Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Cangrelor is approved for oral P2Y12 inhibitor-naïve patients undergoing percutaneous coronary intervention (PCI). Pharmacodynamic (PD) investigations in various clinical settings, with various assays, have shown contrasting data in terms of the extent of platelet inhibition and rates of high residual platelet reactivity (HRPR).
Aims: We aimed to assess the PD effects of cangrelor in all patients receiving it during PCI.
Methods: PharmacOdynaMic Effects of Cangrelor in PatiEnts wIth Acute or chronIc Coronary Syndrome Undergoing Percutaneous Coronary Intervention (POMPEII Registry; ClinicalTrials.gov: NCT04790032) is an investigator-initiated, prospective study assessing PD effects at 4 timepoints with 3 assays. Clinical outcomes at 30 days were also assessed.
Results: From March 2021 to June 2024, 150 patients undergoing PCI and receiving cangrelor were enrolled (64 patients underwent elective PCI; 30 had non-ST-elevation acute coronary syndrome; and 56 had ST-segment elevation myocardial infarction [STEMI], of whom 24 were pretreated with ticagrelor). Most patients switched from cangrelor to either clopidogrel or ticagrelor. Inhibition of platelet aggregation was moderate during cangrelor infusion (light transmittance aggregometry with adenosine diphosphate 20 μM: 57.6±16.5%), with rates of 3.2% for HRPR and 1.3% for bailout tirofiban. Rates of HRPR were relevant at 3 h (37.9%) and 4-6 h (15.3%), and HRPR occurred significantly more frequently in patients switching to clopidogrel compared with ticagrelor. Rates of ischaemic and bleeding events were low.
Conclusions: Cangrelor provided effective platelet inhibition in most patients with ACS or CCS undergoing PCI, including those with STEMI who were pretreated with ticagrelor. Switching from cangrelor to an oral P2Y12 inhibitor, mainly clopidogrel, exposed a large number of patients to a variable period of on-treatment HRPR.
Percutaneous coronary intervention (PCI) is the cornerstone of treatment for patients with acute coronary syndrome (ACS) or chronic coronary syndrome (CCS), and antiplatelet therapy is essential in all these patients to prevent periprocedural and postprocedural thrombotic complications12. In addition to the effects of aspirin, inhibiting the platelet adenosine diphosphate (ADP) P2Y12 signalling pathway reduces the incidence of ischaemic events; however, oral agents require several hours to achieve their full antiplatelet effect, particularly in patients presenting with ACS34567.
Cangrelor is an intravenous P2Y12 receptor inhibitor with rapid onset and offset of platelet inhibition that has been shown to reduce the risk of thrombotic complications compared with clopidogrel in patients undergoing elective or emergent PCI8. It is currently approved in oral P2Y12 inhibitor-naïve patients with CCS or ACS undergoing PCI with a Class IIb recommendation in European guidelines12.
Pharmacodynamic (PD) investigations have been conducted in various clinical settings, with various assays of platelet reactivity assessment and with some contrasting data in terms of the extent of platelet inhibition and rates of high residual platelet reactivity (HRPR), particularly in patients with ST-segment elevation myocardial infarction (STEMI)9101112. Additionally, switching from cangrelor to an oral P2Y12 inhibitor may raise concerns in some patients due to potential drug-drug interactions (DDI), depending on the type of drug selected131415, and in daily practice, cangrelor is used in an off-label setting (i.e., not in P2Y12-naïve patients) and with varying switching strategies16171819; therefore, contemporary PD data are warranted. We conducted a prospective registry to carefully investigate the PD effects of cangrelor in all patients receiving it during PCI.
Methods
Study design and patient population
PharmacOdynaMic Effects of Cangrelor in PatiEnts wIth Acute or chronIc Coronary Syndrome Undergoing Percutaneous Coronary Intervention (POMPEII Registry;
ClinicalTrials.gov: NCT04790032) is an investigator-initiated, prospective, single-centre study conducted at Federico II University of Naples. All adult patients undergoing PCI and receiving cangrelor at the operator’s discretion were eligible2021. Patients were included if they provided consent to blood/data collection, and the study team was available for analyses. No specific exclusion criteria were applied.
All demographic, clinical, procedural and therapeutic data of patients were collected. The Research Electronic Data Capture system (REDCap; developed at Vanderbilt University) was used. The study complied with the Declaration of Helsinki, was approved by the internal ethics committee, and all patients gave their written informed consent.
Medications and procedures
All patients received aspirin, unfractionated heparin, and cangrelor (30 μg/kg bolus followed by 4 μg/kg/min infusion for 2 hours) prior to the start of PCI per standard of care. PCI procedures were performed according to the most recent guidelines and standard of care. If needed as bailout, the glycoprotein IIb/IIIa inhibitor (GPI), tirofiban, was administered at a 25 μg/kg bolus with or without an infusion of 0.15 μg/kg per minute (or 0.075 μg/kg per minute if creatinine clearance was <30 mL/min); the decision to use an infusion and its duration were at the operator’s discretion. Ticagrelor, prasugrel or clopidogrel were administered as loading doses and maintenance doses according to guidelines. The type of oral P2Y12 inhibitor and the timing of its loading dose in the transition from cangrelor were at the operator’s discretion.
Pharmacodynamic assessment
PD assessments were performed with 3 assays: (1) the gold standard light transmittance aggregometry (LTA; 5 μM and 20 μM ADP stimuli); (2) multiplate electrode aggregometry (MEA) ADP test; and (3) VerifyNow P2Y12 test (Werfen).
Blood samples for PD assessments were collected at baseline (before cangrelor bolus administration) as well as at 30 minutes, 3 hours (i.e., 1 h after stopping the cangrelor infusion) and 4-6 hours (i.e., 2-4 h after stopping the cangrelor infusion) after cangrelor initiation. All PD tests were performed within 30 minutes of blood collection by experienced laboratory personnel. HRPR standard definitions were used922.
1) LTA: LTA was performed as previously described923. Briefly, ADP (5 μM and 20 μM) was used as a pro-aggregatory stimulus. The results as a percentage of maximum platelet aggregation (MPA) were collected and used to calculate the percentage of inhibition of platelet aggregation (IPA). HRPR was defined as an MPA >59% (LTA 20 μmol/L ADP) or an MPA >46% (LTA 5 μmol/L ADP).
2) MEA: MEA was assessed in whole blood by the Multiplate analyzer (Roche Diagnostics). Briefly, the ADP test was used to assess the ADP-induced pathway. The mean values of two independent determinations were expressed as the area under the curve (AUC) in arbitrary units (U; 1 U=10 AU min, aggregation unit minutes), maximal aggregation (AU), and velocity (AU/min). HRPR was defined as an AUC >46 U (MEA-ADP).
3) VerifyNow: The VerifyNow P2Y12 assay measures ADP-induced platelet agglutination as an increase in light transmittance and utilises a proprietary algorithm to report values in P2Y12 reaction units (PRU). HRPR was defined as a PRU value >208.
More details are reported in Supplementary Appendix 1.
Study pharmacodynamic and clinical outcomes
The primary outcome was the 30-minute percentage of IPA (%IPA) assessed with LTA after stimulation of platelet-rich plasma with 20 μmol/L ADP as previously described9. The %IPA is defined as follows: 100%×(baseline platelet aggregation − platelet aggregation at time t)/baseline platelet aggregation. Secondary outcomes included all the values of maximum platelet aggregation and %IPA measured at various timepoints with LTA using 20 μmol/L ADP and 5 μmol/L ADP, as well as PRU values measured with the VerifyNow P2Y12 test and AUC values (residual platelet reactivity) measured with the Multiplate analyzer after stimulation with ADP at all timepoints.
Also, data on clinical outcomes (death, cardiovascular death, myocardial infarction [MI], stent thrombosis, stroke, transient ischaemic attack [TIA], urgent revascularisation, bleeding) in the periprocedural period and up to 30 days (telephone or follow-up visit) were collected. Standard definitions were used for these clinical events that were blindly adjudicated by an independent clinical event committee, composed of two cardiologists who were not involved in patient recruitment or management (Supplementary Appendix 1).
Statistical analysis
For the present registry, there was no specific sample size. Up to now, the majority of PD studies on P2Y12 inhibitors, including cangrelor, have enrolled a variable number of patients or group of patients, ranging from 15-50, to explore differences in platelet inhibition3456791011. Given the objective of this registry to include all various clinical scenarios (CCS and ACS subtypes), as well as different combinations of oral P2Y12 inhibitors, we aimed to prospectively enrol at least 100 patients to be able to explore PD effects in various settings.
Data were presented as proportions, medians, or mean±standard deviation, as appropriate. Differences in categorical variables between respective comparison groups were analysed using the chi-square (χ2) statistic test or Fisher’s exact test as appropriate. Continuous variables were compared using the Student’s t-test. IPA was calculated as stated above; for these analyses, ticagrelor-pretreated patients with STEMI (n=24) were excluded, because IPA is calculated using the baseline platelet aggregation, which is affected by ticagrelor pretreatment. In order to assess platelet aggregation after switching from the cangrelor infusion to an oral P2Y12 inhibitor, a dedicated analysis was performed to compare PD values in patients given clopidogrel (n=61) versus ticagrelor (n=61), thus excluding the one patient who did not receive an oral P2Y12 inhibitor due to the need for coronary artery bypass graft (CABG) and the 3 patients receiving prasugrel. Statistical analyses were performed using SPSS, version 29.0 (IBM) and R statistical software, version 4.1.2 (R Foundation for Statistical Computing). A 2-tailed α value of <0.05 was considered significant.
Results
From March 2021 to June 2024, 150 patients undergoing PCI and receiving cangrelor were enrolled in the study, of whom 86 (57%) presented with ACS (STEMI=56 [37%] and non-ST-elevation ACS=30 [20%]) and 64 (43%) with CCS.
The mean age of the study population was 66.7±10.7 years, and 23.3% were female. Overall, 2 patients required bailout with tirofiban due to intraprocedural thrombotic complications. Clinical and procedural characteristics are reported in Table 1 and Table 2.
Among STEMI patients, 24 received pretreatment with ticagrelor 180 mg oral loading dose. Ticagrelor was administered at an average of 41.9±14.7 (min: 10, max: 60) minutes prior to cangrelor bolus administration.
Among the non-pretreated patients (n=126), all switched to an oral P2Y12 inhibitor after the start of cangrelor infusion, apart from one patient who received prolonged cangrelor infusion and was transferred for CABG. Specifically, 61 of 125 (48.8%) patients received ticagrelor (32 STEMI, 23 non-STEMI [NSTEMI], and 6 elective PCI) at a mean time after the start of cangrelor infusion of 51.4±37.2 minutes; 61 (48.8%) received clopidogrel (5 NSTEMI and 56 elective PCI) at the end of cangrelor infusion with a loading dose of 600 mg in 59 patients and 300 mg in 2 patients; and 3 (2.4%) received prasugrel (2 NSTEMI and 1 elective PCI) at the end of the cangrelor infusion.
Table 1. Baseline characteristics of the study population.
| Characteristics | Population (n=150) |
|---|---|
| Age, years | 66.7±10.7 |
| Female | 35 (23.3) |
| Body mass index, kg/m2 | 28.0±4.4 |
| Current smoking | 60 (40.0) |
| Hypertension | 119 (79.3) |
| Diabetes mellitus | 45 (30.0) |
| Hyperlipidaemia | 91 (60.7) |
| Family history of premature CAD | 27 (18.0) |
| Peripheral arterial disease | 9 (6.0) |
| Carotid artery disease | 15 (10.0) |
| Prior MI | 25 (16.7) |
| Prior PCI | 31 (20.7) |
| Prior CABG | 7 (4.7) |
| Prior stroke | 5 (3.3) |
| Prior TIA | 4 (2.7) |
| Previous bleeding requiring medical attention | 3 (0.7) |
| Congestive heart failure | 16 (10.7) |
| Left ventricular ejection fraction, % | 48.3±9.3 |
| Chronic kidney disease (eGFR <60 mL/min) | 19 (12.7) |
| Chronic obstructive pulmonary disease | 23 (15.3) |
| Baseline medications | |
| Aspirin (daily dose ≤100mg) | 82 (54.7) |
| Oral anticoagulant | 10 (6.7) |
| Statins | 73 (48.7) |
| Other lipid-lowering drug | 23 (15.3) |
| ACE inhibitor | 40 (26.7) |
| Angiotensin receptor blocker | 54 (36.0) |
| Beta blocker | 61 (40.7) |
| Amiodarone | 6 (4.0) |
| Ca antagonist | 43 (28.7) |
| Nitrates | 3 (2.0) |
| Diuretics | 42 (28.0) |
| Insulin | 14 (9.3) |
| Oral antidiabetic | 37 (24.7) |
| NSAID | 3 (2.0) |
| Antidepressant drug | 5 (3.3) |
| Proton pump inhibitor | 81 (54.0) |
| Haemoglobin, g/dL | 13.7±1.9 |
| Creatinine, mg/dL | 1.1±0.6 |
| Platelet count, x1,000/mm3 | 228.1±82.7 |
| Data are numbers (%) or mean±standard deviation. ACE: angiotensin II converting enzyme; Ca: calcium; CABG: coronary artery bypass graft; CAD: coronary artery disease; eGFR: estimated glomerular filtration rate; MI: myocardial infarction; NSAID: non-steroidal anti-inflammatory drug; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack | |
Table 2. Clinical presentation and procedural characteristics of the study population.
| Characteristics | Population (n=150) |
|---|---|
| Clinical presentation | |
| Elective PCI | 64 (43) |
| STEMI | 56 (37) |
| NSTE-ACS | 30 (20.0) |
| NSTEMI | 26 (17) |
| Unstable angina | 4 (3) |
| Systolic arterial pressure, mmHg | 136.3±19.9 |
| Diastolic arterial pressure, mmHg | 78.7±13.9 |
| Heart rate, beats/min | 72.2±14.4 |
| Killip class | |
| I | 113 (75.3) |
| II | 32 (21.3) |
| III | 2 (1.3) |
| IV | 3 (2.0) |
| Rhythm at presentation | |
| Sinus rhythm | 146 (97.3) |
| Atrial fibrillation | 3 (2.0) |
| Other rhythm | 1 (0.7) |
| Intraventricular conduction defects | |
| Left bundle branch block | 7 (4.7) |
| Right bundle branch block | 9 (6.0) |
| Other conduction defect | 0 (0) |
| Catheterisation | |
| Radial artery access | 135 (90.0) |
| Haemodynamic support | 2 (1.4) |
| Multivessel disease | 89 (59.3) |
| Vessel treated | |
| LM | 8 (5.3) |
| LAD | 82 (54.7) |
| LCx | 25 (16.7) |
| RCA | 35 (23.3) |
| LIMA/RIMA | 0 (0) |
| SVG | 0 (0) |
| PCI success | 148 (98.7) |
| Total number of lesions treated | 1.4±0.7 |
| Total number of stents implanted | 1.6±1.0 |
| Total length of stents implanted, mm | 40.8±23.9 |
| Data are numbers (%) or mean±standard deviation. LAD: left anterior descending artery; LCx: left circumflex artery; LIMA: left internal mammary artery; LM: left main coronary artery; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; RCA: right coronary artery; RIMA: right internal mammary artery; STEMI: ST-segment elevation myocardial infarction; SVG: saphenous vein graft | |
Pharmacodynamic assessment
PD values were available for analysis in all patients at every timepoint, except for the 2 patients in whom bailout GPI was necessary. For these 2 patients, blood samples at 3 h and 4-6 h were not collected, as the presence of tirofiban may have affected the platelet function results.
Among the 126 patients not pretreated with ticagrelor, at 30 minutes after the start of cangrelor infusion, the mean IPA was 57.6±16.5% at LTA 20 μM ADP (94% of patients with IPA ≤80%) and 62.7±14.9% at LTA 5 μM ADP (89% with IPA ≤80%) (Central illustration). There were 4 cases (3.2%) of HRPR in both assays (Figure 1A). The MEA AUC was 23.9±10.4, while PRU was 40.6±43.1 with both tests showing 2 cases (1.6%) of HRPR (Figure 1A, Figure 2). At 3 h, the mean IPA was 43.9±29.0% at LTA 20 μM ADP and 49.4±30.2% at LTA 5 μM ADP (Central illustration). There were 47 (37.9%) and 46 (37.1%) cases of HRPR, respectively (Figure 1A). The MEA AUC was 30.0±16.1, with 33 (26.6%) cases of HRPR, while the PRU value was 104.7±89.9, with 26 cases (21.0%) of HRPR (Figure 1A, Figure 2). At 4-6 h, the mean IPA was 55.7±26.2% at LTA 20 μM ADP and 63.0±26.6% at LTA 5 μM ADP (Central illustration); there were 19 (15.3%) and 20 (16.1%) cases of HRPR, respectively (Figure 1). The MEA AUC was 22.3±14.5, with 14 (11.3%) cases of HRPR, while the PRU value was 77.2±76.2, with 6 cases (4.8%) of HRPR (Figure 1A, Figure 2). When stratifying the analyses by clopidogrel (n=61) or ticagrelor (n=61) as the switching drug, clopidogrel was shown to be significantly less effective in platelet inhibition at the 3 h and 4-6 h timepoints, with significantly higher rates of HRPR (Figure 1B, Figure 3).
Among the 24 pretreated STEMI patients, at baseline (after ticagrelor pretreatment), MPA was 67.5±22.2% at LTA 20 μM ADP and 53.7±22.0% at LTA 5 μM ADP (Central illustration, Figure 4). HRPR was observed in 18 (75%) and 16 (67%) patients, respectively. Most patients showed low MPA at 30 minutes after the start of cangrelor infusion, with a mean MPA of 34.7±16.4% at LTA 20 μM ADP and 25.4±13.3% at LTA 5 μM ADP (Central illustration, Figure 4), but there were 2 (8.3%) cases of HRPR. Similarly, MPA at 3 h was 35.3±18.3% at LTA 20 μM ADP and 25.0±14.9% at LTA 5 μM ADP and progressively reduced at 4-6 h (27.6±19.3% at LTA 20 μM ADP and 19.0±19.3% at LTA 5 μM ADP) (Central illustration, Figure 4) with 2 (8.3%) cases of HRPR at 3 h and 1 (4.2%) case at 4-6 h with both methods. MEA and VerifyNow showed 13 (54.2%) and 5 (20.8%) cases of HRPR at baseline, with no cases at later timepoints under treatment.
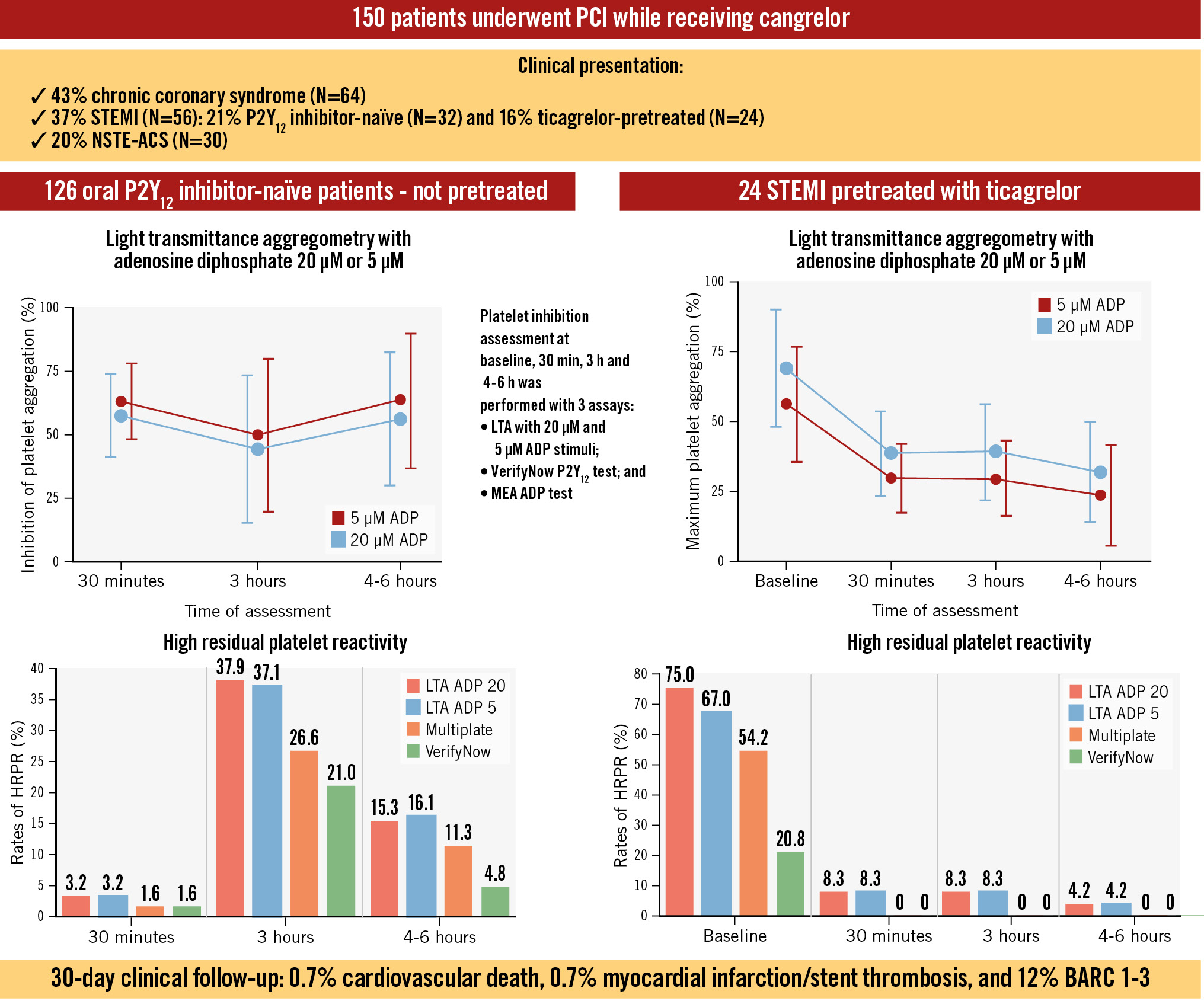
Central illustration. Main findings of the POMPEII Registry with LTA data and HRPR rates among P2Y12 inhibitor-naïve and ticagrelor-pretreated patients. Platelet inhibition assessment at baseline, 30 min, 3 h and 4-6 h was performed with 3 assays: LTA with 20 μM and 5 μM ADP stimuli; VerifyNow P2Y12 test (Werfen); and multiplate electrode aggregometry (MEA) ADP test. Percentage of inhibition of platelet aggregation (left) and maximum platelet aggregation (right) at LTA with ADP 20 μM and5 μM stimulation are reported above the rates of HRPR for P2Y12 inhibitor-naïve and ticagrelor-pretreated patients. ACS: acute coronary syndrome; ADP: adenosine diphosphate; BARC: Bleeding Academic Research Consortium; HRPR: high residual platelet reactivity; LTA: light transmittance aggregometry; NSTE-ACS: non-ST-elevation ACS; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
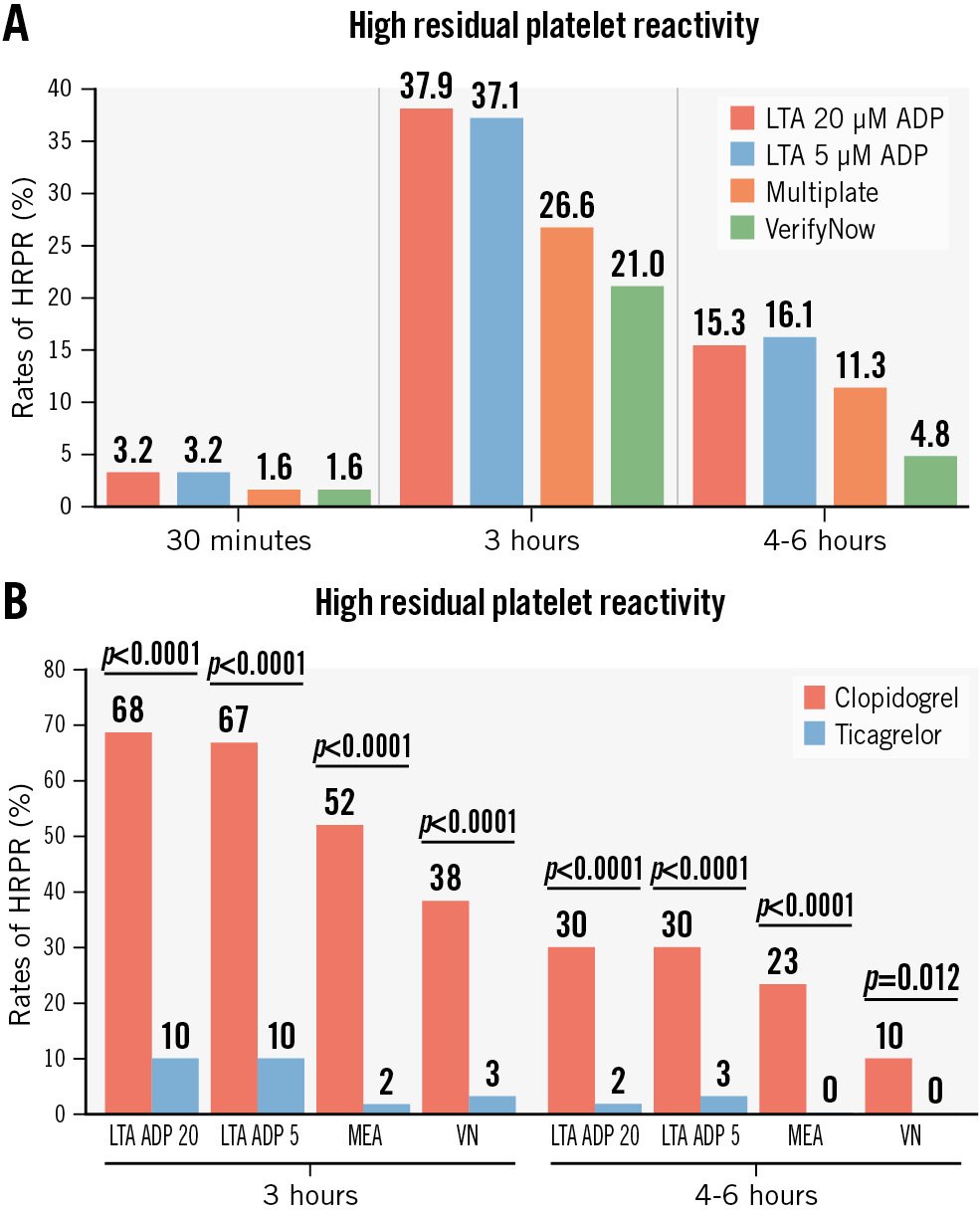
Figure 1. Rates of HRPR in P2Y12 inhibitor-naïve patients. A) Rates of HRPR at 30 minutes, 3 hours and 4-6 hours with all tests defined as follows: 20 µM ADP-induced maximal platelet aggregation >59%, 5 µM ADP-induced maximal aggregation >46% AUC >46 U, and PRU >208, respectively. B) Rates of HRPR at 3 hours and 4-6 hours by clopidogrel or ticagrelor with all tests. ADP: adenosine diphosphate; AUC: area under the curve; HRPR: high residual platelet reactivity; LTA: light transmittance aggregometry; MEA: multiplate electrode aggregometry; PRU: P2Y12 reaction unit; VN: VerifyNow (Werfen)
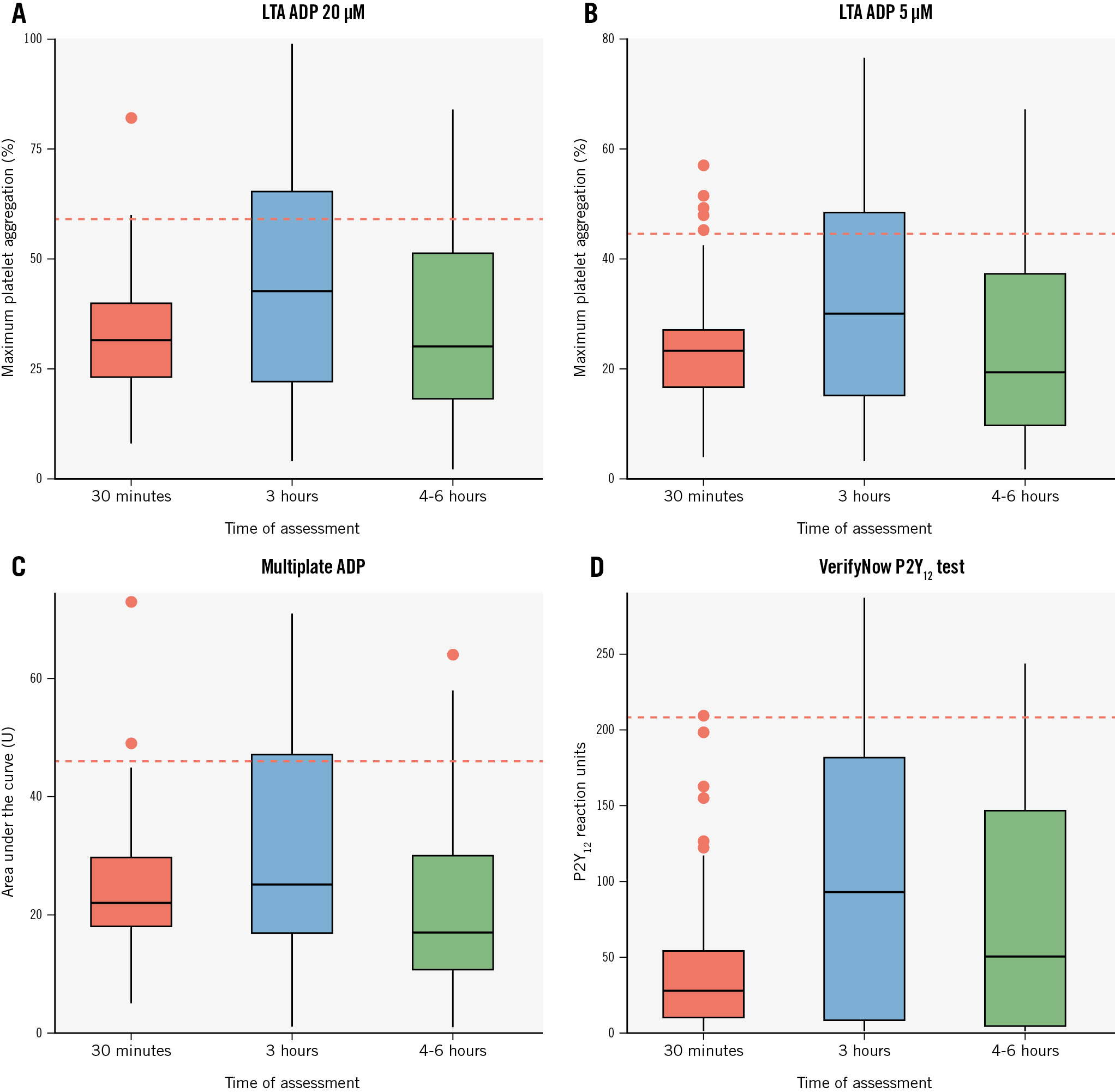
Figure 2. Pharmacodynamic data with all tests at various timepoints in P2Y12 inhibitor-naïve patients. Box plots of values of platelet aggregation at 30 minutes, 3 hours and 4-6 hours as assessed with LTA using ADP 20 µM (A) and ADP 5 µM (B), Multiplate ADP (Roche Diagnostics) (C), and VerifyNow P2Y12 test (Werfen) (D). ADP: adenosine diphosphate; LTA: light transmittance aggregometry
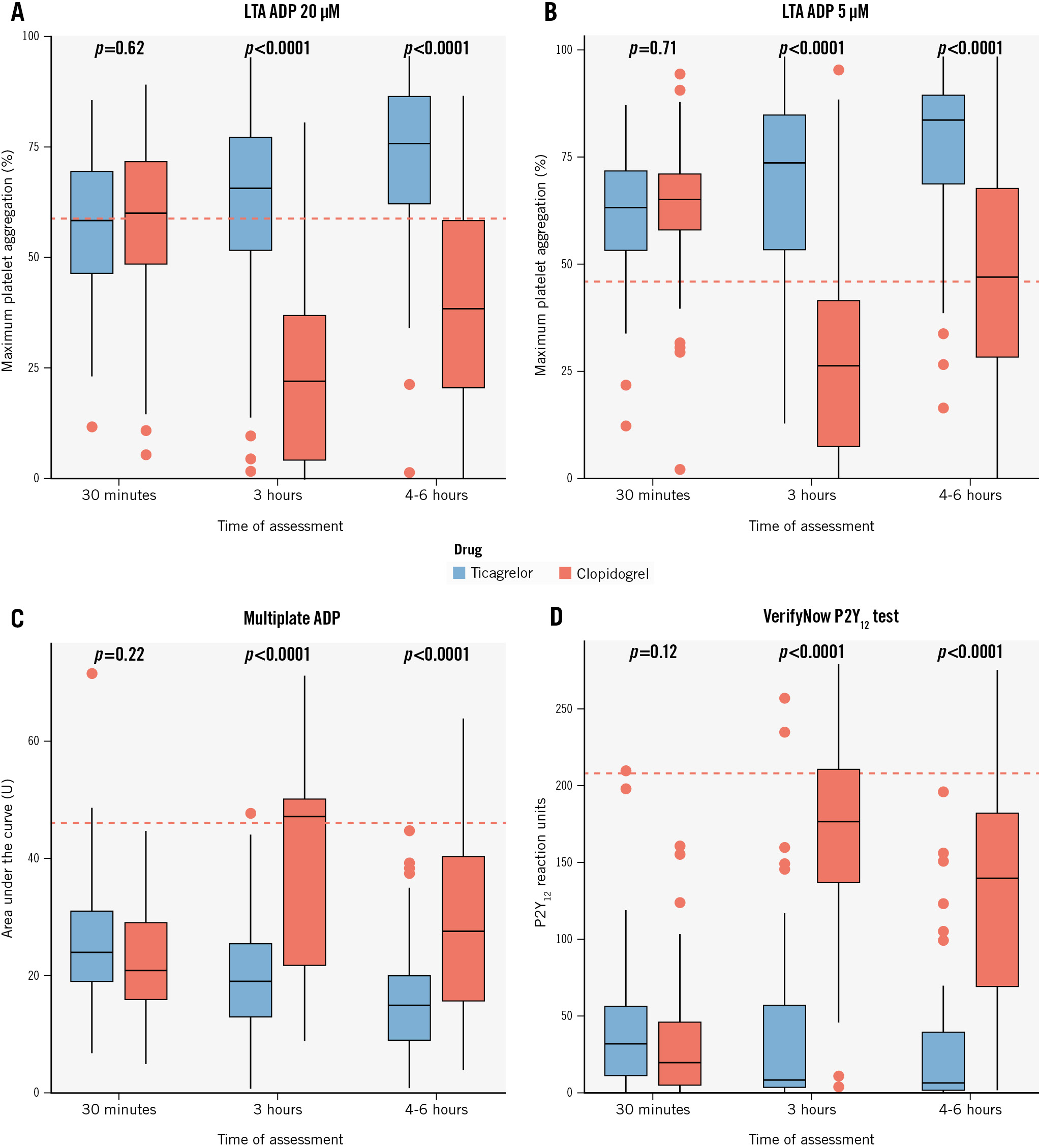
Figure 3. Pharmacodynamic data with all tests after stopping cangrelor by clopidogrel versus ticagrelor in P2Y12 inhibitor-naïve patients. Box plots of values of platelet aggregation at 3 hours and 4-6 hours as assessed with LTA using ADP 20 µM (A) and ADP 5 µM (B), Multiplate ADP (Roche Diagnostics) (C) and VerifyNow P2Y12 test (Werfen) (D). ADP: adenosine diphosphate; LTA: light transmittance aggregometry
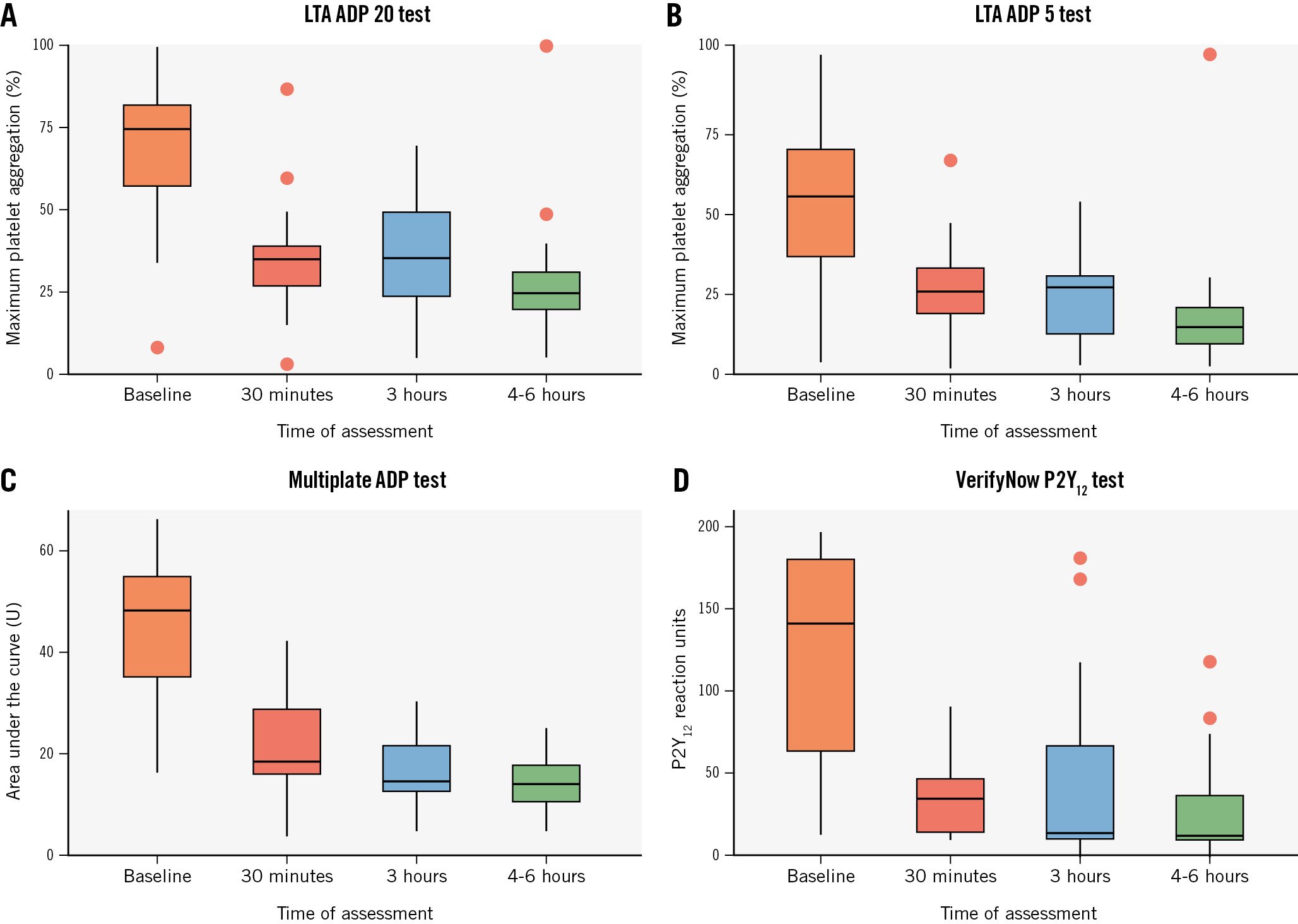
Figure 4. Pharmacodynamic data of the ticagrelor-pretreated STEMI subgroup. Box plots of values of platelet aggregation at baseline, 30 minutes, 3 hours and 4-6 hours as assessed with LTA using ADP 20 µM (A) and ADP 5 µM (B), Multiplate ADP (Roche Diagnostics) (C) and VerifyNow P2Y12 test (Werfen) (D). ADP: adenosine diphosphate; LTA: light transmittance aggregometry; STEMI: ST-segment elevation myocardial infarction
Clinical outcomes
At 30 days, 2 deaths (1.3%) were recorded, 1 (0.7%) of which was a cardiovascular death. One case (0.7%) of MI was observed, and this was at day 1 after elective PCI and defined as periprocedural. No stroke or TIA events were collected. There were 18 (12%) episodes of bleeding, 2 of which required transfusion: 1 (0.7%) was classified as Bleeding Academic Research Consortium (BARC) Type 1 (not access site related), 14 (9.3%) were BARC Type 2 (4 were access site related, of which 3 were radial and 1 femoral), and 3 (2%) were BARC Type 3a (2 were access site related, of which 1 was radial and 1 femoral). Most of these bleeding events (n=15, 10%), including 1 of the BARC Type 3a, occurred within 48 h of the procedure.
Discussion
The POMPEII Registry is a prospective study designed to assess PD profiles in patients undergoing PCI and receiving cangrelor. The main findings are as follows: (1) Cangrelor was shown to be effective in platelet inhibition in most patients with ACS or CCS undergoing PCI. 2) The mean inhibition of platelet aggregation during cangrelor infusion was 57.6%, with most patients showing IPA ≤80%; limited cases of HRPR were seen. 3) Switching from cangrelor to an oral P2Y12 inhibitor exposed a large number of patients to a variable period of on-treatment HRPR, and this was predominantly observed in patients receiving clopidogrel instead of ticagrelor as an oral P2Y12 inhibitor. 4) Ticagrelor-pretreated patients with STEMI undergoing primary PCI showed high rates of HRPR at baseline, but administering cangrelor provided effective platelet inhibition in most patients, with limited HRPR observed. This confirms the absence of DDI and supports the feasibility of such a strategy.
Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over prasugreL: a mUlticenter Randomized Open-label Trial in patientS With ST-elevation Myocardial inFarction Referred for primAry percutaneouS inTERvention (FABOLUS-FASTER) has generated a lot of discussion in terms of the magnitude of platelet inhibition and relevant rates of on-treatment HRPR as assessed with the LTA with cangrelor92425262728. The trial enrolled STEMI patients undergoing primary PCI and compared cangrelor, tirofiban and prasugrel in terms of IPA. The primary hypothesis was that cangrelor may be non-inferior to tirofiban, because preclinical PD data had shown very high degrees (>80%) of platelet inhibition29. However, the trial did not confirm this hypothesis; rather, it showed that cangrelor was inferior to tirofiban with an IPA of 34.1% (as compared with 95.0% with tirofiban) and was also associated with a rate of around 50% for HRPR as assessed by LTA 20 μM ADP. For these reasons, POMPEII was designed to collect contemporary data with different assays of platelet aggregation in various clinical settings. Accounting for differences among patients enrolled, we observed a slightly higher IPA with limited, but detectable, HRPR during cangrelor infusion at LTA, but IPA was moderate and well below 80% in most patients.
The transition from cangrelor to an oral P2Y12 inhibitor is still a debated issue. Indeed, cangrelor and oral P2Y12 inhibitors have different pharmacological properties, thus DDI may potentially occur when concomitantly administrated. Notably, DDI may determine the reduction of platelet inhibition, potentially hindering the prevention of thrombotic complications in the peri-PCI phase. Cangrelor binds platelet P2Y12 receptors during its infusion, which may impede further binding with oral agents such as thienopyridines. The active metabolites of thienopyridines (clopidogrel and prasugrel) have half-lives that are shorter than the duration of cangrelor infusion and, therefore, are mostly cleared once cangrelor infusion is discontinued. Consequently, to prevent DDI, clopidogrel and prasugrel should be administered at the end of the cangrelor infusion. In contrast, ticagrelor has a half-life of 6 to 12 hours and, thus, is still available for P2Y12 receptor binding after cangrelor interruption, allowing for these agents to be concomitantly administered without any DDI. This evidence was recently confirmed by the Switching Antiplatelet (SWAP)-5 and -6 trials. Specifically, the SWAP-5 randomised trial recently ruled out DDI in 20 patients with coronary artery disease who had been pretreated with ticagrelor within 1 h and were receiving cangrelor14. Conversely, the SWAP-6 randomised trial, including 77 patients undergoing PCI, confirmed a DDI between prasugrel and cangrelor by showing that their concomitant administration determined a marked increase in platelet reactivity after stopping cangrelor infusion15. In the POMPEII Registry, we provide data from patients undergoing PCI. Most patients undergoing PCI due to CCS received clopidogrel at the end of cangrelor infusion, and a large proportion of these patients were exposed to HRPR during the next few hours, as previously described. These findings suggest that ticagrelor − preferably administered during cangrelor infusion − might be considered to mitigate this rebound effect20. In the present analysis, we observed an overall HRPR rate of 37.9% at 3 h and 15.3% at 4-6 h in P2Y12 inhibitor-naïve patients who switched from cangrelor to an oral P2Y12 inhibitor. However, HRPR was significantly lower when ticagrelor was used compared with clopidogrel, thus supporting previous evidence that ticagrelor might be the preferable option to overcome the HRPR and DDI issues related to transitioning from cangrelor.
Although evidence from randomised trials on pretreatment in STEMI patients has been discouraging, and European guidelines have recently downgraded this recommendation, pretreatment with oral P2Y12 inhibitors before PCI remains an option that can be considered and is still used in daily practice. A recent observational study refuelled the interest in this strategy by showing that STEMI pretreatment with P2Y12 inhibitors was associated with a significant time-dependent reduction of 30-day major adverse cardiovascular events without increasing bleeding risk30. However, despite it being formally approved only for P2Y12 inhibitor-naïve patients, some registries show that, in daily practice, cangrelor has been used in pretreated patients1617. There is, however, a paucity of PD and clinical data on cangrelor in pretreated patients. The above-mentioned SWAP-5 included patients with stable coronary artery disease, while contemporary data in STEMI patients pretreated with ticagrelor are limited. In a recent preliminary analysis including 13 patients from our POMPEII Registry, we assessed the PD profiles of patients presenting with STEMI and receiving cangrelor after pretreatment with ticagrelor within 1 hour21. Here, we provide an analysis of 24 patients confirming that adding cangrelor was effective and safe, with most patients achieving adequate platelet inhibition during and after primary PCI; however, we observed a limited number of cases of HRPR up to 6 hours. While our study was not specifically designed to assess DDI, these findings may support a strategy already used in clinical practice and, moreover, confirm that this strategy may be a safe and potentially viable option to fill the gap that remains with ticagrelor.
Overall, the rates of ischaemic and bleeding complications were similar to those previously reported. We observed only 1 periprocedural MI in an elective patient, resulting in a low rate (1.6%) for this complication31. The role of elective PCI in stable patients remains debated, and periprocedural MI is crucial in the risk-benefit ratio between medical therapy and myocardial revascularisation. Preventing such a complication might be important to maximise the benefits of PCI, and cangrelor could be an option for this, particularly in the challenging setting of complex PCI, as we previously suggested20. Bleeding events (12%) were higher than those reported in some case series16171832 but lower than in others19. Such discrepancies may be related to different populations and event monitoring/adjudication; however, our bleeding events were mostly non-severe. Therefore, clinical outcomes indicate limited ischaemic complications with an acceptable risk of bleeding.
Limitations
This is an observational study which is underpowered for clinical events. The clinical implications of these findings warrant investigation in adequately powered clinical trials.
This is a single-arm study without a control group. However, in terms of PD assessments, it is intuitive that a placebo group would clearly show absence of platelet inhibition, while it is well-established that an oral P2Y12 inhibitor group would have limited (and inferior to cangrelor) platelet inhibition in the periprocedural phase34910.
This is a single-centre study with its inherent limitations; however, this also represents a strength due to the limited variability in PD assessments. Similarly, some bias was related to the fact that the use of cangrelor as well as the selection of the type and timing of the oral P2Y12 inhibitor was decided by the physicians; however, this too can be considered a strength, as it mirrors daily practice and incorporates new scientific evidence (e.g., the early administration of ticagrelor after cangrelor, despite not yet being approved in Europe, based on the encouraging data from the CANTIC trial, or the use of cangrelor in ticagrelor-pretreated patients after encouraging results from the SWAP-5 trial); this allows a more effective generalisation of the results to current real-world practice.
Some selection bias exists as a result of the enrolment of non-consecutive patients, since cangrelor is not routinely used in all consecutive patients, and PD analysis requires a dedicated team working for up to 6 hours.
Finally, PD assessments during cangrelor infusion were performed only at 30 minutes; however, in the FABOLUS-FASTER trial, samples at 15 minutes, 1 h and 2 h were also analysed, and all timepoints provided overlapping IPA values.
Conclusions
This study provides pharmacodynamic data in a contemporary treated population of ACS or CCS patients undergoing PCI and shows that cangrelor can be an effective and safe option to rapidly inhibit platelet aggregation. Our data confirm that this option could also be considered for ticagrelor-pretreated patients with STEMI undergoing primary PCI. After cangrelor interruption, the transition to an oral P2Y12 inhibitor might expose patients to some risks of platelet aggregation rebound, but, in our study, this issue seemed to be overcome by using ticagrelor as the oral P2Y12 inhibitor instead of clopidogrel; further studies are needed.
Impact on daily practice
Cangrelor was shown to be effective and safe in most patients with acute or chronic coronary syndrome undergoing percutaneous coronary intervention (PCI). The degree of inhibition of platelet aggregation during cangrelor infusion was moderate, with most patients showing inhibition of platelet aggregation ≤80% and limited cases of high residual platelet reactivity (HRPR). Switching from cangrelor to an oral P2Y12 inhibitor exposed a large number of patients to a variable period of on-treatment HRPR; this was predominantly observed in patients receiving clopidogrel instead of ticagrelor as an oral P2Y12 inhibitor. Ticagrelor-pretreated patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI showed high rates of HRPR at baseline. However, the administration of cangrelor effectively inhibited platelet activity in most patients, with limited HRPR observed, thus confirming the absence of drug-drug interactions and supporting the feasibility of such a strategy.
Funding
This study was internally supported by Azienda Ospedaliera Universitaria Federico II and University Federico II of Naples. No direct or indirect external funding was received.
Conflict of interest statement
M. Valgimigli received an institutional grant from Terumo; and consulting fees from AstraZeneca, Terumo, Alvimedica/CID, Abbott, Daiichi Sankyo, Bayer, CoreFlow, Idorsia Pharmaceuticals Ltd., Vifor, Bristol-Myers Squibb SA, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, PhaseBio, and ECRI, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

