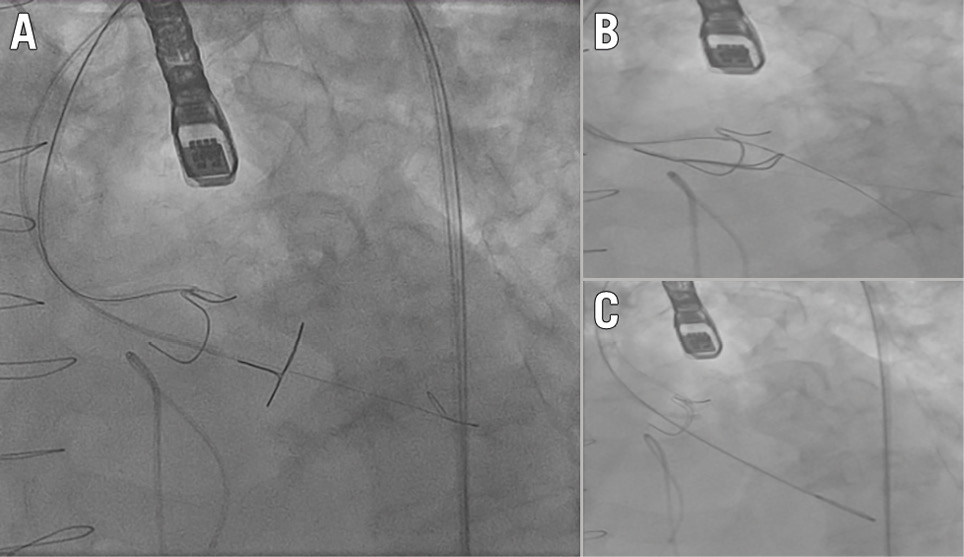
Figure 1. Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA). A) Target leaflet traversal with an electrified Astato wire (Asahi Intecc) inside a telescoping catheter system, and gooseneck snare positioned in the left ventricular outflow tract (LVOT); (B) the distal end of the Astato wire is snared using an Amplatz GooseNeck snare (Medtronic) inside a Multipurpose-1 guide (Merit Medical) in the LVOT; (C) “flying V” in position at the base of the leaflet, ready to lacerate the leaflet.
Balloon-assisted BASILICA
Balloon-assisted BASILICA (BA-BASILICA) was developed in an effort to overcome the issue of inadequate splay after standard BASILICA. This is of particular concern in TAV-in-TAV cases and cases with a short VTC distance or diffusely calcified rigid leaflets. BA-BASILICA uses the same technique as standard BASILICA except for one extra step: after leaflet traversal, the traversal point is dilated with a 5 mm non-compliant balloon or a lithotripsy balloon (before standard laceration with the “flying V”). In benchtop testing, this additional step increased the splay angle (+30%) and splay area (+23%). The first-in-human study for BA-BASILICA included 16 high-risk surgical patients (12 surgical bioprostheses, 4 TAVI) deemed at high risk of inadequate splay (diffuse calcification, VTC distance <2 mm, TAV-in-TAV)15. Leaflet dilation and laceration were achieved in all cases (11 doppios, 5 solos), and the procedure was tolerated well haemodynamically. Three cases required stenting despite BA-BASILICA. Two cases had leaflet prolapse into the left main, treated with orthotopic stenting. The third patient experienced acute coronary syndrome and required left main percutaneous coronary intervention. In each of these cases, the BA-BASILICA made orthotopic stenting feasible, which theoretically should be associated with a long-term lower rate of stent failure than chimney stenting. Although double BA-BASILICA was successful in the 4 TAV-in-TAV cases in this study, caution is still needed with these procedures, particularly when the index TAV has a supra-annular design. Furthermore, benchtop testing on the Evolut PRO (Medtronic) valve has found that BA-BASILICA does not result in a significantly larger splay area than standard BASILICA16.
ShortCut
ShortCut (Pi-Cardia) was the first dedicated transcatheter device for mechanical leaflet splitting to mitigate CO risk (Central illustration)1718. The device has two main components: a splitting element (SE), which divides the leaflet, and a positioning arm (PA) that protects the SE from injuring surrounding structures. A retrograde transfemoral approach is used via a 16 Fr introducer sheath. For the procedure, a stiff preshaped 0.035” wire is placed in the left ventricle, and the ShortCut device is advanced to the aortic root. Once the device has crossed the aortic valve, it is carefully unsheathed to automatically open the PA above the valve. A deflection mechanism allows the device to centre at the valve level. Next, the device is appropriately aligned with the target leaflet using a predetermined fluoroscopic view, and the position is confirmed on the TOE. The SE is then activated, penetrating the leaflet’s base in a controlled manner and engaging with the PA. The PA, located on the aortic side, plays a crucial role in protecting surrounding structures from the activated SE (Figure 2). Splitting of the leaflet is achieved after activating the SE by gently withdrawing the device. If bilateral leaflet splitting is required, these steps are repeated on the contralateral side. The split should be visualised on TOE. Once a split is confirmed, the device is removed, and the valve is delivered over the same 0.035” wire. The device’s 16 Fr sheath provides compatibility with transfemoral TAVI access, eliminating the need for additional vascular entry. By utilising the same access sheath as the TAVI procedure, procedural time and complexity are reduced.
The first-in-human ShortCut Study included 8 patients (high or prohibitive surgical risk) undergoing valve-in-valve TAVI18. Three cases involved bilateral leaflet splitting, and 2 cases were TAV-in-TAV cases. TAVI was ultimately performed in all cases after leaflet splitting was achieved, as planned. No case was complicated by a coronary obstruction, and the median ShortCut procedure time was 19 (interquartile range [IQR] 16-23) minutes. In this series, one patient did develop transient haemodynamic compromise after leaflet splitting. All patients were alive at a median follow-up of 17 months, and all remained free of stroke.
The pivotal prospective ShortCut IDE trial was recently published19. A total of 60 patients (43.8%) from a screened population of 137 patients with a failed bioprosthetic valve (96.7% surgical bioprostheses) deemed at risk of CO underwent the procedure at 22 centres. All cases were transfemoral, and the local Heart Team determined the risk of CO. A cerebral protection device was placed in all cases. The most common risk factors for CO were low-lying coronary ostia (81.7%), leaflet extension above the coronary ostium (70%), a short VTC distance (61.7%), and a short valve-to-sinotubular junction (VTSTJ) distance (45%). The main exclusion reasons were excess leaflet calcium (n=14, 10.2%), anatomy unsuitable for the ShortCut device (n=8, 5.8%), and damaged leaflets (n=8, 5.8%). Patients who had severe renal insufficiency (n=3, 2.2%), unsuitable iliofemoral access (n=4, 2.9%), or anatomy unsuitable for cerebral embolic protection (n=4, 2.9%) were also excluded. Trifecta and Mitroflow, with externally mounted leaflets, accounted for 41.7% of all cases.
Most centres (n=20) had no prior experience with ShortCut (90.9%), and the median number of patients enrolled per clinical site was 2 (IQR 1-4). Leaflet splitting was single in 38 cases (63.3%) and dual in 22 cases (36.7%). The primary efficacy outcome (per-patient leaflet splitting success, determined intraprocedurally by visualisation of leaflet split on TOE or an increase in aortic regurgitation) was achieved in all patients on the first attempt (100%, 95% CI: 94-100%; p<0.001). Furthermore, freedom from the primary safety endpoint of mortality or stroke related to the procedure was achieved in 59 patients (98.3%, 95% CI: 91.1-100%); one patient suffered a stroke on day 4 post-procedure. One case had cardiac tamponade related to left ventricular guidewire placement. ShortCut was well tolerated haemodynamically in all but one patient (1.7%), who had hypotension after dual splitting and ring fracture that resolved after valve implantation. The mean procedure time was 30.6±17.9 mins (single leaflet split: 26.9±19.7 mins, dual leaflet split: 37.0±14.75 mins). Three patients (5%) ultimately required ostial coronary stenting. The ShortCut device recently received U.S. Food and Drug Administration (FDA) approval.
ShortCut has several differences from the more established BASILICA procedure, and each IDE trial had key differences, as shown in Table 1. ShortCut is a dedicated device for leaflet splitting, unlike the off-label use of BASILICA equipment. Furthermore, dual leaflet splitting can be performed with one ShortCut device, and the valve can be delivered using the same sheath and wire. The learning curve also appears shorter, and procedure time is notably shorter than in BASILICA. Complication rates, particularly stroke and major vascular complications, were lower in the ShortCut IDE trial compared to the BASILICA IDE trial but comparable with the BASILICA registry study. The latter finding may be due to the higher-risk population enrolled in the BASILICA IDE trial. BASILICA, however, is more widely studied and has been used in native aortic valve cases, unlike ShortCut. Notably, both devices have minimal data for TAV-in-TAV cases. Some limitations of ShortCut should be considered. The device in its current manifestation is large and bulky. Mechanical cuts may cause leaflet avulsion, more debris liberation, particularly in calcified leaflets, and hypothetically more strokes. Despite this concern, the reported stroke rate remains low, likely because of the mandatory use of cerebral protection devices in most ShortCut procedures and careful patient selection. ShortCut is not indicated for native leaflets. Augmented techniques, such as BA-BASILICA and Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle (UNICORN) are not feasible with this device, limiting its utility in TAV-in-TAV procedures. The device cost is likely to be substantial.
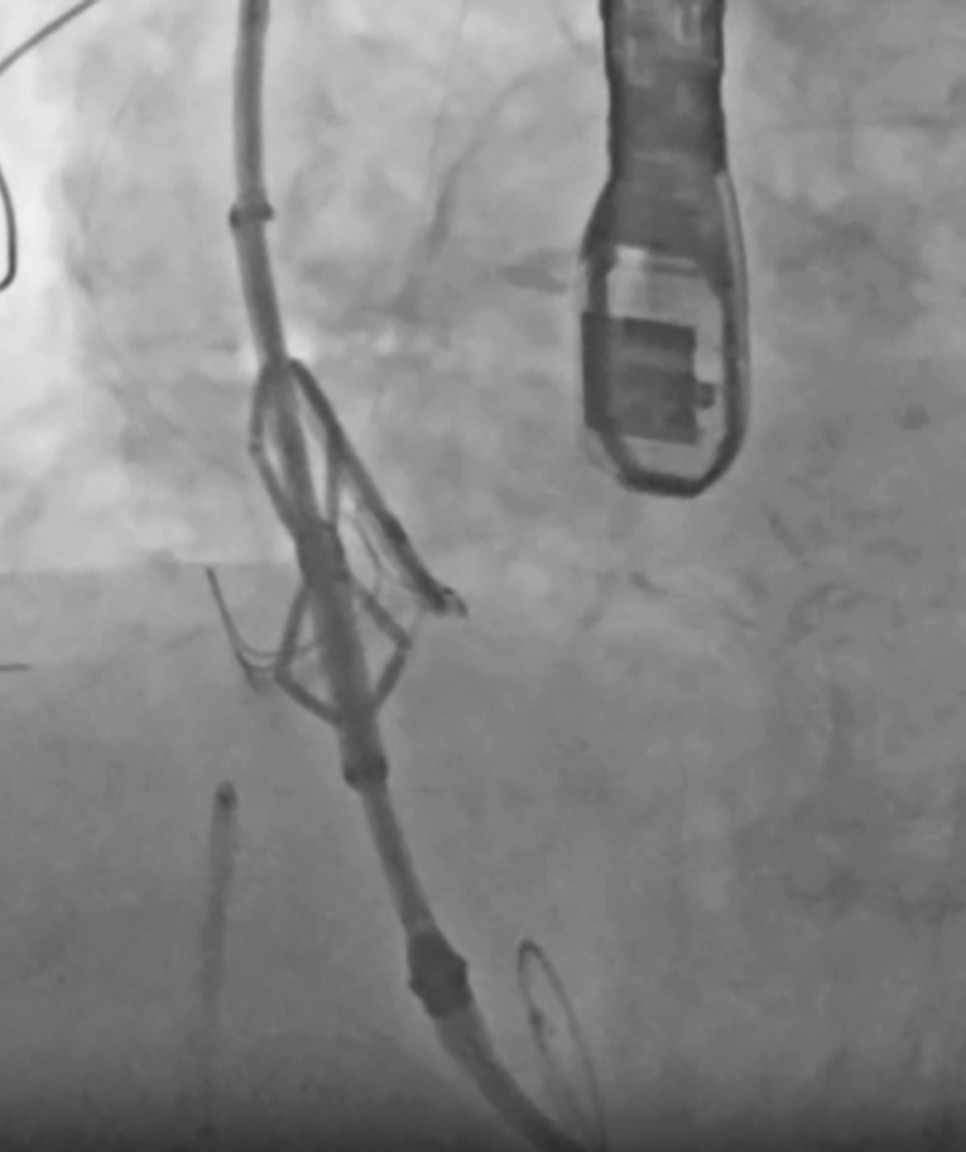
Figure 2. ShortCut. Positioning arm unsheathed above the aortic valve, followed by device alignment with the target leaflet, so the cut will be directly in front of the coronary ostia. The splitting element of the ShortCut device is activated, penetrating the leaflet’s base and engaging the positioning arm, followed by device activation with simultaneous withdrawal to facilitate leaflet splitting.
Table 1. A comparison of leaflet modification studies.
| BASILICA IDE trial (n=30) | ShortCut IDE trial (n=60) | BASILICA registry (n=214) | |
|---|---|---|---|
| Inclusion criteria |
At risk of coronary obstruction |
At risk of coronary obstruction | BASILICA performed between June 2017 and December 2020 for cases deemed at risk of coronary obstruction |
| Main exclusion criteria | Bulky leaflet calcification Referred for SAVR |
Excess calciumUnsuitable for Short Cut Damaged leaflets | Case included in the BASILICA IDE trial |
| STS score | 6 (3-15) | 4.5±2.4 | 6.3±5.3 |
| Surgical risk | |||
| Low | 0 (0) | 0 (0) | 10 (4.7) |
| Intermediate | 0 (0) | 0 (0) | 60 (28.2) |
| High | 30 (100)* | 54 (90) | 115 (54.0) |
| Extreme | - | 6 (10) | 26 (13.1) |
| Bioprosthetic valves total | 17 (57) | 60 (100) | 156 (72.9) |
| Surgical | 30 (100) | 58 (96.7) | 154 (71.9) |
| TAVI | 0 (0) | 2 (3.3) | 2 (0.9) |
| Transfemoral access | 23 (77)# | 60 (100) | 195 (91.1) |
| Cerebral embolic protection | 13 (43)§ | 60 (100) | 102 (47.7) |
| Leaflets split | |||
| Single | 23 (76.7) | 38 (63.3) | 168 (78.5) |
| Double | 7 (23.3) | 22 (36.7) | 46 (21.5) |
| TAVI platform | |||
| Balloon-expandable | 16 (53) | 20 (33.3) | 129 (60.1) |
| Self-expanding | 14 (47) | 20 (66.7) | 85 (39.9) |
| Leaflet laceration | 28 (95) | 60 (100) | 202 (94.4) |
| Haemodynamic compromise | 2 (7) | 1 (1.7) | N/R |
| Additional procedure time, mins | 73 (58-88) | 30.6±17.9 | N/R |
| Complication | |||
| Coronary obstruction | 0 (0) | 3 (5) | 10 (4.7) |
| Death | 1 (3) | 0 (0) | 6 (2.8) |
| Major/disabling stroke | 1 (3) | 1 (1.7) | 1 (0.5) |
| Non-disabling stroke | 2 (7) | 0 (0) | 5 (2.4) |
| Major vascular complication | 6 (20) | 0 (0) | 8 (3.8) |
| Mechanical support needed | - | 0 (0) | 1 (0.5) |
| Cardiac tamponade | 0 (0) | 1 (1.7) | 0 (0) |
| Freedom from reintervention | 30 (100) | 57 (95) | 192 (93) |
| One-year survival | N/R | N/R | 179 (83.9) |
| Data are presented as n (%), median (IQR) or mean±SD. *All BASILICA IDE trial patients were at high or extreme surgical risk. §Embolic debris recovered in 6/13 (46%) of cases. #20% transcaval. BASILICA: Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; IDE: investigation device exemption; IQR: interquartile range; N/R: not reported; SAVR: surgical aortic valve replacement; SD: standard deviation; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |||
Transmural Electrosurgery LeafLet Traversal And Laceration Evaluation (TELLTALE)
The TELLTALE device is the first dedicated electrosurgical system for transcatheter leaflet modification. It comes as a package of devices with a dedicated insulated 0.014” TELLTALE electrosurgical guidewire that has a 1 cm central gold marker that can be denuded and kinked to form the “flying V”. Tailored insulated catheters accompany this. The National Heart, Lung, and Blood Institute TELLTALE IDE pivotal trial is currently ongoing and aims to recruit 75 subjects (60 bioprosthetic, 15 native) in the USA (ClinicalTrials.gov: NCT05666713). The design is predicated on the original BASILICA technique and aims to simplify the procedure for physicians. It has many of the advantages of a small-profile, nimble device that can be used on both aortic and mitral leaflets, both native and bioprosthetic valves, can be used in augmented modification techniques such as BA-BASILICA and UNICORN, and thus may have greater promise in TAV-in-TAV procedures. Furthermore, using electrosurgical laceration may confer an advantage in calcified leaflets in terms of maintaining a linear laceration and reducing debris liberation. The limitations should be considered. The procedure is very similar to the original BASILICA procedure and, although it uses dedicated catheters and wires, may still require more technical skill than a mechanical cut with the ShortCut device. We await data regarding safety and efficacy from the pivotal trial.
Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle (UNICORN)
The UNICORN technique was developed as an alternative to BASILICA, when inadequate leaflet splay is a concern, especially for a failed TAV requiring TAV-in-TAV (Central illustration, Moving image 2). This technique is useful only in cases where one leaflet is at risk of causing CO20. This technique is only feasible with a balloon-expandable valve as the mechanical action of balloon inflation is required to lacerate the leaflet. Self-expanding valves have shown insufficient radial strength to lacerate the pre-existing leaflet in bench testing20.
The initial steps of UNICORN overlap with BASILICA, utilising the same equipment (Table 2). A telescoping catheter system (7 Fr Amplatz Left 1 guide catheter with a 135 cm NaviCross support catheter [Terumo]) is used to direct a wire at the base of the target leaflet (Figure 3A). In contrast to BASILICA, a 0.035” J-tip VersaCross radiofrequency (RF) wire (Boston Scientific) is used to traverse the nadir of the target leaflet. The blunt J-tip of the VersaCross is deemed safer and associated with a lower risk of left ventricular injury. Once the wire traverses the leaflet into the ventricle, the VersaCross is exchanged for a 300 cm balanced heavyweight coronary wire (Abbott). The leaflet is then dilated in two steps, initially with a 3.0 mm non-compliant balloon and then with a 10 mm peripheral balloon over a 0.035” SAFARI XS wire (Boston Scientific) (Figure 3B). Finally, the balloon-expandable valve is delivered over the SAFARI wire to the target implant depth (Figure 3C). Valve inflation results in leaflet laceration, leaflet splay, and leaflet entrapment such that no leaflet obstructs the coronary ostia.
The reported experience with UNICORN is limited to case reports. The first-in-human experience (2022) reported the successful use of the UNICORN technique in a patient with stenosis of a previous 26 mm SAPIEN 3 with a high risk of left main coronary artery occlusion (low sinotubular junction height and VTC distance≈0)20. The authors note that the direction of laceration is less predictable than BASILICA, but leaflet recoil is unlikely because of entrapment by the next valve, especially during TAV-in-TAV. A second case report, in 2024, reported the successful use of UNICORN in treating a patient with stenosis of a 21 mm Carpentier-Edwards bioprosthetic valve (Edwards Lifesciences) at risk of left main obstruction (coronary height 1.2 mm, VTSTJ ≤2 mm, VTC 4.4 mm) with a 20 mm SAPIEN 321. In contrast to the first reported case, a 14x40 mm balloon was required (after the 10x40 mm balloon) to dilate the stenotic valve and facilitate the new valve traversing the leaflet. The larger balloon caused the leaflet to be obliterated. Left main protection was performed with a wire and stent in both reported cases, and cerebral embolic protection was used. Like all leaflet modification techniques, commissural alignment of the existing prosthesis is necessary. No cases of native aortic valves treated with UNICORN have been reported.
Table 2. Required materials for various leaflet modification techniques.
| Technique | Sheath sizes | Wires | Catheters | Energy source |
|---|---|---|---|---|
| BASILICA | 14 to 18 Fr DrySeal Flex introducer sheatha (sized to match intended TAVI valve delivery sheath), contralateral6 Fr sheath | Astato XS 20 (0.014”)b, V-18 ControlWire guidewire (0.018”)c | PiggyBack wire converter (145 cm)d, MP-1 guide catheter (6 Fr)e, AL1-4 guide catheter (6-7 Fr) 120 cmf, Amplatz GooseNeck snare (6 Fr)g sized 1:1 to the LVOT | Electrosurgical “pure cut” (50-70 W) |
| BA-BASILICA | Same as BASILICA | Same as BASILICA | Same as BASILICA plus a non-compliant balloon (5 mm) or lithotripsy balloon | Electrosurgical “pure cut” (50-70 W)+balloon |
| ShortCut | 16 Fr introducer sheath | Preshaped stiff 0.035" wire | ShortCut deviceh (positioning arm, SE) | Mechanical splitting |
| UNICORN | 14 to 18 Fr DrySeal Flex introducer sheath (sized to match intended TAVI valve delivery sheath) | VersaCross RF wire (0.035”)c for crossing, BMW wire (0.014”) and SAFARI XS wire (0.035”)c (for balloon dilatation) | NaviCross catheter (135 cm)i, MP-1 guide catheter (7 Fr), 3 mm coronary balloon and 10 mm peripheral balloon | Radiofrequency+balloon |
| aBy Gore Medical; bby Asahi Intecc; cby Boston Scientific; dby Teleflex; eby Merit Medical; fby Cook Medical; gby Medtronic; hby Pi-Cardia; iby Terumo. AL: Amplatz Left BA-BASILICA: balloon-assisted BASILICA; BASILICA: Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; BMW; balance middleweight; LVOT: left ventricular outflow tract; MP: multipurpose; RF: radiofrequency; SE: splitting element; TAVI: transcatheter aortic valve implantation; UNICORN: Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle | ||||
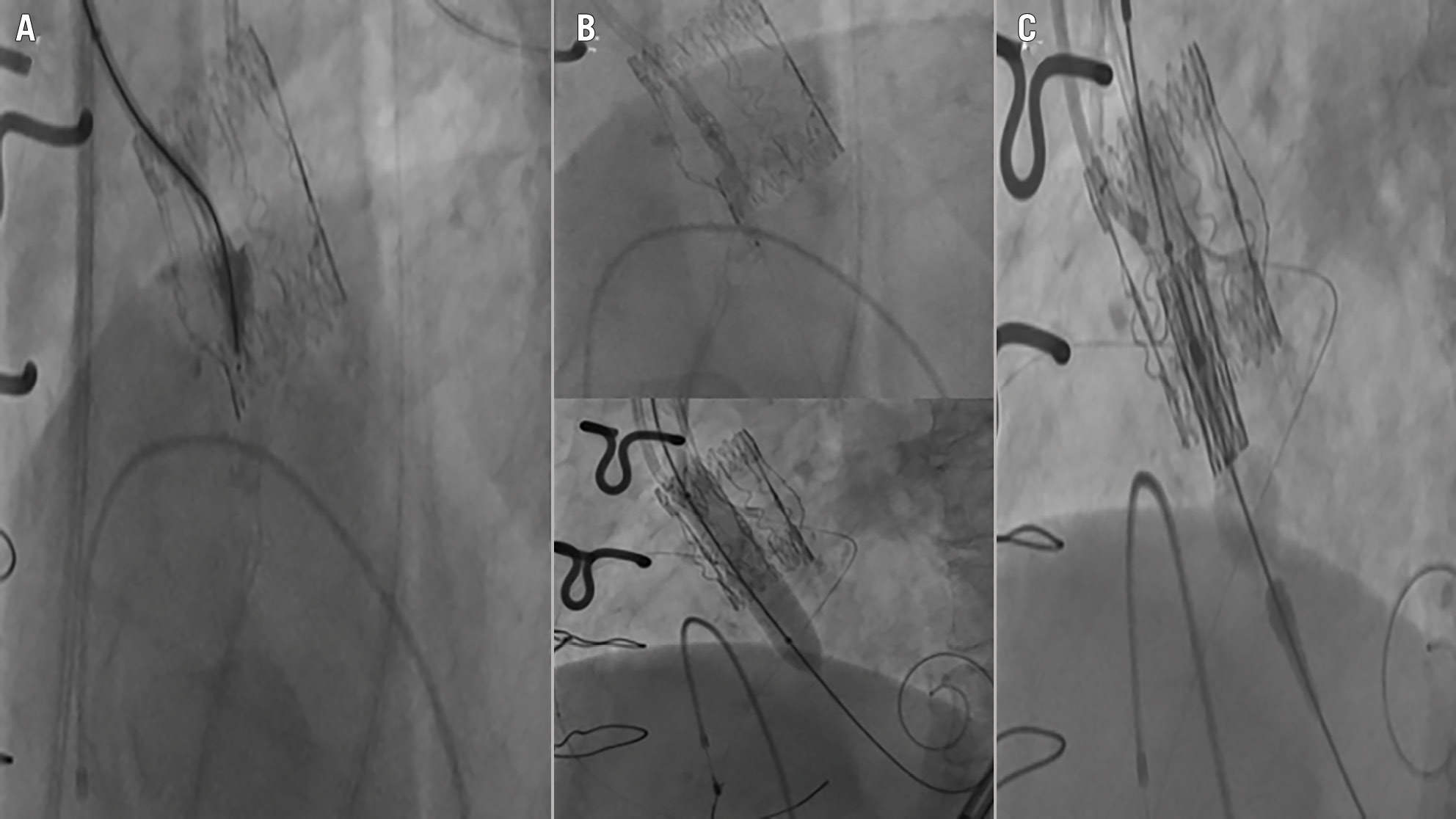
Figure 3. Undermining Iatrogenic Coronary Obstruction with Radiofrequency Needle (UNICORN). A) Leaflet crossing with a J-tip VersaCross radiofrequency wire (Boston Scientific) loaded in a telescoping catheter system; (B) 2-step dilation of the leaflet wire traversal point; (C) intraleaflet deployment of a balloon-expandable valve.
CATHeter Electrosurgical Debulking and RemovAL (CATHEDRAL)
The CATHEDRAL technique was developed by the same team that established BASILICA as a treatment to mitigate the risk of CO (Central illustration). The need for this procedure was driven by a small proportion of patients that may experience CO despite BASILICA. Like BASILICA, the target leaflet is traversed with an electrified Astato XS 20 0.014” wire, and a “flying V” is created between two catheters with the tip of the “V” at the base of the leaflet. However, unlike BASILICA, the exposed section of wire in the “flying V” is not focally denuded but instead is used to grasp, rather than lacerate, the leaflet. Next, the two catheters on the “flying V” are removed, and a 12 Fr sheath is inserted over the “flying V” wires. A guiding catheter is then inserted and used to deliver a loop snare to the leaflet’s base. The prolapsing nature of the leaflet (with torn commissure) and manipulation of the “flying V” facilitates pulling the remainder of the leaflet into the snare. The snare is electrified once the leaflet is confirmed to be in the tightened snare. With simultaneous traction on the “flying V” and the snare, the leaflet is detached. TOE is used to confirm the removal of the leaflet, and the valve can be successfully deployed.
Experience with this technique is very limited. The only described case of this technique is of an 82-year-old man who presented with a failed bioprosthetic valve (23 mm ATS 3F [ATS Medical])22. He was deemed at risk of left main CO due to a prolapsing long left coronary leaflet with a predicted VTSTJ of <2 mm. This technique may only be suitable in cases with a prolapsing leaflet, and it has not been attempted when both coronaries have an obstruction risk.
Splitter
A second dedicated device (Splitter [HVT Medical]) has been studied in benchtop testing and in vivo, with planned animal testing (Central illustration)23. The Splitter device features a steerable catheter delivered over a standard stiff 0.035” wire placed in the ventricle. The electrocuting wire loop, running through the device’s cutting head, makes an intentional U-shaped excision in the target leaflet. The Splitter device is perceived to offer precise control over the cutting site location, controlled excision, and a low risk of embolic complications.
Determining when leaflet modification is sufficient
The leaflet modification techniques mentioned above can be highly effective for mitigating the risk of coronary obstruction in appropriately selected cases. However, their utility depends on specific anatomical and procedural factors. No technique described above can eliminate the risk of CO during TAVI. Moreover, the extensive exclusion criteria from each of the studies mentioned underline the selective nature of the patients in whom these techniques were studied.
Leaflet laceration is most effective when coronary ostia are at a safe height (≥4 mm) from the aortic annulus. In cases where the coronary ostia are extremely low (<2 mm) or have a very eccentric take-off, even successful leaflet laceration may not provide adequate protection. Other techniques, such as chimney stenting, may be required in those cases. Techniques like BASILICA are suitable for thin, non-calcified or moderately calcified leaflets. Bulky, heavily calcified leaflets may resist laceration, reducing the ability to create sufficient coronary clearance. In such cases, alternative strategies, like chimney stenting or BA-BASILICA, which augment leaflet splay with balloon dilation, may be required.
A VTC distance of ≥4 mm is generally considered sufficient to prevent obstruction, provided the leaflet is adequately splayed post-laceration. Proper commissural alignment of the prosthetic valve relative to the coronary ostia is crucial to maximise the efficacy of leaflet modification techniques. In valve-in-valve cases, the presence of pre-existing prosthetic leaflets pinned against the valve frame may limit the efficacy of leaflet laceration. Also, leaflet laceration may not provide adequate coronary protection in procedures involving self-expanding valves due to their larger sealing skirts and the risk of misalignment with the coronary ostia. Techniques like BA-BASILICA, which augment leaflet splay with balloon dilation, may be more effective in these challenging settings.
Future directions
Several areas warrant further investigation to help prevent CO during TAVI.
Leaflet modification for native aortic leaflets
Leaflet modification for native aortic leaflets, especially in challenging anatomies like bicuspid aortic valves, is a growing area of interest. Native leaflets, which are often heavily calcified and asymmetrical, pose a significant risk for CO due to their unpredictable motion during the TAVI valve deployment. Techniques like BASILICA, originally developed for bioprosthetic leaflets, are now being used to modify native leaflets to prevent CO. However, native leaflets present added complexity, such as dense calcifications, which can make wire traversal difficult, potentially reducing the procedural success rates and creating uncertainty. Given the anatomical differences between native tricuspid and bicuspid aortic valves, further research is needed to refine these modification techniques, optimise outcomes, and expand their applicability in patients undergoing TAVI.
Improving risk prediction
Advanced imaging techniques and artificial intelligence-driven models can refine patient selection and better identify those at risk for CO. These tools offer the potential to enhance preprocedural planning and procedural safety by predicting anatomical risks more accurately.
Optimisation of existing techniques
Further optimisation of existing leaflet modification techniques, such as BASILICA and UNICORN, is necessary to simplify these procedures, reduce complexity, and improve reproducibility across operators. Dedicated devices like the ShortCut, TELLTALE, and Splitter should continue to evolve, focusing on reducing procedural times and complications while enhancing outcomes.
Challenges in valve-in-valve TAVI
Valve-in-valve TAVI poses specific challenges, and developing refined techniques for managing stentless or highly calcified leaflets is critical. Exploring alternative energy sources, such as lasers or radiofrequency, may also enhance precision in leaflet modification.
Larger studies and long-term follow-up
Larger studies with long-term follow-up are needed to assess the durability and safety of these interventions, particularly in younger patients with extended life expectancies. These studies will provide robust data to guide clinical decision-making and device development.
Addressing gaps in TAV-in-TAV procedures
Data on TAV-in-TAV procedures remain limited. Current evidence does not demonstrate the superiority of BA-BASILICA or UNICORN over BASILICA or ShortCut in TAV-in-TAV scenarios. More research is necessary in this growing population to determine the best strategies for managing coronary obstruction risks.
Operator training and standardised protocols
Enhancing operator training and developing standardised protocols will help ensure safer and more effective application of these advanced techniques. Simulation-based training programmes and structured curricula can equip operators with the skills necessary to manage high-risk cases effectively.
Laser-based leaflet modification
Laser-based techniques have the potential to transform leaflet modification strategies by offering high precision and the ability to target calcified areas with minimal collateral damage. Lasers can be used to ablate calcified or fibrotic tissue, softening or reshaping the leaflets to reduce the risk of coronary obstruction. These techniques can complement existing methods such as BASILICA or ShortCut, especially in patients with complex anatomies or extensive calcifications. While currently in benchtop testing, laser-based approaches could emerge as a promising tool in leaflet modification24.
Conclusions
Procedures to mitigate the risk of CO during TAVI continue to evolve. To date, these techniques have proved effective in splitting or modifying bioprosthetic leaflets that are at risk of causing CO. However, no device can eliminate the risk of coronary obstruction, and each procedure has technical challenges. Moreover, only BASILICA has been examined for native aortic valve leaflet modification, and there is a paucity of published experience with TAV-in-TAV leaflet modification. Further research is needed to establish if the wider TAVI physician community can safely adopt dedicated leaflet modification techniques.
Conflict of interest statement
G.H.L. Tang has received speaker honoraria and served as a physician proctor, consultant, advisory board member, TAVI publications committee member, RESTORE study steering committee member, APOLLO trial screening committee member, and IMPACT MR steering committee member for Medtronic; has received speaker honoraria and served as a physician proctor, consultant, advisory board member and TRILUMINATE trial anatomic eligibility and publications committee member for Abbott; has served as an advisory board member for Boston Scientific and JenaValve; has been a consultant and physician screening committee member for Shockwave Medical; has been a consultant for NeoChord, Peijia Medical, and Shenqi Medical Technology; and has received speaker honoraria from Siemens Healthineers. J.M. Khan has received proctoring/consulting fees from Abbott, Edwards Lifesciences, and Medtronic; he has equity in Transmural Systems; and he is a co-inventor on patents, assigned to the NIH, of leaflet laceration devices. S. Khera is a physician proctor and consultant for Medtronic; a consultant and proctor for Abbott; a consultant and serves on the advisory board for EastEnd Medical; a consultant and proctor for W. L. Gore & Associates; serves on the speaker’s bureau for Zoll Medical and Edwards Lifesciences; and also serves as the Global PI for the Teleflex ACCESS MANTA Trial (no compensation). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Leaflet modification with BASILICA.
Moving image 2. Leaflet modification with UNICORN.

