Cory:
Unlock Your AI Assistant Now!
Abstract
BACKGROUND: The MitraCut procedure employs beating heart transapical (TA) cannulation and endoscopic scissors for dividing the anterior mitral leaflet (AML) to prevent left ventricular outflow tract (LVOT) obstruction in transapical transcatheter mitral valve replacement (TA-TMVR).
AIMS: We present the first multicentre experience of the MitraCut procedure prior to TA-TMVR to prevent LVOT obstruction.
METHODS: In 6 European centres, the clinical outcomes of all 13 high-risk patients who had undergone the MitraCut procedure during TA-TMVR procedures were retrospectively reviewed regarding technical success, procedural details and outcome.
RESULTS: The MitraCut procedure was successfully completed in 11 patients with 1 cutting attempt, while 2 patients had 2 cutting attempts, with an average procedure duration of 9.0±5.4 min. No patient demonstrated postoperative LVOT obstruction, and all mitral valve (MV) prostheses were competent throughout the follow-up period. However, 1 patient developed a MitraCut-related paravalvular leak (PVL; technical success rate: 12/13). The mean LVOT gradient was 3.9±4.4 mmHg directly after valve expansion and 3.6±3.1 mmHg at follow-up. In-hospital and 30-day mortality were 0%. One patient experiencing MitraCut-related PVL was successfully treated by interventional PVL closure (reintervention rate: n=1). One patient died at 47 days due to cardiac arrhythmia, unrelated to the AML-directed procedure. The mean follow-up at the time of data analysis was 52±34 days.
CONCLUSIONS: The MitraCut procedure was effective and reproducible for preventing potential LVOT obstruction in TA-TMVR patients during its initial exploration in 6 European hospitals. Considerations regarding the scissors’ characteristics, their handling and cut length are mandatory for safe performance of the procedure.
Severe mitral valve (MV) disease is associated with high mortality rates when untreated1. In patients presenting as frail or with multiple comorbidities preventing conventional MV surgery, several transcatheter interventional approaches have emerged as potentially valuable therapeutic options2. The decision by the Heart Team between interventional MV repair or replacement (TMVR) remains challenging. Many of the evaluated patients do not meet the inclusion criteria of market-release TMVR studies3456. While interventional repair techniques continue to expand to patients with complex MV pathologies4, various anatomical predictors indicate unfavourable outcomes of transcatheter edge-to-edge repair; such poor prognostic indicators include mitral annular calcification (MAC)7, restricted leaflet motion of the posterior MV leaflet8, a small MV opening area9, and a high baseline MV mean pressure gradient (MPG)7.
Because of expected unsatisfactory outcomes and/or contraindications for interventional repair (e.g., stenotic MV disease), there is a trend towards liberal screening for TMVR6. Though TMVR has been shown to be highly effective in reducing mitral regurgitation (MR)1610, up to 69% of the screened patients are considered anatomically ineligible for TMVR, mainly due to unsuitable annular dimensions and unacceptably high risk for left ventricular outflow tract (LVOT) obstruction1. Despite specific screening processes, including assessment of the neo-LVOT by cardiac multislice detector computed tomography (MDCT), LVOT obstruction may occur in >10% of patients11. Further, (dynamic) LVOT obstruction has been reported with the Tendyne (Abbott) valve system even in presence of an acceptable neo-LVOT612 above 250 mm2.
While transseptal electrosurgical techniques for splitting the anterior mitral leaflet (AML), including laceration of the anterior mitral leaflet to prevent outflow tract obstruction (LAMPOON), have been shown to be effective for avoiding systolic anterior movement of the AML and obstruction of the LVOT by the subvalvular portion of the prosthesis stent frame, such techniques can be difficult to perform131415. The MitraCut procedure in TA-TMVR opens new paths to address the AML, as this leaflet can be easily reached by endoscopic scissors without requiring an additional access site or expensive equipment. Based on the success of previous published case reports of the novel MitraCut procedure from different centres16171819, this retrospective study describes the relative safety and efficacy of the MitraCut procedure prior to TMVR across a larger study population within this first European, multicentre experience.
Methods
This is a retrospective report of a European, multicentre evaluation regarding the early surgical results of the beating heart MitraCut procedure incorporating a transapical large-bore sheath to enable scissor-mediated AML division. To our best knowledge, all European centres applying this technique prior to TA-TMVR in the initial period (11/2022-05/2023) were invited to participate. Finally, patients from 6 heart centres in Austria, Germany, Italy, and Spain were included. Each centre followed the standards of the local ethical boards. Data collection was performed according to Mitral Valve Academic Research Consortium (MVARC) criteria.
MITRACUT PROCEDURE
The Heart Team elected to perform the MitraCut procedure in patients with a small predicted LVOT (but above the cutoff for Tendyne implantation), in patients with a long anterior mitral valve leaflet or if a potential risk for fixed or dynamic LVOT obstruction based on preoperative or intraoperative imaging was perceived (Supplementary Table 1). The MitraCut procedure was performed using endoscopic shafted scissors and a manually shortened large-bore sheath (Moving image 1). The diameter and the length of the sheath were chosen based on the size and length of the endoscopic scissors’ shaft; the sheath was typically shortened to a length of between 10 and 15 cm. After carefully flushing the shortened sheath, the endoscopic scissors were introduced inside the sheath to check the length (Figure 1A).
After performing a standard apical access, heparinisation, and “flossing” manoeuvre to exclude any entanglement of the guidewire in the subvalvular apparatus20, the modified sheath and its indwelling dilator were passed through the apical myocardium into the left ventricle while maintaining the guidewire tip’s position in the pulmonary vein. This dilator was later replaced by the scissors through the sheath. The sheath was advanced into the left atrium to approximately 2 cm above the MV plane (Figure 1B). The tip of the scissors was pushed out of the sheath and opened to demonstrate appropriate alignment under three-dimensional (3D) echocardiography (Figure 1C). Once correct alignment in the centre of the MV was achieved, the open scissors and the sheath were simultaneously retracted into the left ventricle. Guided by an X-plane view of the left ventricle (intercommissural view, LVOT view), the AML was trapped between the scissor blades and sharply transected in the A2 segment (Central illustration, Figure 1D). The effectiveness of the transection was assessed based on a 3D en-face view of the MV (Figure 1E). After the cuts were complete, the scissors were retracted into the sheath, facilitating their removal without risk of tissue damage. The MitraCut procedure was followed by TMVR with the Tendyne valve system, as previously described in detail520.
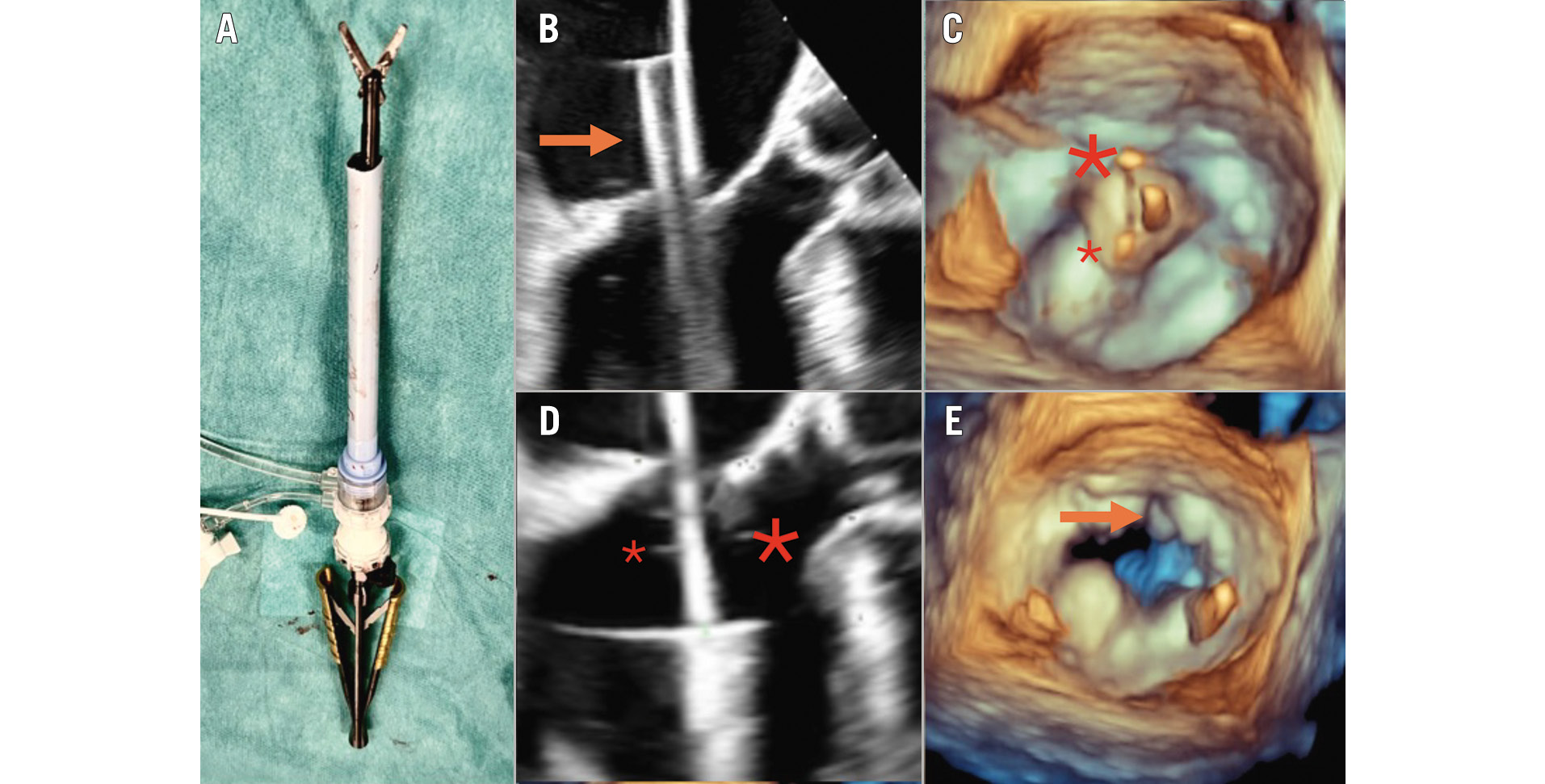
Figure 1. The MitraCut procedure. Illustration of the off-table preparation after manual shortening of the large-bore sheath and insertion of the endoscopic scissors (A). The sheath and the covered scissors (orange arrow) are inserted up to approximately 2 cm above the MV plane (B), followed by alignment of the scissors (C; big asterisk above the AML, small asterisk above the posterior leaflet). The scissors together with the sheath are carefully retracted into the left ventricle, enabling the grasping of the AML (D). The post-MitraCut three-dimensional en-face view demonstrates successful AML division (E). Adapted and changed from Andreas et al16. AML: anterior mitral leaflet; MV: mitral valve
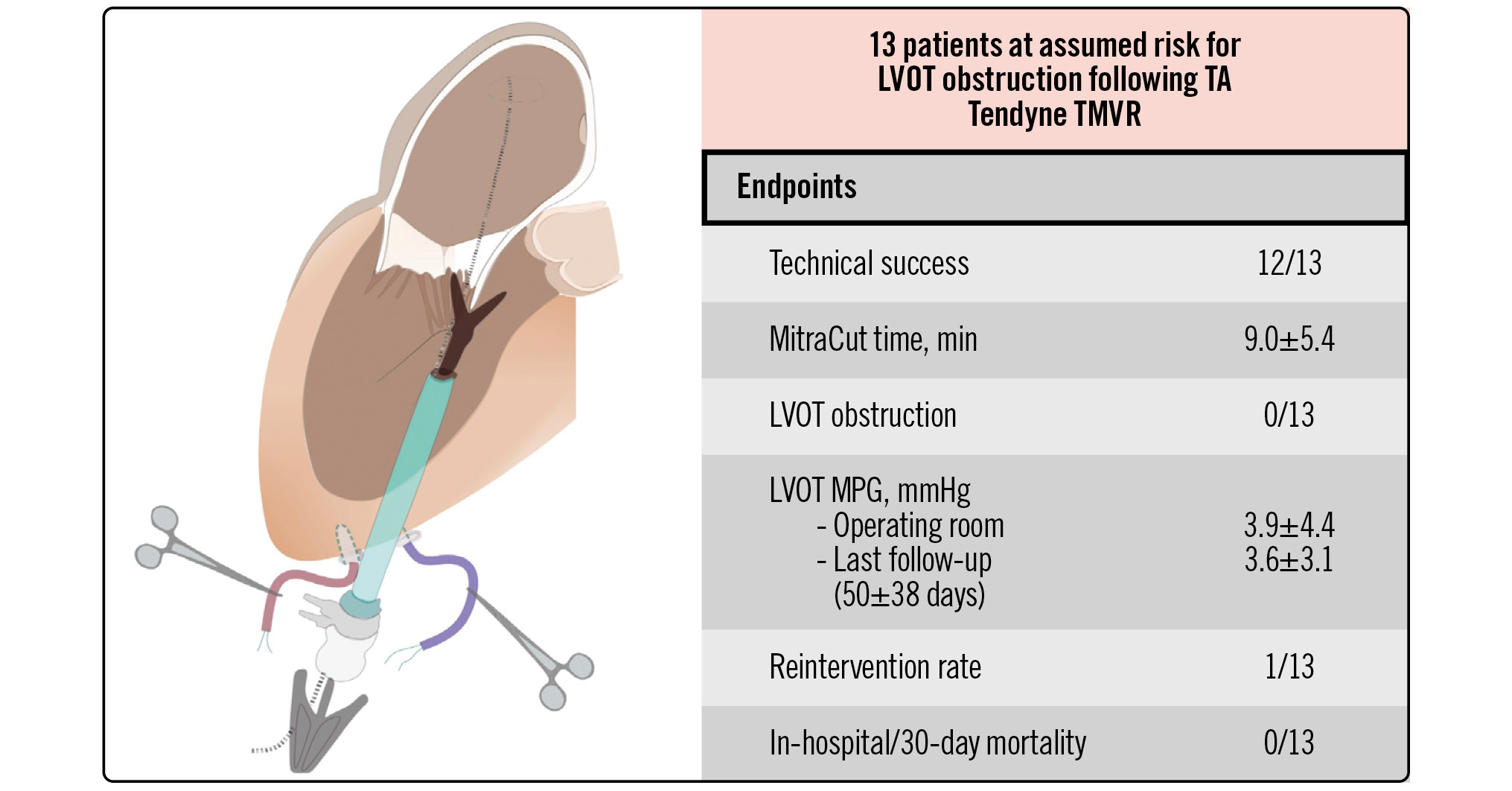
Central illustration. Multicentre experience with a novel surgical technique to prevent LVOT obstruction in TMVR: the MitraCut procedure. LVOT: left ventricular outflow tract; MPG: mean pressure gradient; TA: transapical; TMVR: transcatheter mitral valve replacement
ECHOCARDIOGRAPHIC ASSESSMENT
The LVOT gradients were measured directly after complete valve expansion in the operating room by transoesophageal echocardiography (TOE) and at follow-up by transthoracic echocardiography. Prosthetic mitral valve function (MV MPG and paravalvular leakage [PVL]) was assessed according to the recommendations of the American Society of Echocardiography2122.
MDCT CALCULATIONS
The calculations from the screening MDCT scan were conducted using the commercially available MV workflow of 3mensio, version 10.3 (Pie Medical Imaging). The standard triangle language file of the respective Tendyne valve was snapped to the mitral annulus to achieve a coaxial alignment. The narrowest part between the simulated valve and the LVOT was traced (neo-LVOT) in end-systole and end-diastole (Figure 2A, Figure 2B). Additionally, the distance between the stent frame and the LVOT/interventricular septum (AML clearance in end-systole and end-diastole) (Figure 2C, Figure 2D) as well as the length of the AML were measured in an LVOT view (Figure 2E). MAC was graded according to Guerrero et al (Figure 2F)23.
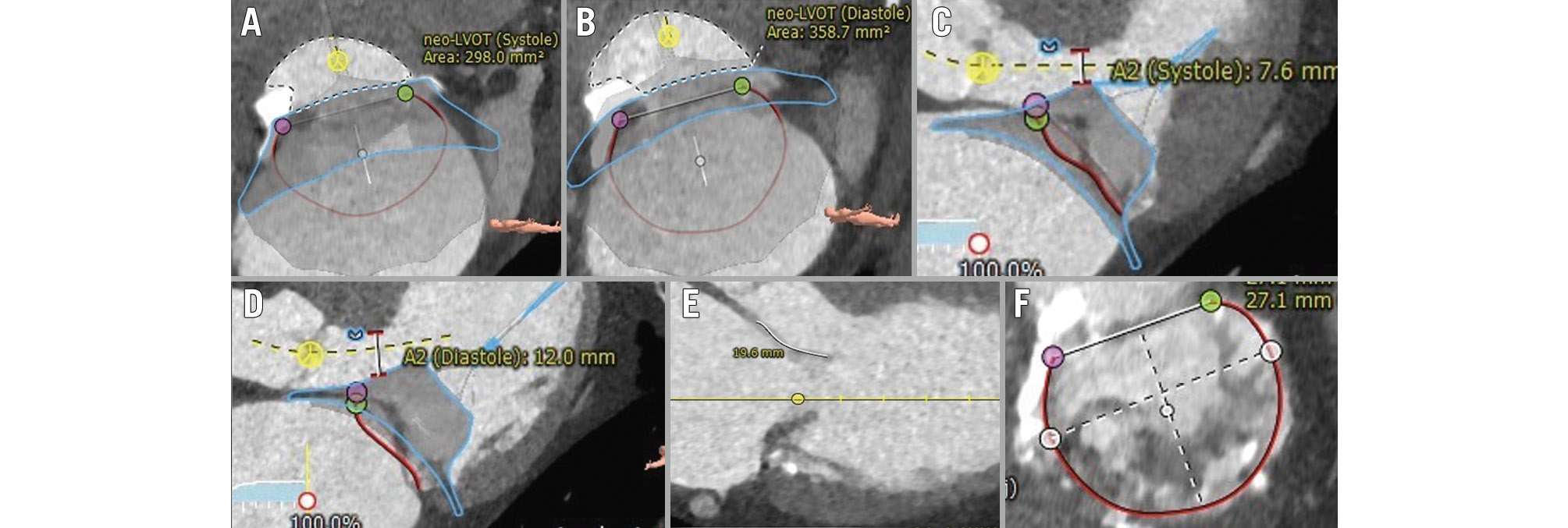
Figure 2. MDCT-derived assessment. Calculation of the neo-LVOT and the distance between the stent frame and the interventricular septum (A2 clearance) in end-systole (A,C), and end-diastole (B,D), respectively. Further, the anterior length on an LVOT view (E), as well as the degree of mitral annular calcification on an en-face view (F), were determined. Adapted and changed from Andreas et al16. LVOT: left ventricular outflow tract; MDCT: multislice detector computed tomography
ENDPOINTS
The primary endpoint was the technical success of the MitraCut procedure, which was defined as successful insertion and removal of the sheath and scissors, an echocardiographically confirmed division of the AML, successful valve implantation without LVOT obstruction, freedom from clinically significant PVL related to the AML cutting and embolic events, freedom from apical access and bleeding complications with a need for reintervention and/or haemodynamic instability, as well as periprocedural death. The number of AML cutting attempts, the time from skin incision to closure (procedural time), insertion and removal of the sheath for the MitraCut and the time from AML division to complete valve expansion were recorded. Secondary endpoints within the index hospitalisation and the last available follow-up were reported according to the criteria defined by the MVARC24.
Numerical and categorical data were reported as mean±standard deviation and number (%), respectively. Descriptive statistics were applied using SPSS Statistics, version 29.0 (IBM) and Excel for Mac (Microsoft).
Results
Thirteen patients who underwent the MitraCut procedure prior to TA-TMVR to prevent LVOT obstruction were included in this analysis. The Heart Team decision against conventional surgery was determined based on age, frailty or cardiac cachexia (n=6), immobility (n=1), severely reduced left ventricular systolic function (n=1), prior cardiac surgery (n=4), and previous thoracic endovascular aortic repair hindering aortic clamping (n=1) (Supplementary Table 1). Two patients were enrolled for valve-in-ring TMVR due to recurrent MR after surgical annuloplasty. The reasons for rejecting transcatheter MV repair are depicted in Figure 3.
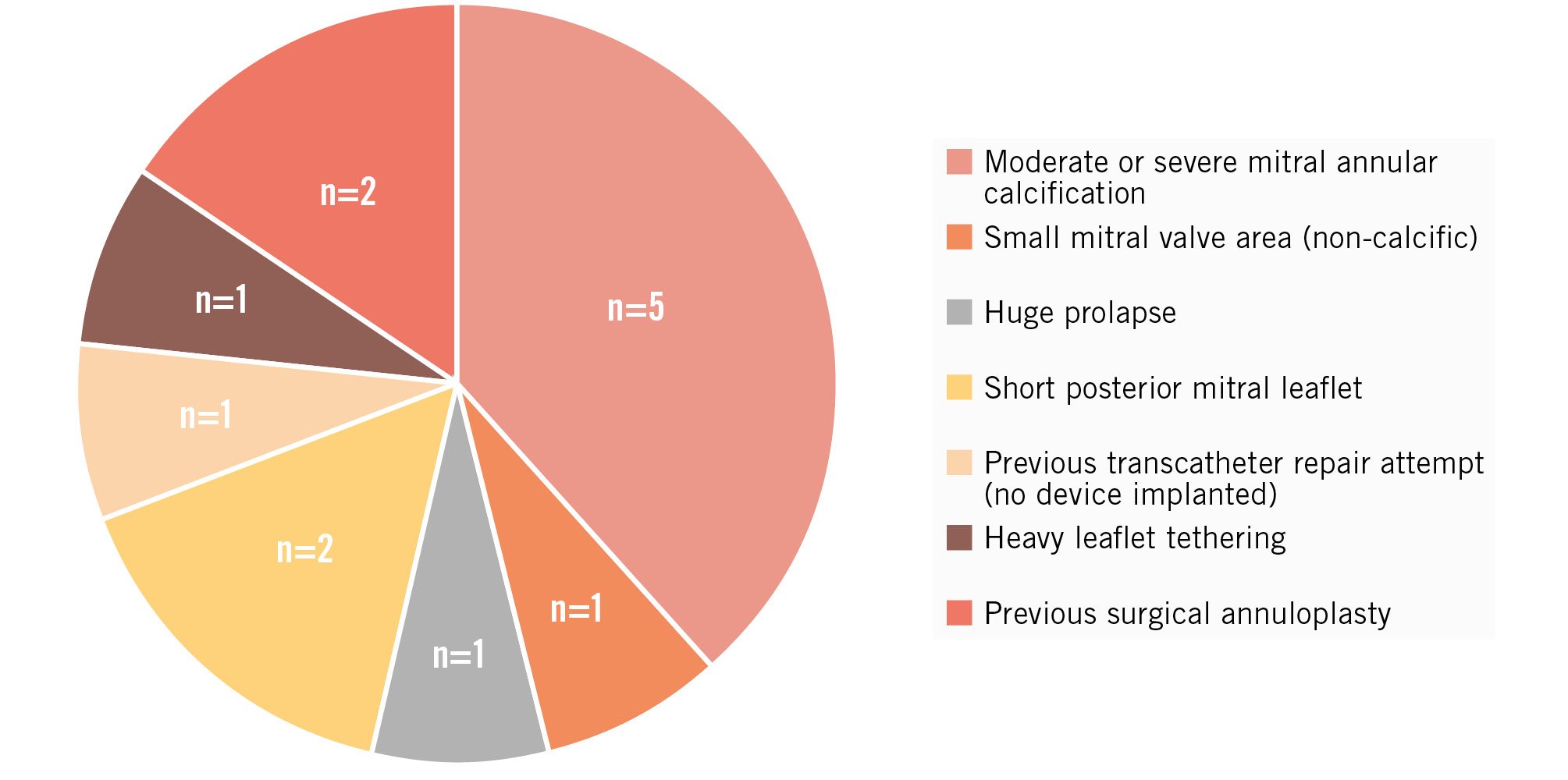
Figure 3. Reasons against transseptal transcatheter mitral valve repair. Seven patients were anatomically ineligible for repair. One patient had a previous repair attempt with an unsatisfactory interventional result and abandonment of the procedure. In 5 patients, an inadequate repair result was expected because of moderate or severe mitral annular calcification.
BASELINE DATA
The multiple comorbidities of this patient cohort are reflected within the demographic data (Table 1). All patients were symptomatic and had been previously hospitalised due to heart failure prior to TMVR (mean count of heart failure hospitalisations: 1.4±0.9). Nine patients were treated for primary MV disease. In 12 patients, MR was the leading pathology, while 1 patient underwent TMVR due to severe calcific mitral stenosis (MPG 12 mmHg). The left ventricular ejection fraction ranged from 30% to 70%. Septal hypertrophy (>11 mm) was evident in 8 of 13 patients. Four patients had concomitant severe tricuspid regurgitation. The advanced heart failure status was reflected by the elevated mean systolic pulmonary artery pressure (59±18 mmHg).
Moderate or severe MAC was evident in 5 patients (Table 2). The median neo-LVOT were 358±77 mm2 and 405±156 mm2 in end-systole and end-diastole, respectively, and decreased in 12/13 patients during systole. The AML length ranged from 20 mm to 29 mm. The end-systolic distance from the sealing-body of the prosthesis to the interventricular septum was <6 mm in 2/13 patients, <8 mm in 5/13 patients, <10 mm in 8/13 patients and ≥10 mm in 5/13 patients. The surgeon’s reasons for the AML-directed procedure are given for each patient in Supplementary Table 1.
The median procedure time was 111±44 min, of which 9.0±5.4 min were attributed to the MitraCut procedure. The labelled diameter of the manually shortened, transapically inserted sheaths were 20 Fr (n=1), 24 Fr (n=3), and 26 Fr (n=9). The size of the sheath was based on the size of the endoscopic scissors used at each centre (Ceramo HCR, MRT-2 [Fehling Surgical Instruments]; CLICKLINE no 33151 [KARL STORZ]; ValveGate PRO [Geister]; ValveGate classic line 15° with 25 cm working length [Geister]; AdTec mini [Aesculap/B. Braun]). The AML division was achieved after 1 (n=11) or 2 cutting attempts (n=2) in the 13 treated patients. The technical success rate of the MitraCut procedure was 92%. One patient experienced significant PVL in the anterior portion of the AML, most likely related to a MitraCut extending into the aortomitral continuity due to excessive scissor blade length or too high pressure during the AML cut. The increase in MR after cutting the AML prior to prosthetic placement was well tolerated by all patients, with no haemodynamical instability nor need for circulatory support. Sheath removal, insertion of the Tendyne valve system, and complete valve expansion took a total of 7.0±7.4 min. There was no evidence of LVOT obstruction, as demonstrated by the patients’ LVOT MPG of 3.9±4.4 mmHg directly after complete valve expansion.
All patients were free from procedural and in-hospital mortality. One patient had a prolonged in-hospital stay (43 days; 20 days in the intensive care unit), which might have been related to the high surgical risk (European System for Cardiac Operative Risk Evaluation [EuroSCORE] II 15.8%) and postprocedural acute kidney injury. The prosthesis showed mild PVL, unrelated to the AML cut, and an MPG of 7 mmHg. One bleeding complication, not related to the MitraCut procedure, without indication for intervention/surgery, was recorded. Further endpoints during the index hospitalisation are summarised in Table 3. The mean clinical and echocardiographic follow-up periods were 52±34 days and 50±38 days, respectively. The patient who experienced the prolonged and complicated postoperative course died 47 days after the procedure due to cardiac arrhythmia, without evidence of LVOT obstruction. All other patients were free from mortality during the follow-up period.
The patient with the MitraCut-related PVL (n=1), which was graded as mild immediately after TMVR, required hospitalisation because of heart failure within the 30-day follow-up period. Subsequent imaging and clinical assessment detected an increase of the PVL to severe with significant haemolysis. This patient’s PVL was successfully addressed by 3 interventional PVL closure devices, reducing the PVL to mild 28 days after the index procedure. Accordingly, this study cohort had a reintervention rate of 8% within the follow-up period. All patients were otherwise free from residual MR ≥1+ and improved by at least 1 New York Heart Association (NYHA) Class at the last clinical follow-up compared to baseline (Table 4).
While the average gradient in the LVOT remained low (MPG: 3.6±3.1 mmHg, peak pressure gradient: 5.9±5.8 mmHg), 4 patients had an MV MPG ≥5 mmHg (MV MPG 5-7 mmHg), which appears attributable to the use of low-profile valves (prosthesis area 2.2 cm2).
Table 1. Baseline subject characteristics.
| Study population (n=13) |
|
|---|---|
| Age, years | 77.1±11.4 |
| Female | 6 (46) |
| Body mass index, kg/m2 | 25.6±5.5 |
| EuroSCORE II, % | 6.1±4.7 |
| STS-PROM score, % | 5.1±2.7 |
| NYHA Class ≥III | 13 (100) |
| Previous HFH | 13 (100) |
| NT-proBNP, ng/dL | 4,230±5,295 |
| Atrial fibrillation or flutter | 8 (62) |
| Glomerular filtration rate, ml/min/1.73 m2 | 62.4±20.8 |
| COPD | 2 (15) |
| Stroke | 2 (15) |
| Coronary artery disease | 7 (54) |
| Previous myocardial infarction | 4 (31) |
| Previous PCI | 2 (15) |
| Previous CABG | 1 (8) |
| Previous TAVI | 1 (8) |
| Previous SAVR | 1 (8) |
| Previous MV intervention | 1 (8) |
| Previous MV surgery | 2 (15) |
| Valve-in-ring TMVR | 2 (15) |
| Valve-in-MAC TMVR* | 5 (39) |
| Pacemaker | 1 (8) |
| Heart failure medication | |
| ACE inhibitor/ARB | 9 (69) |
| Beta blocker | 10 (77) |
| SGLT2 inhibitor | 3 (23) |
| Diuretics | 12 (92) |
| CRT | 0 (0) |
| Echocardiographic characteristics | |
| Mitral valve disease aetiology | |
| Primary | 9 (69) |
| Secondary | 4 (31) |
| Mitral regurgitation ≥3+ | 12 (92) |
| Stenotic mitral valve disease | 4 (31) |
| Mitral valve mean pressure gradient, mmHg | 4.2±3.1 |
| LVEF, % | 56.0±10.3 |
| LVEDD, mm | 51.5±6.4 |
| Interventricular septum, mm | 11.5±2.7 |
| TAPSE, mm | 17.8±5.9 |
| Tricuspid regurgitation ≥3 | 4 (31) |
| sPAP, mmHg | 58.7±17.8 |
| Numerical data are given as n (%), and metric variables are given as mean±standard deviation. *MAC ≥moderate according to Guerrero et al [23]. ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HFH: heart failure hospitalisation; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MAC: mitral annular calcification; MV: mitral valve; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SAVR: surgical aortic valve replacement; SGLT: sodium-glucose linked transporter; sPAP: systolic pulmonary artery pressure; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAPSE: tricuspid annular plane systolic excursion; TAVI: transcatheter aortic valve implantation; TMVR: transcatheter mitral valve replacement | |
Table 2. Computed tomography-derived measurements.
| Study population (n=13) |
|
|---|---|
| End-systolic neo-LVOT, mm2 | 358±77 |
| End-diastolic neo-LVOT, mm2 | 405±156 |
| AML length, mm | 23.6±2.4 |
| End-systolic AML clearance, mm | 9.2±3.0 |
| End-diastolic AML clearance, mm | 11.8±2.3 |
| Mitral annular calcification | |
| None | 7 (54) |
| Mild | 1 (8) |
| Moderate | 4 (31) |
| Severe | 1 (8) |
| AML calcification | 1 (8) |
| Numerical data are given as n (%), and metric variables are given as mean±standard deviation. AML: anterior mitral leaflet; LVOT: left ventricular outflow tract | |
Table 3. Procedural and in-hospital outcomes.
| Study population (n=13) |
|
|---|---|
| Procedural time, min | 111.4±44.4 |
| Total MitraCut time, min | 9.0±5.4 |
| AML division attempts | |
| n=1 | 11 (85) |
| n=2 | 2 (15) |
| Technical success of MitraCut | 12 (92) |
| Time from MitraCut to valve expansion, min | 7.0±7.4 |
| Technical success according to MVARC criteria | 12 (92) |
| Use of neuroprotection device* | 2 (15) |
| Low-profile valve | 13 (100) |
| LVOT obstruction | 0 (0) |
| LVOT gradient directly after valve expansion, mmHg | 3.9±4.4 |
| Postprocedural PVL | 2 (15) |
| Postprocedural MitraCut-related PVL† | 1 (8) |
| Valve retrieval | 0 (0) |
| Major cardiac structural complication at the apex | 0 (0) |
| Conversion to full sternotomy | 0 (0) |
| Heart-lung machine/ECMO | 0 (0) |
| Intraprocedural death | 0 (0) |
| In-hospital adverse events | |
| In-hospital death | 0 (0) |
| Reintervention related to access | 0 (0) |
| Bleeding complication | 1 (8) |
| BARC 3a | 1 (8) |
| Transfusion of packed red blood cells, units | 1.0±1.2 |
| Myocardial infarction | 0 (0) |
| Stroke | 0 (0) |
| Acute kidney injury | 1 (8) |
| Dialysis | 0 (0) |
| Sepsis | 0 (0) |
| In-hospital stay, days | 12.2±9.7 |
| Time in intensive care unit, days | 2.8±5.4 |
| Discharge location | |
| Home | 11 (85) |
| Other hospital | 1 (8) |
| Rehabilitation centre | 1 (8) |
| Numerical data are given as n (%), and metric variables are given as mean±standard deviation. *Neuroprotection also covering the left vertebral artery was used in both patients. †MitraCut-related PVL was graded mild immediately after TMVR in the operating room but increased to severe with an indication for interventional PVL closure within 30 days after the procedure. AML: anterior mitral leaflet; BARC: Bleeding Academic Research Consortium; ECMO: extracorporeal membrane oxygenation; LVOT: left ventricular outflow tract; MVARC: Mitral Valve Academic Research Consortium; PVL: paravalvular leakage; TMVR: transcatheter mitral valve replacement | |
Table 4. Short-term follow-up.
| Study population (n=13) |
|
|---|---|
| Follow-up (follow-up time: 52±34 days) | |
| 30-day mortality | 0 (0) |
| Overall mortality | 1 (8) |
| Heart failure hospitalisation | 3 (23) |
| NYHA Class* | |
| ≤II | 11 (85) |
| Reintervention† | 1 (8) |
| Bleeding event between discharge and follow-up | 0 (0) |
| Haemolysis | 1 (8) |
| Valve thrombosis | 0 (0) |
| Valve migration or embolisation | 0 (0) |
| Myocardial infarction | 0 (0) |
| Stroke | 0 (0) |
| Echo (follow-up time: 50±38 days) | |
| LVEF, % | 53.5±6.5 |
| LVEDD, mm | 46.9±6.3 |
| LVOT obstruction | 0 (0) |
| LVOT mean pressure gradient, mmHg | 3.6±3.1 |
| LVOT peak pressure gradient, mmHg | 5.9±5.8 |
| Mitral regurgitation | |
| None | 10 (77) |
| Mild | 3 (23) |
| No PVL | 11 (85) |
| Mild PVL | 2 (15) |
| Mitral valve mean pressure gradient, mmHg | 4.5±1.5 |
| Mitral valve peak pressure gradient, mmHg | 13.2±4.1 |
| Numerical data are given as n (%), and metric variables are given as mean±standard deviation. *Only assessed in the survivors (n=12). †Interventional treatment of severe paravalvular leakage (haemolysis) using 3 closure devices. LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PVL: paravalvular leakage | |
Discussion
This report describes the first successful multicentre experience of the MitraCut procedure for surgical division of the AML to prevent LVOT obstruction in patients undergoing TA-TMVR. Our initial clinical MitraCut experience can be summarised as effective, with no consecutive LVOT obstruction after TMVR, a fast procedure time, and a low complication rate based on this early clinical experience.
The MitraCut procedure does not require any specialised technology. It can be conducted using standard shafted endoscopic scissors inserted through a large-bore sheath. Though we are currently exploring more optimised options, each surgeon in this early experience manually shortened the sheaths to accommodate the endoscopic scissors. The modification of this equipment required special considerations (further discussed in Moving image 1). The necessary sheath length is determined by the distance from the left ventricular apex access to approximately 2 cm above the MV; this distance will vary between patients and can be accurately assessed by segmentation of the screening MDCT scan (Figure 4). It is important to note that the sheaths used clinically so far include a spiral wire along their length. When cutting the sheath for the MitraCut procedure, the surgeon must ensure that the sharp wire is not protruding from the cut sheath tip. This consideration is one reason why we are evaluating alternative options (Supplementary Figure 1).
The endoscopic scissors used for the MitraCut procedure must have a working length greater than the length of the sheath to enable the division of the AML (Figure 1D). While inserting the endoscopic scissors through the apex, left ventricle, and MV, the scissors remain securely retracted within the sheath to avoid injury to delicate tissue structures. Most important for safe performance of the MitraCut is adequate TOE imaging quality, because the grasping of the AML and estimation of the cut length is performed under precise X-plane LVOT view guidance. Care must be taken to avoid folding of the AML between the scissor blades, which is an indicator of too much push with the scissors towards the aortomitral continuity, and to account for the guidewire to prevent guidewire transection by the scissors. For safe performance of the procedure, especially for scissor positioning, specific training and proctoring is advised. In the reported MitraCut-related PVL, too much push on the scissors and/or use of scissors with scissor blades that were too long relative to the AML was suggested. Subsequently, we inferred empirically a maximal cut length of one-half of the TOE-derived leaflet length, controllable by the choice of the scissors and push during the cut towards the AML hinge point. This threshold should be sufficient to prevent LVOT obstruction, as part of the anterior leaflet is covered by the Tendyne outer stent graft anyway. Further simulations may investigate if there is a cut length-dependent effect of the MitraCut. Independent of the length of the AML cut, the anaesthesiologist should be prepared for an acute increase in MR following division of the AML, and therefore, close communication between the surgeon and the anaesthesiology staff must be maintained.
In our first experience, a single cut was sufficient in 11/13 patients. Even though no thromboembolic event was recorded in our first multicentre experience, multiple cuts may theoretically increase the thromboembolic risk due to potential cutting of free parts of the leaflets or chords. A relatively high portion of patients (5/13) were treated for calcific (≥moderate MAC) MV disease. Neuroprotection devices also covering the left vertebral artery were used in the only patient with AML calcification and in one patient with severe MAC. Complete neuroprotection is strongly recommended particularly in the presence of AML calcification and should be considered in patients with extensive MAC.
Time is a valuable resource in hybrid operating rooms, and it has been reported that shorter anaesthesia times for comorbid patients may confer additional benefits25. Even in this first multicentre experience report, the time required for the MitraCut procedure and the time from dividing the AML to complete valve expansion were remarkably low: 9.0±5.4 min and 7.0±7.4 min, respectively. The LAMPOON technique is primarily utilised in transseptal TMVR131426, but the technique has also been employed prior to TA-TMVR27. In TA-TMVR, the MitraCut procedure appears to offer advantages for dividing the AML due to its straightforward TA approach with no need for additional femoral access. Compared to European real-world data with the Tendyne prosthesis, our early experience with the MitraCut procedure did not demonstrate an increase in the incidence of bleeding complications (Bleeding Academic Research Consortium class 2, 3, 4: 11% without MitraCut in the real-world data vs 8% in our experience)6.
Alcohol septal ablation (ASA) represents an effective strategy to prevent LVOT obstruction, primarily in patients with septal hypertrophy2829. However, in patients without severe septal hypertrophy, we favour an AML-directed prevention strategy. Unlike with the MitraCut procedure, patients require at least a 1-month recovery period between ASA and TMVR. In addition to the physical, psychological, and economic burden of multiple hospitalisations, this waiting period is a significant delay in treating an ill patient’s MV disease. ASA prior to TMVR has also been associated with a significant incidence of complete heart block and need for pacemaker implantation of up to 35%29. Techniques that address the AML likely avoid this potential complication. The BATMAN technique, a balloon-mediated rupturing of the AML to facilitate transseptal or TA-TMVR, represents a further AML-directed transcatheter technique to prevent LVOT obstruction3031. We agree with Denti et al that this technique should be restricted to the in valve-in-ring setting due to the unpredictable tissue damage of the ballooning30. Notably, we propose the MitraCut procedure in TA-TMVR, but transseptal LVOT obstruction preventive techniques should be employed in transseptal TMVR to avoid unnecessary access.
In our MitraCut procedure patients undergoing TA-TMVR with the Tendyne valve system, the MDCT-derived neo-LVOT areas were significantly higher than those reported for patients receiving LAMPOON and ASA prior to TMVR with the SAPIEN 3 (S3) valve (Edwards Lifesciences)1314262829. The neo-LVOT was not below 250 mm² in any of our patients32. However, a potential risk for LVOT obstruction was assumed in all patients, as summarised in Supplementary Table 1. Importantly, the acceptable burden of the neo-LVOT for TA-TMVR with the Tendyne valve has to be larger than for the S3 valve33, as the subvalvular component of the Tendyne valve stent frame is covered by the pericardium, which limits the potential effect of leaflet cutting compared to the open stent frame which is present in the S3 valve. Thus, the concept of the “skirt” neo-LVOT is not applicable. Further, dynamic variables throughout the entire cardiac cycle, for example, leaflet tethering, should also be taken into account within the procedural planning because of experiences with dynamic LVOT obstruction, especially in case of poor AML tethering12. While balloon-expandable valve implantation depth can be readily controlled, the position of the self-expanding Tendyne valve with its sealing body is influenced by the pull on the tether in addition to the position of the apical access. An unintended deviation of the calculated apical target access anteriorly may further push the sealing body into the LVOT, with a subsequent increased risk of LVOT obstruction. Importantly, even if systolic anterior motion occurs in an anterior mitral valve leaflet previously cut in the A2 segment, LVOT obstruction may be avoided due to the cut.
Kohli et al showed the beneficial haemodynamic effect of AML division with the LAMPOON technique prior to TMVR in a silicon model; this beneficial effect on the haemodynamics increased with a smaller predicted neo-LVOT34. A similarly designed study for assessing the haemodynamic effect of AML division prior to Tendyne valve implantation is necessary. Such a study would help to define the anatomical characteristics of patients who could benefit from AML division to prevent complete or dynamic LVOT obstruction and to determine the smallest acceptable neo-LVOT for the Tendyne system. This might help to reduce the screening failure rate related to risk for LVOT obstruction. Further clinical evaluation of haemodynamics and short- to intermediate-term outcomes in matched left ventricular and mitral anatomies between patients with and without additional MitraCut are awaited.
The MitraCut procedure is technically straightforward. In addition, it appears to be effective and time efficient in our early experience. However, simulator-based surgical training and proctoring is advised for the first cases. A dedicated device to address the AML transapically, which was originally designed to prevent coronary obstruction in valve-in-valve transcatheter aortic valve replacement, mimics the effect of the MitraCut but is restricted for use in a few centres in the context of clinical evaluation studies and might be more expensive35.
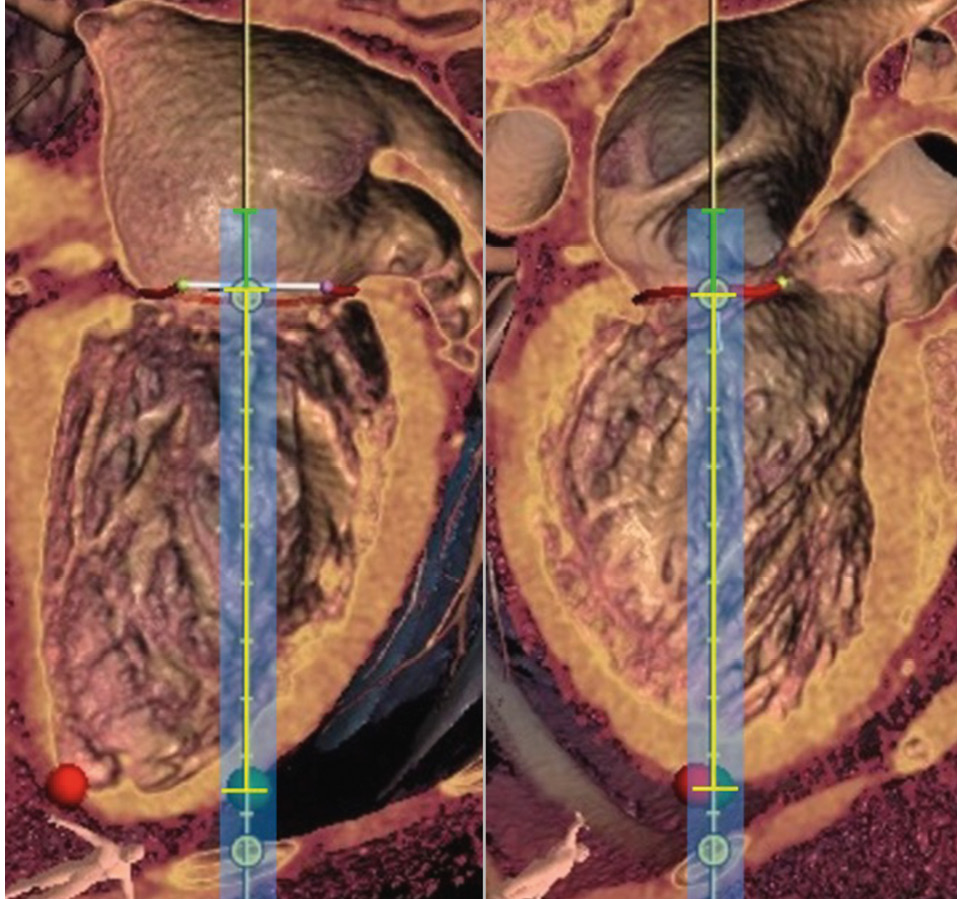
Figure 4. Assessment of the length of the large-bore sheath by segmentation of the cardiac computed tomography scan (left: intercommissural view, right: LVOT view). The length of the sheath is calculated from the distance between the target access (green dot) and MV plane (yellow bracket) plus approximately 2 cm (green bracket). This is important to enable complete covering of the scissors during the insertion to avoid structural cardiac damage with the sharp scissor tips. Red dot: true apex. LVOT: left ventricular outflow tract; MV: mitral valve
Limitations
Due to the novelty of the MitraCut procedure, the sample size of this study was limited to 13 patients. Risk assessment for LVOT obstruction is not standardised yet, and a composite of MDCT-derived measurements and TOE-acquired AML imaging mainly focusing on AML length and tethering was used. Therefore, the indication to perform MitraCut was an individual decision made based on expert opinion without a control group. The study is limited by its retrospective study design.
Conclusions
This initial multicentre experience evaluating the effectiveness and reliability of the MitraCut procedure showed promising results regarding LVOT obstruction avoidance in TA-TMVR. The procedure was technically straightforward and did not require specialised equipment. We believe that it has the potential for widespread applicability to lower LVOT obstruction risk in TA-TMVR.
Impact on daily practice
The MitraCut procedure may improve results after transapical transcatheter mitral valve replacement (TA-TMVR) and increase the rate of patients successfully screened for TA-TVMR by reducing the risk of left ventricular outflow tract obstruction.
Funding
Preclinical imaging for this work was funded by the Christian Doppler Society (Laboratory for Microinvasive Heart Surgery at the Medical University of Vienna), supported by the Austrian Federal Ministry of Labour and Economy and the National Foundation for Research, Technology and Development. The authors have no funding relevant to the contents of this multicentre experience to disclose.
Conflict of interest statement
M. Andreas is a proctor/consultant/speaker for Edwards Lifesciences, Abbott, Medtronic, Boston Scientific, Zoll, and B. Braun; and received institutional research grants from Edwards Lifesciences, Abbott, Medtronic, and LSI. T. Kerbel received speaker honoraria from Abbott. M. Mach has received institutional grants, research support, speaker honoraria, and travel compensation from Edwards Lifesciences, Symetis SA, JenaValve, Boston Scientific, Medtronic, Abbott, and Novartis. A. Zierer serves as a proctor for Tendyne; and received speaker fees and educational grants from Abbott. H. Ruge is an Abbott board member and serves as a physician proctor and speaker for Abbott and Edwards Lifesciences. A. Colli is a proctor/consultant for Abbott. E. Kuhn is a proctor for Medtronic and Abbott. J.S. Sauer is President and CEO of LSI Solutions. A. Regueiro is a proctor and consultant for Abbott.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Step-by-step explanation of the MitraCut procedure prior to transapical transcatheter mitral valve replacement.

