Cory:
Unlock Your AI Assistant Now!
Abstract
After extensive debate, the percutaneous closure of patent foramen ovale (PFO) has been established as a first-line treatment for the secondary prevention of PFO-related stroke in patients between 18 and 60 years old, whereas the role of PFO closure for primary prevention remains controversial. Additionally, in selected cases, PFO closure may be considered beyond these age limits and for other indications such as the treatment of systemic deoxygenation syndromes and the secondary prevention of systemic embolism or decompression sickness, when the PFO has been determined to be causative in the condition. In all cases, an in-depth diagnostic work-up, requiring collaboration among different specialists, is necessary to estimate the likelihood of PFO being related to the clinical condition. Since the first percutaneous closure of an atrial septal defect in 1976, the technique has been adapted and simplified for PFO. It is now well standardised with double-disc occluders, which are widely adopted because of their ease of use and evidence-based efficacy and safety. The procedure is generally straightforward, but some anatomical characteristics may be challenging. The choice of device and drug therapy after the procedure is currently empirical and guided by patient characteristics. Early and late complications of the procedure are infrequent but require early diagnosis. Further evidence is eagerly awaited to improve diagnosis, define other indications, make better procedural choices, and prescribe the most effective drug therapy after closure.
The foramen ovale is a valve-like structure situated in the interatrial septum (IAS) that permits right-to-left shunt (RLS) of blood from the right atrium (RA) to the left atrium (LA) during foetal circulation, mainly to supply the upper body. Although the foramen ovale in most individuals spontaneously closes postnatally, approximately 25% of the general adult population has a patent (or more appropriately, persistent) foramen ovale (PFO).
The clinical impact of a PFO was previously uncertain, except for rare cases of systemic desaturation due to RLS or direct visualisation of large venous emboli trapped in the PFO tunnel. However, evidence over the last decade has demonstrated the benefit of PFO closure in preventing recurrent cryptogenic ischaemic stroke in patients younger than 60 years of age, with these strokes subsequently reclassified as PFO related, i.e., paradoxical embolic strokes or in situ thrombosis. This has led to an increase in the number of catheter-based procedures for PFO closure in this condition and a growing interest in other conditions associated, or potentially so, with PFO.
While PFO closure is, on average, a relatively simple intervention, the global process of its management is complex and requires an in-depth knowledge to understand which patients may benefit from the procedure, how to plan the procedure using imaging, how to execute the procedure safely and effectively with the available devices even in challenging technical situations, how to address potential complications, and how to manage patients after the intervention (Central illustration).
This state-of-the-art article aims to describe these topics, focusing on readers who are involved with patients undergoing interventions for structural heart diseases.
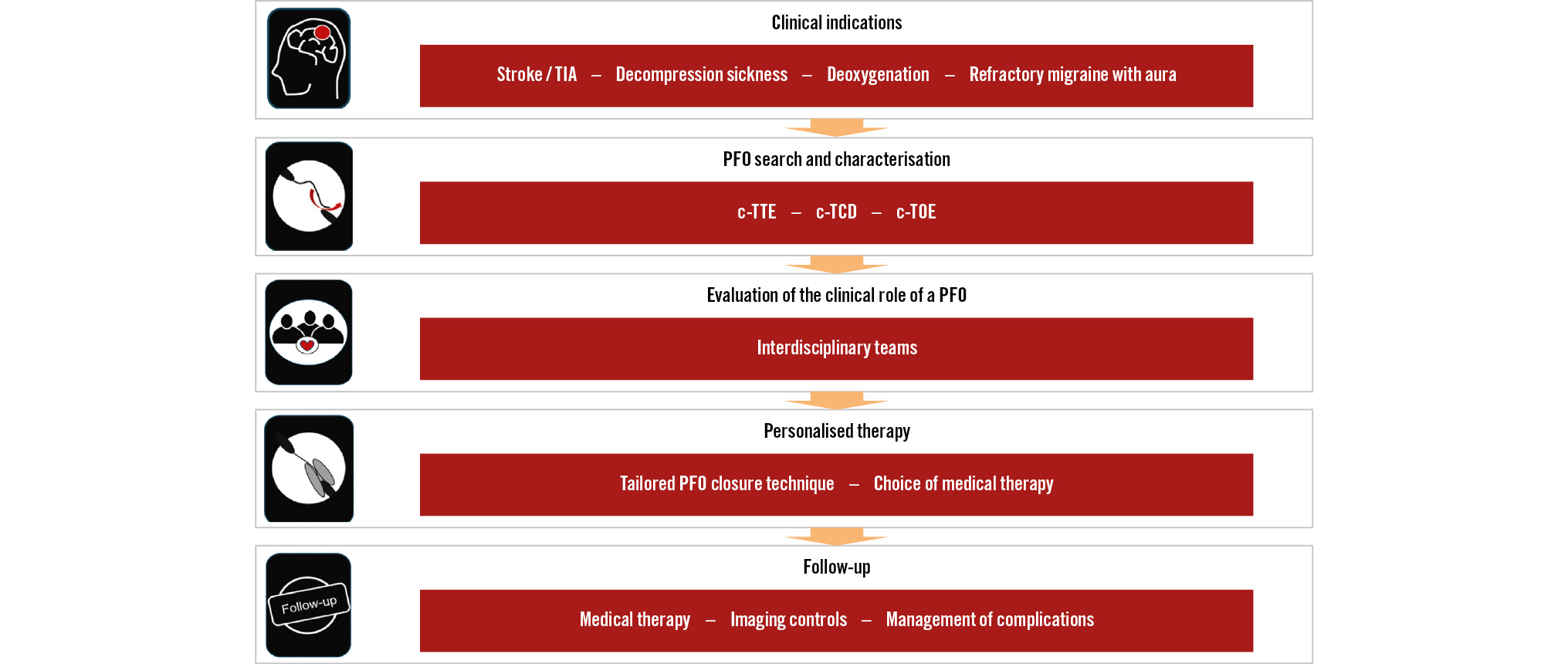
Central illustration. Algorithm for the management of PFO-associated syndromes. c-TCD: contrast-enhanced transcranial Doppler; c-TOE: contrast-enhanced transoesophageal echocardiography; c-TTE: contrast-enhanced transthoracic echocardiography; ICE: intracardiac echocardiography; PFO: patent (persistent) foramen ovale; TIA: transient ischaemic attack
When to search for, how to diagnose, and how to characterise a shunt and a PFO
PFO characterisation and closure should be performed only in clinical conditions where it has been proven to improve prognosis, as the primary prevention of PFO-associated conditions presently cannot be recommended1. In such situations, accurately searching for PFO shunting is crucial but challenging due to the intermittent nature of RLS and various modifying variables that must be considered (Figure 1).
Although commonly used diagnostic tests are based on microbubble contrast administration to enhance accuracy, no single test has emerged as a gold standard for diagnosis (Table 1)23. Contrast-enhanced transcranial Doppler (c-TCD) has high accuracy for diagnosing and quantifying RLS, but echocardiography is required to confirm that the shunt occurs through the PFO. Although contrast-enhanced transthoracic echocardiography (c-TTE) can allow the diagnosis of a PFO, its sensitivity for RLS is limited. Contrast-enhanced transoesophageal echocardiography (c-TOE) provides the detailed anatomical information necessary to characterise the PFO, to rule out other possible embolic sources, to guide clinical decisions and interventional planning (Table 2), at the price of an uncomfortable procedure for the patients, more difficulty in patients performing provocation manoeuvres, and a less than optimal diagnostic rate1. Thus, the combination of multiple tests, performed by experienced operators, is often necessary to achieve a sufficient and accurate diagnostic assessment.
If non-invasive findings are inconclusive or controversial, intracardiac echocardiography (ICE) or transoesophageal echocardiography (TOE) during cardiac catheterisation may be necessary to accurately quantify the true width of the PFO during its opening with a guidewire deflecting the septum primum (SP) or a sizing balloon across the tunnel.
The European Stroke Organisation’s 2024 guidelines advise developing local RLS and PFO diagnostic algorithms2. To help with this process, we propose a scheme to serve as a guide in different realms (Figure 2).
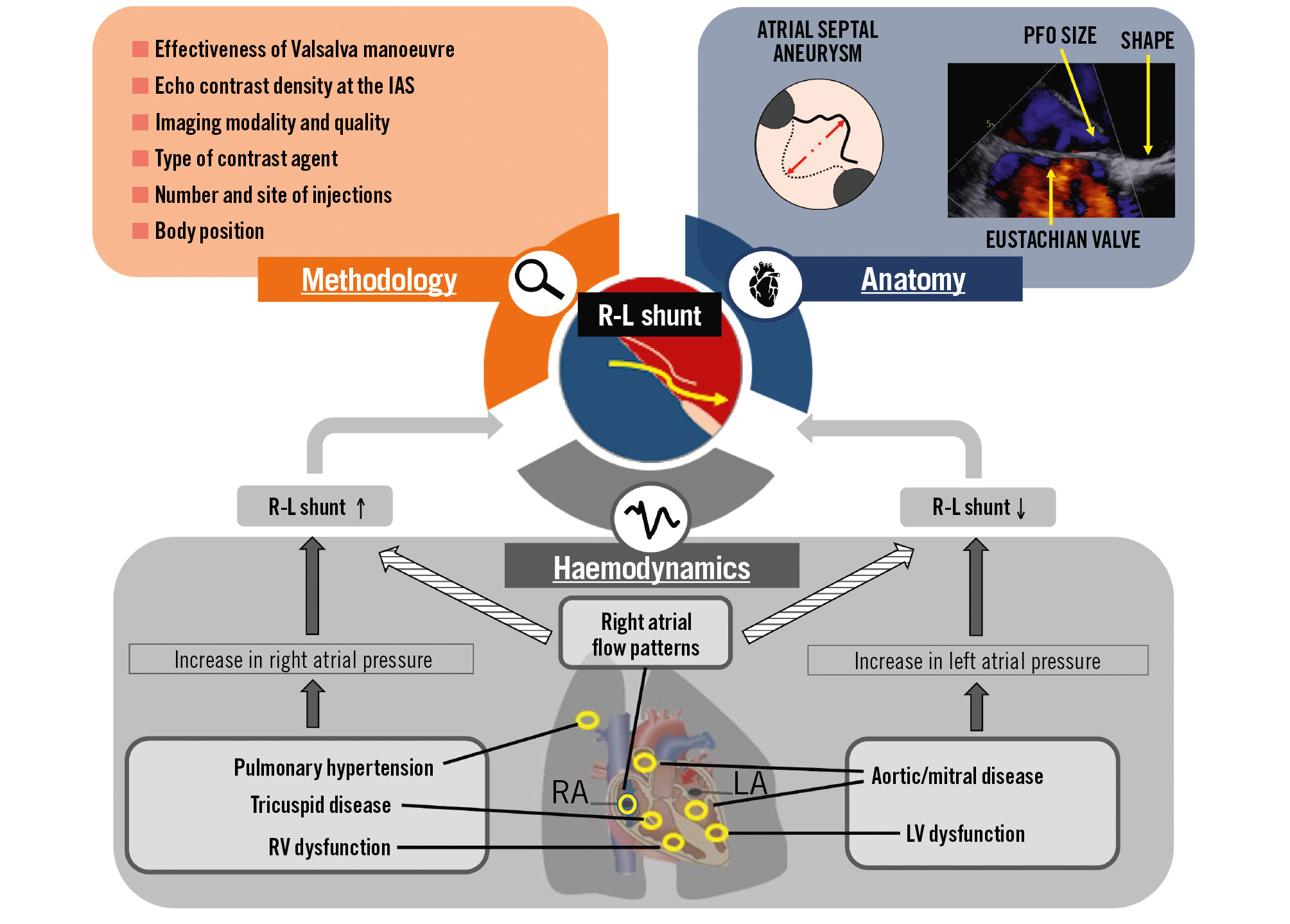
Figure 1. Factors that influence the magnitude of a right-to-left shunt. Right atrial flow patterns are complex four-dimensional parameters that depend on the dynamics of atria and can increase or decrease RLS. Breathing impacts right atrial flow patterns and pulmonary pressures, with varying influences on RLS. IAS: interatrial septum; LA: left atrium; LV: left ventricle; PFO: patent (persistent) foramen ovale; RA: right atrium; R-L: right to left; RLS: right-to-left shunt; RV: right ventricle
Table 1. Methods for PFO diagnosis.
| Diagnostic method | Main applications | Diagnostic criterion | Main advantages | Major limitations |
|---|---|---|---|---|
| c-TTE | Evaluation of cardiac structuresEvaluation of pathologies that cause increased LA or RA pressuresEvaluation of IAS mobilityEvaluation of potential sources of embolism (e.g., left atrial or ventricular masses, thrombi, vegetations)Diagnosis of a clinically relevant shunt (provocation manoeuvres+echo contrast needed) | Appearance of microbubbles in the LA within 3 cycles following complete opacification of the RA (no consensus on semiquantitative assessment of shunt magnitude)578990 | Well tolerated by the patientsWidely availableCost-effectiveReproducibleAllows adequate execution of provocation manoeuvresLocalisation and semiquantification of RLSComparative follow-up method | Decreased sensitivity in the detection of small shuntsSufficient imaging quality requiredTraining requiredSemiquantitative shunt assessment not validated |
| c-TOE | Evaluation of potential sources of embolism (e.g., LA or LA appendage thrombi, intracardiac masses, complex aortic plaques, vegetations)Detailed assessment of morphological IAS and PFO characteristics relevant to intervention (Table 2) | Appearance of microbubbles in the LA within 3 cycles following complete opacification of the RA:<20 microbubbles —> mild/moderate shunt≥20 microbubbles —>significant shunt1314 | Localisation and semiquantification of RLSGold standard for the evaluation of cardiac and aortic embolic sourcesBased on the morphological IAS and PFO characteristics, the device selection and implant strategy can be determined | Inconvenient for the patientProvocation manoeuvres often cannot be performed adequatelyLower sensitivity regarding PFO detection10Training requiredOnly semiquantitative shunt assessment possible |
| c-TCD | Diagnosis of RLS (provocation manoeuvres+echo contrast needed) | Detection of HITSafter echo contrast injection:<10 HITS —> mild/moderate shunt≥10 HITS —> (shower/curtain) significant shunt12 | Well tolerated by the patientsCost-effectiveReproducibleAllows adequate execution of provocation manoeuvresHigh sensitivity in the diagnosis of any RLSSemiquantification of RLSComparative follow-up method | Unable to localise the RLSTranscranial acoustic window required (absent in ~20%)Training requiredOnly semiquantitative shunt assessment possible |
| Adapted with permission from EuroIntervention1. c-TCD: contrast-enhanced transcranial Doppler; c-TOE: contrast-enhanced transoesophageal echocardiography; c-TTE: contrast-enhanced transthoracic echocardiography; HITS: high intensity transient signal; IAS: interatrial septum; LA: left atrium; PFO: patent (persistent) foramen ovale; RA: right atrium; RLS: right-to-left shunt | ||||
Table 2. Morphological characteristics to be assessed by TOE prior to a PFO closure procedure.
| Morphological characteristics to be assessed | Procedural impact | ||
|---|---|---|---|
| Device size | Device type | Implantation strategy | |
| PFO | |||
| PFO size (entry/exit) | The device size is selected according to the PFO size | PFO occluders or self-centring occluders, as required | Repeat measurement after stiff wire advancement through the PFOConsider balloon sizing if equivocal |
| PFO tunnel length and tissue compliance | Appropriate to cover the device-induced deformation of the septum, maximising stability | Select a device that minimises the “concertina effect” (e.g., less rigid) | Consider balloon sizing, septostomy or TSP |
| Multiple PFO outflows | Adequate to cover all outflows | Non-self-centring occluder with symmetrical discs | Probing of the most appropriate exit for device implantation |
| IAS | |||
| Multifenestrated septum | Adequate to cover all fenestrations | Non-self-centring occluder with symmetrical discs | Probing of the most appropriate fenestration for device implantationMultiple devices may be needed |
| Thickness and mobility of the septum primum | Consider a larger device size if the septum primum is thin and floppy | Consider a device with stronger support or pinching force in a thin and floppy IAS Consider the use of self-centring devices | Achieve stability of the device and of the SP with implantation |
| Thickness of septum secundum | Based on the foreseen adherence of discs to the SS (larger and more compliant devices in a thick SS) | Select a device apt to follow the septal profile (e.g., less rigid) | Embrace the SS with the two discs |
| Atrial septum aneurysm | Adequate to prevent septal excursion | Select a device apt to follow the septal profile to avoid a “concertina effect” (e.g., less rigid) | Evaluate residual septal excursion before release |
| Total septal length | Adequate to accommodate the IAS | - | - |
| Presence of pacing leads, prominent Eustachian valve or Chiari network | The smallest size of the right atrial disc compatible with other procedural needs | - | Avoid entrapping structures during the release |
| Evaluation of circumferential rims and distances of surrounding structures (aortic root/CS/SVC/RUPV/AV valves/free wall of the atria) | |||
| Based on the distance from other cardiac structures (aorta, valves, roof of the atrium) | Symmetrical or asymmetrical discs | Secure device anchorage without impinging neighbouring structures should be achieved | |
| AV: atrioventricular; CS: coronary sinus; IAS: interatrial septum; PFO: patent (persistent) foramen ovale; RUPV: right upper pulmonary vein; SP: septum primum; SS: septum secundum; SVC: superior vena cava; TOE: transoesophageal echocardiography; TSP: transseptal puncture | |||
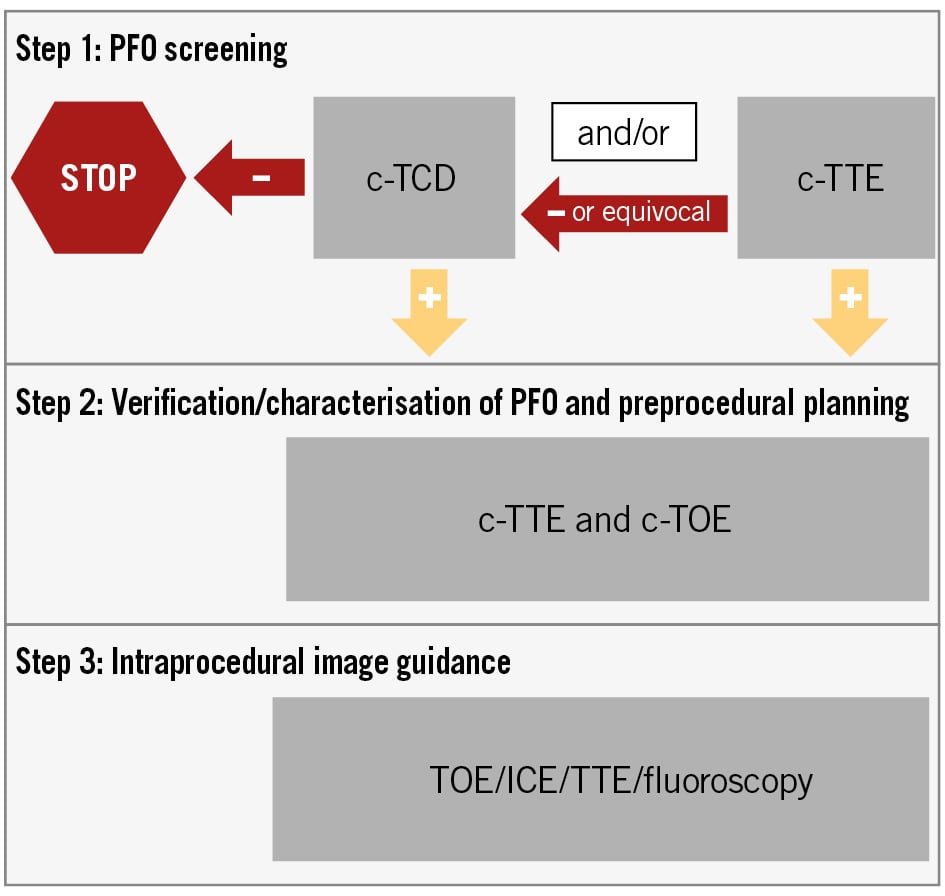
Figure 2. Master scheme for the development of diagnostic algorithms. Disparate local algorithms can be derived from this scheme in different realms2. If other cardiovascular conditions need to be simultaneously screened, c-TOE may also be used to screen for RLS/PFO. However, if c-TOE is negative or equivocal, c-TCD could be warranted. c-TCD: contrast-enhanced transcranial Doppler; c-TOE: contrast-enhanced transoesophageal echocardiography; c-TTE: contrast-enhanced transthoracic echocardiography; ICE: intracardiac echocardiography; PFO: patent (persistent) foramen ovale; RLS: right-to-left shunt
Key technical aspects for the interpretation
The contrast observed in ultrasound and Doppler tests is generated by the injection of microbubbles, which traverse the venous system to reach the heart. These microbubbles create contrast due to the difference in densities between the bubbles and blood at the boundary layer4. Microbubbles are created by mixing air with a saline-blood mixture or varying echo contrast media (e.g., polygelatine, dextrose solutions), which each have different visualisation advantages and detection rates for RLS567. Echo contrast agents are safe if no large bubbles of air are injected8.
The visualisation of >3 microbubbles in the left atrium within 3 cycles following complete opacification of the right atrium usually indicates interatrial RLS, but it is necessary to consider some pitfalls (Supplementary Table 1).
The majority of RLS may only be detected with provocation manoeuvres such as Valsalva or coughing; however, the variability of these manoeuvres results in inconsistencies in the diagnostic accuracy. It is important to understand the proper performance of the Valsalva manoeuvre by the patient and be aware that it is during the release phase that the rapid inflow of venous blood into the right atrium transiently enhances RLS9. PFO detection during echocardiography is reliable only if the IAS bulges into the LA during the Valsalva manoeuvre and there is adequate echo contrast quantity at the level of the IAS10. Performing the Valsalva manoeuvre by blowing into a party balloon (i.e., party balloon technique) has been shown to allow better control of the strength of the manoeuvre and to enhance its efficacy and reproducibility11.
The strength of the provocative manoeuvre can be assessed using the peak flow velocity of the Doppler curve in c-TCD12. Large RLS is defined as ≥20 microbubbles in the LA within 3 cycles following complete opacification of the right atrium during c-TOE1314 and >10 high-intensity transient signals during c-TCD (Table 1)12. Factors affecting RLS magnitude must be considered during assessment and interpretation (Figure 1). Specifically, injection from the femoral vein improves diagnostic accuracy as compared to an injection from a brachial venous access, since the blood flow from the inferior vena cava (IVC) is directed towards the fossa ovale, whereas the superior vena cava (SVC) flow is not15,16]. Injection from the inferior vena cava should be considered in patients with a high clinical suspicion and equivocal RLS obtained with traditional brachial venous access using the party balloon technique.
Assessing PFO causality
PFO is associated with various clinical conditions, with ischaemic stroke being the most frequent. However, since PFO is present in approximately 25% of the general population, its incidental coexistence must be considered. Therefore, estimating the probability of PFO relatedness (i.e., causal involvement) in each case is essential.
PFO-RELATED STROKE
Identifying PFO as the cause of stroke can be challenging, utilises probabilistic logic, and requires a comprehensive aetiological work-up by experienced cerebrovascular disease physicians to estimate the probability of PFO as the cause compared to other potential causes. The standard work-up and additional investigations, depending on the context, are summarised in Table 3. The ASCOD classification system can aid in assigning the likelihood of causal relationships for potential causes of ischaemic stroke17. Among all possible causes, occult paroxysmal atrial fibrillation (AF) deserves particular attention. In patients with cryptogenic stroke and inconclusive in-hospital short-term electrocardiogram (ECG) monitoring, the need for longer-term monitoring has been established, potentially including an implantable loop recorder (ILR) in those with an increased pretest probability of having paroxysmal AF1819. Younger patients, without any risk factors for AF, have a very low probability of having paroxysmal AF but still may require several weeks of monitoring using various technologies other than ILR. European scientific societies involved in PFO management have shared a rational approach to selecting patients for ILR in PFO-associated stroke (Supplementary Table 2)1. However, ILR findings should be interpreted with caution and clinical judgment. Indeed, although ILR monitoring leads to higher AF detection rates and higher rates of oral anticoagulation after stroke, this does not necessarily translate into improved outcomes202122, implying that other factors, such as a high-risk PFO, may have a more probable causal role than some low-risk AF episodes detected during ILR monitoring1.
Studies have shown that a PFO with a large shunt23 and/or a PFO associated with an atrial septal aneurysm (ASA; so-called “high-risk PFO”)24 are more likely to be causally related to “cryptogenic” stroke than incidental findings. The risk of stroke recurrence is higher in patients who have both a large shunt and an ASA than in patients with only one or none of these PFO features25. Other structures that modify right atrial flow patterns toward the PFO, such as a prominent Eustachian valve, Chiari network, and acute angle between the IVC and the PFO, have been associated with greater shunts through a PFO26272829 (Figure 1) and with cryptogenic stroke3031. Yet these structures were not studied in the major trials of PFO closure and, therefore, do not have a high level of evidence to impact decision-making.
In addition to these PFO features, non-cardiac characteristics included in the Risk of Paradoxical Embolism (RoPE) score (Table 4) such as embolic infarct topography and the absence of traditional vascular risk factors can help assess the likelihood of a causal relationship. The PFO-Associated Stroke CAusal Likelihood (PASCAL) classification system32 (Figure 3) combines non-cardiac characteristics and high-risk PFO features to categorise patients into three groups of causal relatedness: unlikely, possible, and probable.
Other features that may support and potentially strengthen the causal relationship between PFO and cryptogenic stroke, even for patients categorised as “unlikely” by the PASCAL criteria2, include deep vein thrombosis or pulmonary embolism occurring close to the ischaemic stroke, circumstances that promote venous thrombotic events (e.g., prolonged travel or recent surgery with immobility, venous thrombophilia, and diseases or medications associated with a hypercoagulable state, i.e., certain cancers and hormonal therapies), stroke onset coincident with a Valsalva manoeuvre, a persistently increased right-to-left pressure gradient (due to chronic pulmonary hypertension, or right heart diseases), a history of non-cerebral embolism, a history of migraine with aura, May-Thurner syndrome and decompression illness.
Table 3. Aetiological work-up of ischaemic stroke in young and middle-aged adults.
| Standard aetiological work-up | Aim |
|---|---|
| Brain MRI (DWI, FLAIR, T2* gradient echo sequences) or brain CT scan if MRI not possible | To confirm the diagnosis of ischaemic strokeTo help in defining the embolic or non-embolic type of the infarct |
| Imaging of extracranial (cervical) and intracranial arteries supplying the brainUsing CT angiography or MRI angiography (including axial cervical slices on T1 fat-suppression sequences to look for dissection) in addition to ultrasound examinationArterial investigations should be performed soon after stroke to avoid missing transient angiopathies | To look for common (e.g., dissection, atherosclerosis, RCVS) or rare inflammatory/infectious or non-inflammatory angiopathies (fibromuscular dysplasia, carotid web…) |
| c-TCD (as a screening tool for PFO)c-TTE (as a screening tool for PFO)c-TOE if (i) no other cause of stroke has been detected, (ii) a right-to-left shunt/PFO has been detected at screening OR an unusual cause is suspected (e.g., intracardiac thrombus, infective endocarditis) | To look for major and minor cardiac sources of embolismTo look for a PFO and an ASA and to assess the RLS size and the size of the ASA |
| ECG, ECG monitoring during stroke unit stayProlonged cardiac rhythm monitoring (Supplementary Table 2) | To look for paroxysmal AF |
| Biological work-up including blood count, ESR, CRP, fasting blood glucose, lipid analysis, serum creatinine, ASAT, ALAT, PT, APTT | To detect rare causes of stroke such as haematological, thrombotic, or inflammatory disordersTo assess biological risk factors for stroke |
| Other examinations to confirm a cause suspected based on clinical data and/or the initial aetiological work-upOther tests to be performed on a case-by-case basis depending on anamnestic information and results of the initial work-up | |
| Search for recreational drugsParadoxical embolism: search for deep vein thrombosis, pulmonary embolism, pulmonary arteriovenous fistulaCoagulation disorders: antiphospholipid syndrome (LA, IgG and IgM aCL and aß2GPI testing); disseminated intravascular coagulation; deficit of coagulation factorsDrepanocytosis: haemoglobin electrophoresisHyperhomocysteinaemiaSearch for occult malignancy: thoracic and abdominal CT scan, positron emission tomography, etc.Infectious, inflammatory (e.g., isolated angiitis of the CNS, CNS vasculitis associated with autoimmune diseases) and non-inflammatory angiopathies (e.g., Moya-Moya disease): CSF examination, Rx cerebral angiography, etc.Genetic diseases (e.g., Fabry disease, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, etc.): enzymatic diagnosis, search for genetic mutation, etc. | |
| aß2GPI: anti-ß2-glycoprotein I; aCL: anticardiolipin; AF: atrial fibrillation; ALAT: alanine aminotransferase; APTT: activated partial thromboplastin clotting time; ASA: atrial septal aneurysm; ASAT: aspartate aminotransferase; c-TCD: contrast-enhanced transcranial Doppler; c-TOE: contrast-enhanced transoesophageal echocardiography; c-TTE: contrast-enhanced transthoracic echocardiography; CNS: central nervous system; CRP: C-reactive protein; CSF: cerebrospinal fluid; CT: computed tomography; DWI: diffusion-weighted imaging; ECG: electrocardiogram; ESR: erythrocyte sedimentation rate; FLAIR: fluid-attenuated inversion recovery; IgG: immunoglobulin G; IgM: immunoglobulin M; LA: lupus anticoagulant; MRI: magnetic resonance imaging; PFO: patent (persistent) foramen ovale; PT: prothrombin time; RCVS: reversible cerebral vasoconstriction syndrome; RLS: right-to-left shunt; Rx: radiographic | |
Table 4. The Risk of Paradoxical Embolism (RoPE) score calculator.
| Characteristic | Points |
|---|---|
| No history of | |
| Hypertension | 1 |
| Diabetes | 1 |
| Stroke or transient ischaemic attack | 1 |
| Non-smoker | 1 |
| Cortical infarcts on imaging | 1 |
| Age, years | |
| 18-29 | 5 |
| 30-39 | 4 |
| 40-49 | 3 |
| 50-59 | 2 |
| 60-69 | 1 |
| ≥70 | 0 |
| Score (sum of individual points) | = |
| The RoPE score assesses the probability that a PFO discovered in the setting of an otherwise cryptogenic stroke was pathogenically related to the stroke rather than an incidental finding. The RoPE score is based on clinical and imaging variables and ranges from 0 to 10, with scores of 0 to 3 indicating a negligible likelihood that the stroke is attributable to the PFO and a score of 9 or 10 indicating an approximately 90% probability that the stroke is attributable to the PFO. PFO: patent (persistent) foramen ovale | |
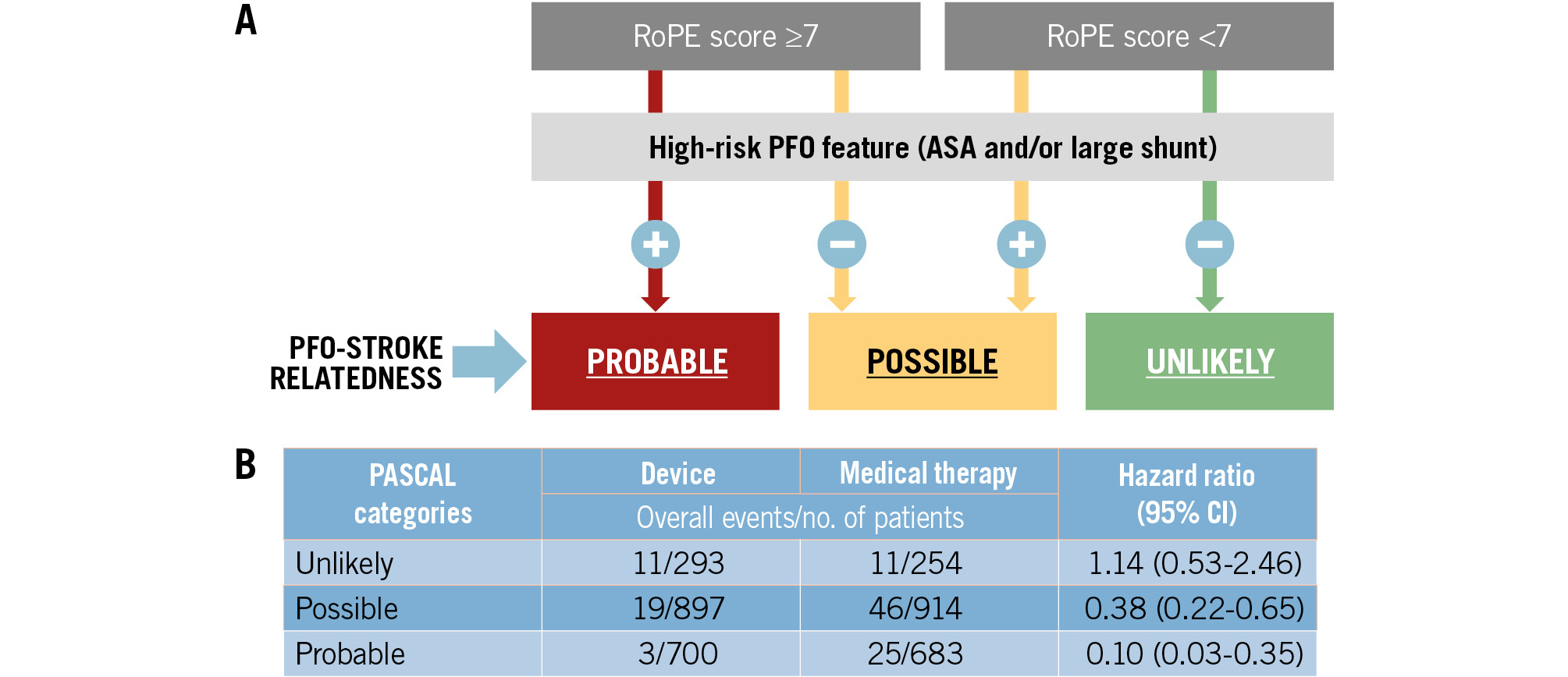
Figure 3. The PASCAL classification system (simplified version). A) The PFO-Associated Stroke CAusal Likelihood (PASCAL) classification system combines the RoPE score (a 10-point scoring system in which higher scores reflect younger age and the absence of vascular risk factors) with the presence or absence of high-risk PFO features (either an atrial septal aneurysm or a large-sized shunt) to classify patients into three categories of causal relatedness to the index stroke: unlikely, possible, or probable. A large shunt size was defined in the database as >20 bubbles in the left atrium on transoesophageal echo; an ASA was defined as ≥10 mm of excursion from the midline. B) Performance of the PASCAL classification system from an individual patient-data meta-analysis43. ASA: atrial septal aneurysm; CI: confidence interval; PFO: patent (persistent) foramen ovale; RoPE: Risk of Paradoxical Embolism
Other conditions
When the role of PFO in systemic desaturation syndromes like platypnoea-orthodeoxia syndrome (POS) and exertional desaturation (ED) is not straightforward (i.e., when massive RLS is not present), measuring oxygen saturation across the IAS and in the four pulmonary veins in the catheterisation laboratory can help. However, other factors like pulmonary embolism, parenchymal pulmonary diseases, intrapulmonary shunts, and severe pulmonary hypertension must be considered, as PFO typically exacerbates existing desaturation33.
In a recent meta-analysis, decompression sickness (DCS) had a strong association with RLS (odds ratio [OR] 5.63, 95% confidence interval [CI]: 3.14-10.09)33. However, more common factors, such as errors in diving technique, must be thoroughly considered by a hyperbaric physician when assessing the characteristics of previous DCS and the presence of abnormalities that increase DCS risk independently of PFO. A PFO’s causal role is more likely when it is large, when DCS occurs after a low-risk dive, when neurological symptoms are present (including cutis marmorata), and when an isometric effort has been made shortly before DCS onset33.
Although the causal role of PFO in migraines has not yet been confirmed clinically, a recent mechanistic study has demonstrated a causal link34. While association studies are inconclusive, PFO appears to be associated with migraines with aura, and PFO size may be instrumental for a causal assessment33. However, further studies are needed to inform patient selection, to distinguish when a PFO is pathogenic versus incidental, and to predict the degree of migraine relief for individual patients from PFO closure.
When should PFO closure be considered?
PFO-RELATED STROKE IN PATIENTS AGED 18 TO 60 YEARS
Recent evidence shows that percutaneous closure in combination with antithrombotic therapy is more effective in preventing recurrent PFO-related strokes than antithrombotic therapy alone. This finding was the result of six randomised clinical trials35363738394041 including patients up to the age of 60 years (mean age approximately 45 years) who had recently (usually within six months) experienced an unexplained ischaemic stroke (Table 5). One of these trials41 included patients up to 80 years old, but only a small percentage were aged over 60 years, with a mean age of 51.8 years. Four of the trials3536373841 compared PFO closure followed by antithrombotic therapy (mainly antiplatelet therapy) to a control group treated with antiplatelet or anticoagulant agents based on physician preference. Two trials3940 compared PFO closure followed by antiplatelet therapy to antiplatelet therapy alone.
Of the six trials, four3536373841 included patients with any type of PFO, while two3941 only enrolled patients with both PFO and ASA, or those with a large PFO without ASA. These features have been associated with an increased likelihood of cryptogenic stroke being related to a PFO, and the presence of an ASA has been associated with a higher risk of stroke recurrence.
A study-level meta-analysis1 of the six trials showed that PFO closure was associated with a 62% lower risk of recurrent stroke (OR 0.38, 95% CI: 0.18-0.80) compared to antithrombotic therapy alone (antiplatelet therapy or anticoagulation) (Supplementary Figure 1A). Another study-level meta-analysis42 showed that the benefit of PFO closure was moderate overall, with an approximately 1% absolute reduction per year, with a reduction from 1.27 per 100 person-years (95% CI: 0.84-1.78) with antithrombotic treatment alone to 0.29 per 100 person-years (95% CI: 0.02-0.76) after PFO closure plus antithrombotic treatment. Subgroup analyses of randomised controlled trials (RCTs) performed in the aforementioned meta-analyses consistently suggested that patients with high-risk PFO features benefit more from PFO closure than patients without those features (Supplementary Figure 1B)142. In a patient-level meta-analysis of the six trials43, risk reduction for recurrent stroke with device closure varied across the PASCAL classification subgroups. Patients who had experienced a stroke classified as “probably” or “possibly” PFO related benefited from PFO closure, whereas patients in whom strokes were classified as “unlikely” to be PFO related were unlikely to benefit, although with wide confidence intervals (Figure 3B). The patient-level meta-analysis of the 6 trials also showed that patients with both an ASA and a large PFO benefit substantially more from device closure than patients with only one or none of these PFO features25. Based on the aforementioned studies, since 2019, major professional medical societies have recommended combining PFO closure and antithrombotic therapy after a stroke that has a high probability of being PFO related. Tailoring the approach to each patient is emphasised in these guidelines, utilising the available evidence at the time of their publication (Table 6).
Table 5. Summary of the design and results of randomised controlled trials comparing transcatheter PFO closure with antithrombotic treatment in patients with an otherwise unexplained ischaemic stroke.
| RCT | n | Age range; mean, years | Stroke characteristics; Rankin score; time from stroke to inclusion; PFO characteristics | Comparison | Mean FU, years | Recurrent stroke (n); HR (95% CI); p-value |
|---|---|---|---|---|---|---|
| CLOSURE I (2012)35 | 909 | 18-60; 46.0 | IS or TIA; Rankin <3; <6 months; unselected PFO (small [1-10 mb], 47.1%; moderate [10-25 mb] or large [>25 mb], 52.9%) | PFO closurea vs ATTe | 2 | 12 vs 13; 0.90 (0.41-1.98); p=0.79 |
| PC-Trial (2013)36 | 414 | <60; 44.5 | IS; Rankin <3; median 4.4 months; unselected PFO (small [1-5 mb], 34.4%; moderate [6-20 mb], 43.9%; large [>20 mb], 21.7%) | PFO closureb vs ATTe | 4.1 | 1 vs 5; 0.20 (0.02-1.72); p=0.14 |
| RESPECT (2013, 2017)3738 | 980 | 18-60; 45.9 | IS; Rankin <3; <9 months; unselected PFO (small [1-9 mb], 22.7%; moderate [10-20 mb], 26.4%; large [>20 mb], 48.8%) | PFO closureb vs ATTe | 2.1/5.9 | 9 vs 16; 0.49 (0.22-1.11); p=0.0818 vs 28; 0.55 (0.31-0.999); p=0.046 |
| CLOSE (2017)39 | 663 | 16-60; 43.4 | IS; Rankin 3; <6 months; PFO+ASA >10 mm or PFO >30 mb | PFO closurec vs APTf | 5.3 | 0 vs 14; 0.03 (0.00-0.26); p<0.001 |
| REDUCE (2017)40 | 664 | 18-60; 45.2 | IS; Rankin <3; <6 months; unselected PFO (small [1-5 mb], 19%; moderate [6-25 mb], 40%; large [>25 mb], 41%) | PFO closured vs APTf | 3.2 | 6 vs 12; 0.23 (0.09-0.62); p=0.002 |
| DEFENSE-PFO (2018)41 | 120 | 18-80; 51.8 | IS; Rankin 3; <6 months; PFO+ASA ≥10 mm or PFO ≥2 mm | PFO closureb vs ATTe | 2.8 | 0 vs 6; log-rank p=0.013 |
| aSTARFlex Septal Closure System (NMT Medical); bAmplatzer PFO Occluder (Abbott); cmultiple devices; dHELEX Septal Occluder or CARDIOFORM Septal Occluder (both W. L. Gore & Associates); epatients randomised into the medical group were treated with antiplatelet drugs or oral anticoagulants at the discretion of the investigator in charge of the patient up to the end of the study; fpatients randomised into the antiplatelet group were treated with antiplatelet drugs up to the end of the study. The following antithrombotic treatments were recommended in patients treated with PFO closure: CLOSURE I: clopidogrel (75 mg) for 6 months and aspirin (81-325 mg) for 2 years; PC-Trial: aspirin (100-325 mg) for at least 5-6 months and ticlopidine (250-500 mg) or clopidogrel (75-100 mg) for 1-6 months; RESPECT: clopidogrel for 1 month and aspirin for 6 months, then antiplatelet therapy at the discretion of the investigator; CLOSE: clopidogrel and aspirin for 3 months, then antiplatelet therapy up to the end of the study; REDUCE: clopidogrel 300 mg before or after the intervention, then clopidogrel 75 mg for 3 days, then antiplatelet therapy up to the end of the study; DEFENSE-PFO: clopidogrel and aspirin for at least 6 months, then antiplatelet therapy or anticoagulant therapy at the discretion of the investigator. APT: antiplatelet treatment; ASA: atrial septal aneurysm; ATT: antithrombotic treatment; CI: confidence interval; FU: follow-up; HR: hazard ratio; IS: ischaemic stroke; mb: microbubbles; n: number of patients; PFO: patent (persistent) foramen ovale; RCT: randomised controlled trial; TIA: transient ischaemic attack | ||||||
Table 6. Indications to PFO closure according to published guidance papers.
| Evidence-based recommendations | |||
|---|---|---|---|
| Type of document | Diagnosis | Patient selection | Age |
| ESO 2024 guidelines2 | PFO-associated cryptogenic stroke | PASCAL classification possible or probable | 18-60 years old |
| SCAI 2022 guidelines91 | PFO-associated stroke | RoPE score ≥7 | 18-60 years old |
| AHA/ASA 2021 guideline19 | Non-lacunar ESUS | High-risk PFO anatomy | <60 years old |
| AAN 2020 guideline92 | Embolic, appearing ESUS | Ruling out other mechanisms of stroke | <60 years old (if <30 years old, only in a single, small and deep infarct without any risk factor for small vessel disease) |
| Canadian 2017 best practice recommendation93 | Non-lacunar stroke/TIA with diagnostic imaging or cortical symptoms | Expert stroke neurologist identifying PFO as most likely cause | 18-60 years old |
| Expert consensus statements | |||
| ESO 2024 guidelines2 | PFO-associated cryptogenic stroke | PASCAL classification possible or probable+clinical judgement | >60 years old |
| ESO 2024 guidelines2 | PFO-associated cryptogenic stroke | High-risk PFO anatomy | <18 years old |
| ESO 2024 guidelines2 | PFO-associated cryptogenic stroke | PASCAL classification unlikely with other high-risk factors for clinical causality | 18-60 years old |
| EAPCI/ESO/ESC2018 intersocietal position paper1 | PFO-associated left thromboembolism (stroke/TIA or systemic) | High-risk PFO anatomy and clinical evaluation | 18-60 years old>65 years old after careful assessment of the role of other possible alternative comorbid causes |
| AAN: American Academy of Neurology; AHA: American Heart Association; ASA: American Stroke Association; EAPCI: European Association of Percutaneous Cardiovascular Interventions; ESC: European Society of Cardiology; ESO: European Stroke Organisation; ESUS: embolic stroke of undetermined source; PASCAL: Patent Foramen Ovale-Associated Stroke CAusal Likelihood; PFO: patent (persistent) foramen ovale; RoPE: Risk of Paradoxical Embolism; SCAI: Society for Cardiovascular Angiography & Interventions; TIA: transient ischaemic attack | |||
PFO-related stroke in patients over 60 years of age
Around one-third of ischaemic strokes in patients aged 60 years or older are cryptogenic. The stroke recurrence rate is about 5% per year, and approximately two-thirds of recurrences are cryptogenic44. Although there is an association between PFO, ASA, and cryptogenic stroke, it is weaker in older patients (OR 2.5) than in younger patients (OR 5)4546. Elderly patients often have alternative sources of cerebral embolism, such as atrial cardiomyopathy or subcritical atherosclerotic plaques. More research is needed to evaluate the risk/benefit ratio of PFO closure and anticoagulants in this age group. Currently, no superior treatment option has been demonstrated and an RCT is warranted. Pending results from these trials, in carefully selected patients where other possible stroke causes have been ruled out and the stroke appears PFO related, current expert position statements suggest that percutaneous closure may be proposed with a strict shared decision-making process12.
Other indications
In patients without a previous stroke, PFO closure may be considered for some patients with several other clinical syndromes. However, PFO closure remains controversial and not well studied as a primary treatment strategy in these other conditions.
• Systemic embolism to locations other than the brain may be related to paradoxical embolism via a PFO. Therefore, after an evaluation of the role of other potential embolic causes, a patient with a systemic embolism judged to be PFO related can be offered percutaneous closure1.
• A recent meta-analysis of non-randomised studies of PFO closure in desaturation syndromes (POS and ED) found a statistically significant improvement of approximately 10% in oxygen saturation in the blood (SaO2) after closure33. Other observational studies showed that PFO closure resulted in a durable improvement of symptoms in symptomatic POS and ED. Therefore, PFO closure can reasonably be considered for patients with symptomatic POS or ED that is clearly PFO related.
• Any DCS should primarily be prevented by changes in scuba activity, such as ceasing activity, regardless of PFO. However, for scuba divers with a history of carefully assessed PFO-related DCS who cannot achieve effective behavioural changes to prevent venous gas emboli, or when the risk of DCS remains unacceptable even after behavioural changes, PFO closure can be offered after consulting with a physician expert in DCS33.
• PFO closure remains controversial in patients with migraines in the absence of stroke. However, it may be considered in patients who experience migraines with aura as a compassionate treatment when all other available therapies have failed in expert centres and the patient’s quality of life is severely affected33.
Interdisciplinary professional teams
According to the different clinical conditions associated with PFO, a close coordination between cardiologists and different specialists (e.g., neurologists, pneumologists, hyperbaric or aerospace physicians, haematologists) is essential to provide patient-centred care with a comprehensive approach1. Given the complexity of assessing the role of any PFO in each individual clinical condition, the objectives of this approach include the interdisciplinary choice and assessment of the most appropriate diagnostic and aetiological work-up, the evaluation of the PFO’s anatomical and physiological features, the empowerment of patients regarding different therapeutic strategies’ risks and benefits based on available evidence, and the active engagement of patients in shared decision-making that considers their understanding, values, and preferences. Recent observational reports showed that formally structured, physically present heart-brain teams can improve patient selection, empowerment, and engagement4748.
How to close a PFO
The first percutaneous closure of an IAS defect was performed in 1976 by King and Mills using a clamshell device49. Since then, the technique has been adapted for use in PFO closure, simplified, and standardised. However, most of the devices currently used for PFO closure are still based on the same principle.
Choice of echocardiographic assistance and of anaesthesia
To ensure a safe and effective PFO closure procedure, echocardiography is necessary in most cases to allow for a comprehensive assessment of the anatomy and guidance of the implantation stages. Various imaging modalities, such as conventional TOE, mini and micro TOE, or ICE, can be employed during the procedure.
When ICE is used, only local anaesthesia is required. When TOE is used to guide the procedure, many sites will choose general anaesthesia to avoid inconvenience and reduce discomfort for the patient. However, the procedure can be performed with a mini/micro TOE probe, or even with classic TOE, with local anaesthesia (Table 7) and light sedation, without the presence of an anaesthetic team.
Table 7. Imaging techniques for intraprocedural guidance.
| Imaging technique | Major advantages | Major disadvantages |
|---|---|---|
| TOE | Widely available3D imaging modalities availableAppropriate defect sizing with 3D imaging modalities possibleComparable images if a TOE was also used in the preprocedural evaluationWell standardised imagingReal-time imaging | Semi-invasivePatient discomfortTraining neededNeed for sedation/anaesthesiaRequires oesophageal (±endotracheal) intubationPotential risk of oesophageal trauma/aspirationNeed for an echocardiographer |
| Mini/Micro-TOE | Better tolerated by the patients than conventional TOE probesTransnasal access possibleLow sedation usually sufficientComparable images if a TOE was also used in the preprocedural evaluationReal-time imaging | Semi-invasiveTraining neededNeed for an echocardiographerPure manual operation3D imaging modalities available only for micro-TOEExtra costs for the probe |
| ICE | Patient comfortCan be used if anatomical conditions do not allow oesophageal passageNo additional echocardiographer necessaryImaging quality comparable with TOE3D imaging modalities availableAppropriate defect sizing with 3D imaging modalities possiblePosterior rim sometimes better to assess than with TOENo additional sedation necessaryNo risk of aspiration/oesophageal traumaReal-time imaging | InvasiveTraining neededExtra costs for the ICE probeCosts not reimbursable in some countriesRisk of vascular complications due to an additional 8-10 Fr venous sheath3D imaging only recently introduced (role needs to be defined)Adequate short- and long-axis views difficult to achieve in some patients with 2D imagingSingle plane imaging with 2D modalitiesImages not directly comparable if examined by TOE before the intervention |
| 2D: two-dimensional; 3D: three-dimensional; ICE: intracardiac echocardiography; TOE: transoesophageal echocardiography | ||
Venous access and closure
Safe and effective vascular access and closure mitigate the risk of complications and shorten hospital stays. The use of ultrasound (US)-guided vascular puncture during structural heart disease interventions has significantly decreased the rate of vascular complications. The ultrasound probe, connected to a monitor, is covered with a sterile plastic bag and allows visualisation in the long- and short-axes of the suitable puncture site of the vein with sufficient calibre and distance from bifurcations and arteries. US can also be used to accurately place local anaesthesia superficial to the vein’s anterior wall before puncture.
Suture-based closure may be more efficient than manual compression for achieving haemostasis, especially after removal of an introducer sheath or delivery system with larger French (Fr) sizes, in obese patients, or when full anticoagulation is present. A single ProGlide/ProStyle (Abbott) device is typically used for closure, inserted after vascular puncture but before the large-core delivery sheath or system is inserted50. The closure device is tightened once the delivery sheath or system is removed, and absorbable sutures are cut below the skin level. Alternatively, a surgical suture can be used to create a “figure-of-8” technique that is removed 4-6 hours later51.
Guidance of the procedure and assessment of the result
Prior to the intervention, intracardiac masses and thrombi should be ruled out. Additionally, anatomical features − including PFO tunnel length, septum secundum (SS) thickness, and the presence of additional septal openings, fenestrations, septal aneurysm (Figure 4A, Figure 4B)43, a Eustachian valve or Chiari network − should be assessed52. Measurement of circumferential rims and distances to neighbouring structures is needed to select the device and guide the implantation (Figure 5).
The procedural stages to be closely followed by echocardiography are similar for double-disc devices (DDDs), as follows:
• Confirm, or guide in case of challenging anatomies, the correct probing of the defect and repeat the defect measurement with the stiff guidewire across the PFO channel to hold open the SP.
• Confirm the guidewire position in the chosen pulmonary vein.
• During balloon sizing, if used, confirm the “stop flow” during inflation and perform measurements.
• Provide a safe and continuous visualisation of the delivery sheath and discs throughout the procedure.
• Confirm the correct position of the deployed device, with the capture of all rims by the discs.
• Confirm a stable device position during a “wiggle test”.
• Evaluate for residual shunt, proper device orientation and interaction with adjacent structures.
Immediate postprocedural echo contrast injection with a provocation manoeuvre is generally unnecessary after an uncomplicated device implantation, as effective or complete closure of a PFO requires a neoendothelisation process over the device, needing weeks or months. However, it is mandatory in suture-based closure for assessing immediate effectiveness because early patency cannot be resolved by spontaneous neoendothelisation with this technique. Furthermore, imaging is crucial to detect procedural complications.
The procedure can be facilitated using fused echocardiography/radiology imaging systems in case of challenging anatomies53.
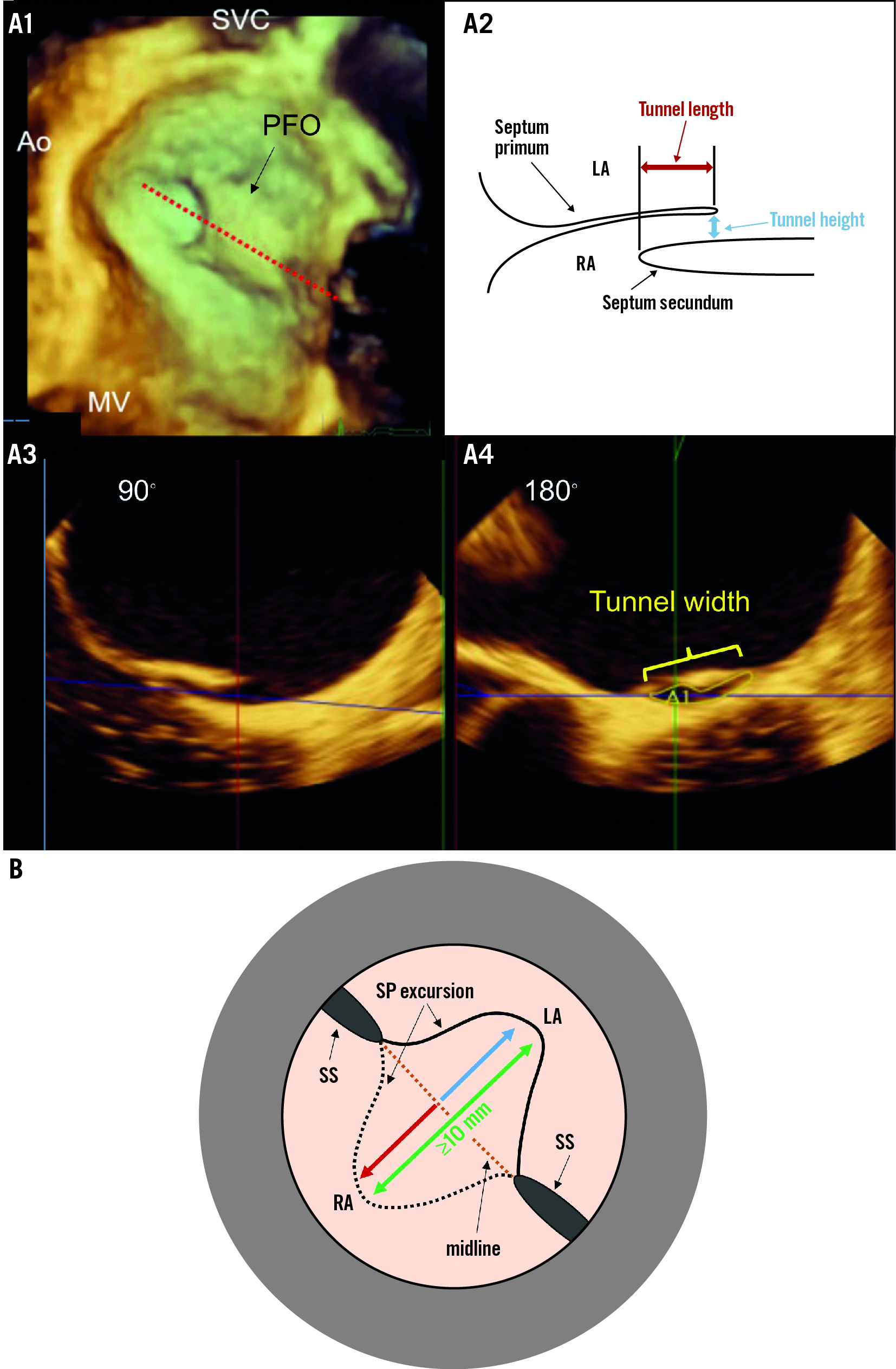
Figure 4. Key PFO and ASA anatomical parameters to be appraised. A) Measurement parameters for the evaluation of the PFO tunnel. Due to the arcuate shape of the PFO tunnel (A1), the measurement of the height and length of the tunnel (A2) should be performed in the middle of the PFO tract (A1; red dotted line), which can be done accurately with a 3D multiplanar reconstruction. The width of the PFO tunnel and the area of the exit is measured in this example at the exit of the PFO in an orthogonal plane (A3,A4). B) Schematic illustrating the measurement of a total excursion to define an atrial septal aneurysm. The total excursion (green double arrow) is the sum of the left atrial (blue arrow) and right atrial (red arrow) protrusion of the septum primum, measured from an imaginary midline. Since most studies have used this definition for an atrial septal aneurysm, we recommend a uniform use of this definition. 3D: three-dimensional; Ao: aorta; ASA: atrial septal aneurysm; LA: left atrium; MV: mitral valve; PFO: patent (persistent) foramen ovale; RA: right atrium; SP: septum primum; SS: septum secundum; SVC: superior vena cava
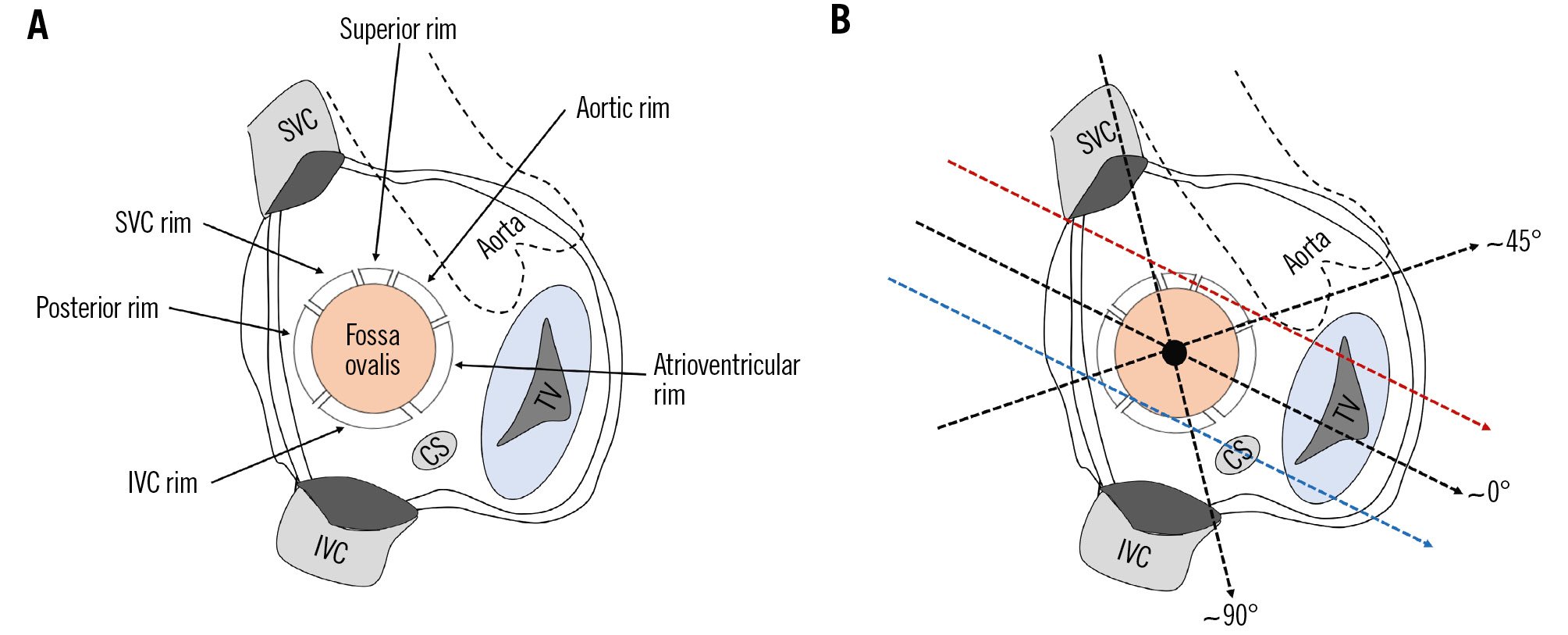
Figure 5. Circumferential rims and neighbouring structures. A) Right atrial en face view of the interatrial septum. B) Mid-oesophageal TOE planes in which the rims can be assessed. The aortic and the posterior rim and the distance to the aortic valve can be assessed in a short-axis view (~45°), the IVC and SVC rim and the distance to both caval veins in the bicaval view (~90°), and the atrioventricular rim and the distance to the mitral and tricuspid valve in a four-chamber view (~0°). The superior rim and the distance to the atrial roof can be visualised by retracting the probe at ~0° to a high-oesophageal position and rotating it anteriorly (red dotted arrow). The right upper pulmonary vein serves as landmark and should appear in the image. The distance to the CS can be most easily assessed by manoeuvring the TOE probe at ~0° to a deep oesophageal position until the CS appears in the image (blue dotted arrow). CS: coronary sinus; IVC: inferior vena cava; SVC: superior vena cava; TOE: transoesophageal echocardiography; TV: tricuspid valve
Device-specific operational procedures
1. Amplatzer and similar devices
The Amplatzer PFO Occluder (Abbott) has been the most used device for the closure of PFO since its invention by Dr Kurt Amplatz and first use by Dr Bernhard Meier in 199754. It is the device with the most available clinical data and has inspired several variants.
The Amplatzer Talisman PFO Occluder is a preassembled device consisting of an implantable occluder (available in sizes of 18, 25, 30, and 35 mm), a delivery catheter (8-9 Fr), and a flexible delivery cable (Trevisio [Abbott]) (Supplementary Figure 2A). The occluder has a double-disc design with a central connector and asymmetrical disc sizes to minimise the left atrial disc. The self-expanding discs are made of a nitinol wire mesh (treated with Intaglio [Abbott] to reduce nickel leaching) and polyester fabric. The device aligns with the PFO without a locking mechanism and is easily recapturable and repositionable while the delivery cable is attached.
The PFO closure procedure with the Amplatzer PFO Occluder typically takes 30 minutes and is performed under full heparinisation (activated clotting time [ACT] >250 seconds). Fluoroscopy and echocardiography usually guide the procedure. Measurement of atrial pressures is useful in order to reveal intravascular volume depletion. A saline bolus can help reduce the likelihood of air entry and prevent any significant vasovagal reaction. The delivery catheter is inserted into the femoral vein over a stiff guidewire, which has been previously positioned in the left upper pulmonary vein through the PFO. The dilator and guidewire are removed when the delivery catheter is in the left atrium, allowing back bleeding to prevent air entry. The loader is attached to the delivery catheter using saline flushing while keeping the system as low as possible to prevent air entry.
The device is advanced by pushing the cable inside the delivery catheter, tracked by fluoroscopy as it reaches the heart. The left atrial disc is then expanded by unsheathing it from the tip of the delivery catheter (Moving image 1, Moving image 2). The assembly is then pulled back until the disc adheres to the IAS. The right atrial disc is then unsheathed and apposed to the PFO by pushing the delivery cable (Moving image 3, Moving image 4). A “wiggle” manoeuvre of pushing and pulling the delivery cable is performed to verify the stability of the device (Moving image 5, Moving image 6). Attention is focused on whether the superior aspect of the right atrial disc prolapses into the PFO, which indicates device undersizing. The distance between the two discs is often greater where the SS is captured, giving rise to the “Pac-Man” sign in the left anterior oblique-cranial projection (Supplementary Figure 2A). Finally, the delivery cable is rotated counterclockwise until it is released from the right atrial disc, and the final assessment is performed (Moving image 7, Moving image 8).
Before release, recapture can easily be performed by advancing the delivery catheter to the right atrial disc, pulling the cable to recapture the right atrial disc inside the catheter, and, after further advancement into the left atrium, regrasping the left atrial disc.
Recapturing after release can be performed with a goose-neck snare which must firmly grasp the right atrial hub, i.e., where the delivery cable was attached, retracting the device inside an upsized catheter (usually ≥2 Fr larger than the delivery catheter).
The other Amplatzer-like systems differ in some technical characteristics of the device (e.g., elasticity, presence/absence of a distal hub, amount of metal mass, sizes) and the delivery system, but the principles of use are basically the same. The devices available in Europe at the time of writing this article are displayed in Supplementary Figure 2B.
2. Loop double-disc device
The GORE CARDIOFORM Septal Occluder (W. L. Gore & Associates) is composed of a frame made of five nickel-titanium (nitinol) wires filled with platinum and covered with expanded polytetrafluoroethylene (ePTFE) (Supplementary Figure 2C). An intrinsic locking mechanism passing through the centre of the device fixes the device in place by forming a loop on the right side. The GORE CARDIOFORM is available in three sizes, with disc diameters of 20 mm, 25 mm, and 30 mm and can be used in defects with a maximum diameter of 11 mm, 14 mm, and 17 mm, respectively.
The occluder is premounted on a handheld delivery catheter, which uses a contained slider mechanism to load and deploy both the left and right atrial discs (Supplementary Figure 3A). The flush port on the handle is flushed with heparinised saline. The occluder is then positioned in heparinised saline and pulled into the delivery catheter using the slider mechanism. This is followed by a second flush of the flush port on the handle for de-airing.
To implant the device, a femoral vein is accessed with a 12 Fr short sheath, and a stiff guidewire is positioned in the left upper pulmonary vein after crossing the PFO. The delivery monorail catheter is inserted over the guidewire, flushing the port once again (Supplementary Figure 3B).
Once the delivery catheter is in the left atrium, the guidewire is removed, and, guided by echocardiography, the distal tip of the delivery catheter is placed in the first half of the left atrium, beyond the PFO. The left atrial disc is then deployed, advancing the slider on the handle (Supplementary Figure 3C), carefully enough to avoid contact between the hard distal metallic tip of the device and the atrium wall. Then, the delivery catheter is pulled back until the disc adheres to the IAS. Next, the slider is advanced further forward to deploy the right atrial disc (Supplementary Figure 3D). If necessary, the occluder can be repositioned by reversing the steps of deployment to bring the right or both discs into the delivery catheter.
When both discs have been delivered, and the device position is found to be correct on echocardiography, the locking mechanism is deployed by pulling the red occluder lock back on the handle while maintaining the handle in a neutral position (Supplementary Figure 4A). This separates the occluder from the delivery catheter. The device and IAS are then free of any tension from the delivery catheter, and further assessment of positioning can be performed. At this stage, the device can still be retrieved, if necessary, by unscrewing the retrieval luer between the delivery catheter and the handle, fixing the delivery catheter, and sliding the handle back to bring the device into the delivery catheter (Supplementary Figure 4B). However, if the position is satisfactory, the retrieval cord lock is flipped up and twisted (Supplementary Figure 4C), which allows the cord to be gently pulled out to release the occluder from the delivery catheter (Supplementary Figure 4D).
3. Other techniques
Since the introduction of PFO closure, several closure techniques and devices have been proposed. However, most of these systems are no longer available, and some new techniques such as bioresorbable double discs and other concepts are still under development and/or testing.
Currently, the only available alternative to the double-disc concept is the NobelStitch EL (HeartStitch), a percutaneous suture system that does not leave any device in the heart. This technique was introduced in 2011 and overcomes some limitations of the DDDs, such as the need for intraprocedural echocardiographic guidance, the risk of erosion, embolisation of large devices, potential allergy issues due to nickel, and it is usually well accepted by patients. To date, some observational studies have reported on its feasibility with a good safety profile5556, but only non-controlled studies are available, and no randomised studies have been performed. Therefore, its clinical efficacy remains undetermined in any condition so far. The current technique appears to achieve acceptable closure rates only in selected patients with anatomically simple PFOs; therefore, patient selection is paramount57. Complications include partial stitch detachment, suture thrombosis, atrial tear, and knot embolisation58. The procedure is technically demanding, and still dependent on a specific operator’s experience, despite undergoing some technical and procedural refinements over time.
The technique requires a 14 Fr femoral vein sheath and consists of a four-stage procedure with radiographic guidance only. In the first stage, the PFO tunnel is defined with contrast injection and balloon sizing, and two guidewires are positioned, one in the left atrium and the other in the SVC. In the second stage, the first suture is applied by advancing a dedicated catheter over the SVC guidewire, capturing the SS under fluoroscopic guidance by opening a small arm and puncturing it with a needle which draws the 4-0 polypropylene thread across the tissue. The thread is subsequently captured, unrolled all the way down to the femoral vein and finally has its distal end extending out of the sheath for future use. In the third stage, the suture is applied to the SP, advancing another dedicated catheter over the PFO guidewire with a similar technique to the previous stage. In the fourth stage, the distal end of the sutures, extending out of the sheath, are loaded through another dedicated catheter, the KwiKnot (HeartStitch). This is gently advanced over the sutures up to the IAS to release a small knot system to bind the sutures together, subsequently cutting the thread just before the knot, and finally removing the cut portion of the sutures from the sheath.
Choice of procedure and closure device
PFO closure techniques should be selected based on evidence, anatomical features, patient-specific considerations, and operator experience.
For secondary prevention of PFO-related stroke, only Amplatzer and CARDIOFORM devices have shown efficacy and safety in RCTs1, with evidence of up to 92-98% complete closure rates beyond 1 year in the real world1, low complication rates, and trivial rates of reintervention during follow-up425960. Amplatzer-like double-disc devices can also reasonably be used because of their similar concept, but the completely different principle of suture-based closure cannot be unequivocally and broadly recommended because of the lack of evidence for secondary stroke prevention and suboptimal closure rates, especially in high-risk PFO which are most likely to be causal for stroke57.
In other clinical conditions related to PFO, no technique has demonstrated clear effects, so the choice of closure technique should be based on individual anatomical characteristics and operator experience, with DDDs being the only option with available efficacy and effectiveness data at the time of writing this article33.
The clinical relevance of nickel allergy is very controversial in this context61. In the extremely rare cases where nickel allergy may be a concern, devices with less nickel exposure and/or release (e.g., CARDIOFORM, Ceraflex [Lifetech Scientific], Cocoon [Vascular Innovations], Amplatzer Talisman) or percutaneous suture techniques may be used. Suture closure can also be considered in cases of contraindications to antiplatelet therapy62 or, according to local regulatory requirements, for aircraft crews or professional divers to avoid possible disqualification from their professional activity33.
Technical choices guided by anatomical features are currently based only on expert opinions.
The length of the PFO is best measured by TOE or ICE without any invasive interference with the tunnel in most cases.
On the contrary, due to the physiological intracardiac pressure variations, which may cause an underestimation of the PFO width, the choice of device should only be based on the maximal PFO opening, as obtained when it is gently held open by a stiff guidewire. The use of routine balloon sizing is avoided in most centres because it may unnaturally, and sometimes irreversibly, alter the true anatomy of the PFO, leading to false conclusions. However, balloon sizing may be considered in the infrequent cases of a suspected stiff and long PFO tunnel (especially if associated with a thick SS) where it may be difficult to assess true PFO dimensions, when concealed multiple septal defects are suspected, or when an uncertainty on the length or width of the tunnel persists after a comprehensive conservative assessment6263. The technique consists of advancing a compliant sizing balloon (18 mm if the PFO is suspected to be up to 20 mm wide, 24 mm if the PFO is suspected to be larger) over the guidewire across the PFO and gently inflating it with saline mixed with dye. The inflation must be interrupted when colour Doppler flow through the PFO is stopped at echocardiography (ICE or TOE) or until a fixed waist is observed at fluoroscopy, whichever of the two appears first. The PFO can then be measured in width and length by measuring the waist of the balloon by echography and/or radiography using the markers on the balloon for calibration. When a stop flow is observed through the PFO, other septal defects may become apparent, such as cribriform atrial septal defects (ASDs).
As larger device sizes have been associated with persistent shunt and late erosions after closure, all efforts should be made to select the smallest devices compatible with each anatomy6465.
Table 2 and Figure 6 summarise some anatomical and echocardiographic features suggesting specific approaches.
Simple anatomies are the most frequent, and double-disc devices with a diameter of ≤25 mm are usually sufficient.
A redundant SP with a wide ASA excursion (≥20 mm) and/or a thick SS (>10 mm) typically requires resilient discs (e.g., DDDs with “soft” or elastic discs, such as loop DDDs [GORE CARDIOFORM], Amplatzer-like devices with a monolayer left atrial disc [Ceraflex, Cocoon, Hyperion [Comed B.V.], Nit-Occlud [pfm medical], Ultrasept [Cardia]] or soft Amplatzer-like devices [e.g., MemoPart [Lepu Medical Technology] without a distal hub, Figulla Flex II PFO Occluder [Occlutech]]) and larger sizes of both discs (>25 mm) to symmetrically embrace the involved cardiac structures that anchor to the aortic aspect of the tunnel (Supplementary Figure 5A), thus avoiding compression and shortening of the redundant tissue in between the two rigid discs (“concertina effect”, often resulting in a residual shunt) (Supplementary Figure 5B)62.
Self-centring devices (atrial septal defect or muscular ventricular septal closure devices) may be used to minimise the risk of persistent shunt in cases of a very large PFO (>15 mm)66, or a very thick SS (>15 mm)67. A malalignment of the SP with respect to the SS, with a resulting abnormal angulation and a wider gap between the two structures, is often due to an acquired or congenital displacement of the aortic plane due to thoracic or aortic deformations and is frequently associated with POS68. Again, the use of self-centring devices may be preferred to gain precise anchoring of the discs on both sides and gain greater stability of the device to fully occlude the gap.
In cases of additional IAS defects along with the PFO, multiple devices may be required. Detunnelisation (performed by a balloon septoplasty69 or by inflating a sizing balloon in the PFO and gently pulling it out70) may be necessary in case of a severely stiff SP before implantation of an Amplatzer-like device with enhanced radial strength (diameter ≤25 mm). As an alternative in these cases, a transseptal puncture can be performed into the fossa ovalis in a position near the PFO to implant a DDD to close the PFO without negotiating the stiff tunnel.
Current percutaneous suture closure may be suitable for selected cases where there are no additional IAS defects, the PFO width is small (<5 mm and no spontaneous preprocedural shunt at TOE), there is no ASA, and >10 mm of overlap between the SP and SS is present577172. Transseptal access for left atrial or mitral interventions is easy in the presence of a suture, but it is also usually feasible after the implantation of DDDs737475.
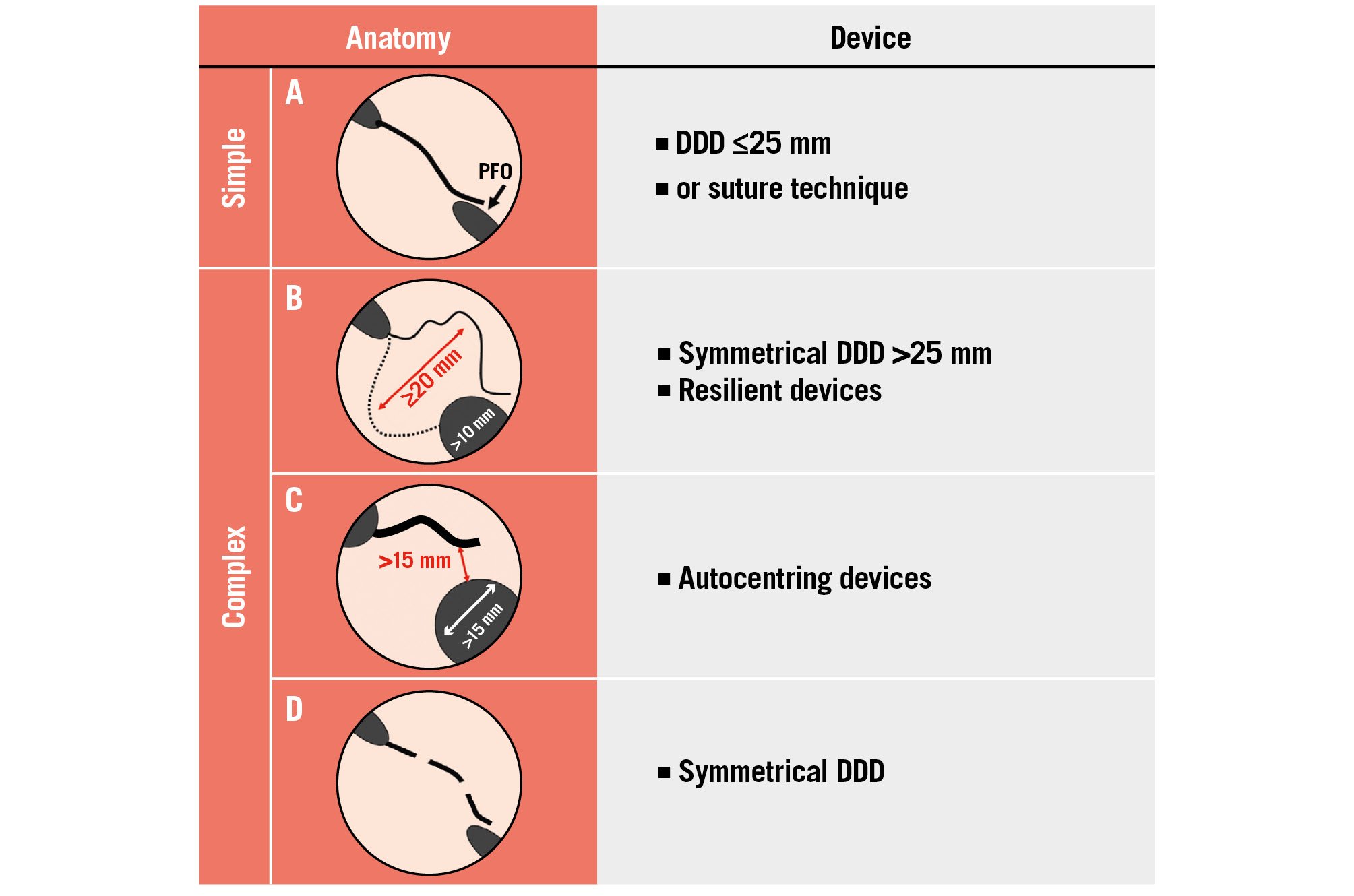
Figure 6. Principles to guide the choice of a PFO closure technique or device according to anatomical characteristics. A) A simple anatomy typically consists of a PFO with <5 mm opening and no ASA, with good overlap between the SS and SP; (B) a thin and floppy SP with a wide ASA and a thick SS usually require a large, symmetrical and resilient DDD; (C) a wide PFO and/or a very thick SS may require ASD or VSD devices especially if a stiff SP is present; (D) a multifenestrated SP requires a symmetrical DDD. Inspired with permission from62. ASD: atrial septal defect; DDD: double-disc device; PFO: patent (persistent) foramen ovale; SP: septum primum; SS: septum secundum; VSD: ventricular septal defect
How to deal with difficult PFO crossing
Crossing the PFO with a guidewire is typically a simple procedure, but it can become challenging under certain circumstances.
Atypical orientation of the PFO
If the PFO is oriented more posteriorly, superiorly, or inferiorly than expected, using echocardiographic guidance may be helpful. Additionally, rotating the C-arm to a more left anterior-oblique position can help access the PFO. If difficulty in crossing the PFO is still encountered, advance the multipurpose catheter-guidewire combination up to the lower superior vena cava and slowly retract and rotate it, as if performing a transseptal catheterisation. Often a small, visible movement is noted when the limbus is crossed, and then advancement of the guidewire will cross the PFO.
Small or unexpectedly absent PFO
Place the multipurpose catheter-guidewire combination in the standard location and use the catheter to engage the right atrial entry point with the J-wire. The multipurpose catheter can then be gently rotated, usually in a clockwise direction, to cross the PFO. If crossing fails, replace the J-wire with a slightly curved or straight hydrophilic guidewire and gently probe to avoid perforation. Echocardiography and microbubble injection should be used to confirm the absence of the PFO if the above-mentioned tips are ineffective. Consider a pulmonary RLS if an intracardiac RLS is not present.
Long serpiginous tunnels
The technique described for a small PFO can be used with the multipurpose catheter and hydrophilic guidewire approach, combined with a gentle twisting or forward torquing of the catheter. Once the multipurpose catheter is in a left pulmonary vein, insert a stiff guidewire, such as an Amplatzer, to exchange for the delivery catheter of the chosen PFO closure device. Use fluoroscopy for the exchange with visualisation of the guidewire tip, using only gentle forward pressure on the guidewire to maintain its position.
Occasionally, navigation to the pulmonary veins may not be quick. There is no major downside to placing a preshaped guidewire with wide loops in the left atrium.
Procedural and periprocedural complications
Nowadays the in-hospital complications of percutaneous closure are infrequent.
Bleeding complications have been reported in 1.7% of cases, specifically major haematomas in 0.1% of cases60.
Early DDD embolisation was reported at between 0.9% and 1.3% in older studies76 but has become even rarer with procedural and device improvements, with a reported rate of 0.4% in one meta-analysis60. Moreover, when it occurs during the procedure, it is easily detected and the device can often be retrieved by interventional techniques; more rarely, it requires surgery. A routine predischarge echocardiogram can rule out early postprocedural embolisation.
A pericardial effusion has been previously reported in 0.5-1% of cases1 and may evolve into tamponade in 0.2% of cases when caused by an intraprocedural perforation or early erosion (<48 h)6077, but it is mild if due to an allergic reaction7879.
Early supraventricular arrhythmias as a direct complication of the procedure are rare80.
Follow-up
Routine follow-up recommendations
Following PFO closure, patients should avoid heavy lifting and vigorous exercise for a week to allow for complete healing of the femoral venous access site. Most patients will not require further subspeciality care.
Follow-up appointments assess the completeness of PFO closure, new-onset AF, and the need for long-term antithrombotic therapy. Routine follow-up c-TCD is reasonable, at least at 6 months, to accurately assess closure, and/or c-TTE can verify device position. In case of PFO suture closure, more frequent visits are suggested in the first year due to higher asymptomatic failure rates.
Unrestricted diving can be resumed after demonstration of PFO sealing.
Based on clinical trials, dual antiplatelet therapy for 1-6 months, followed by at least 5 years of single antiplatelet therapy for secondary stroke prevention, is recommended for most patients12. Single antiplatelet therapy may be reasonably stopped after a year if closure has been performed for non-stroke indications and no residual shunt has been confirmed. Full anticoagulation can be used as an alternative to antiplatelet therapy if required by other concomitant conditions.
Subacute bacterial endocarditis prophylaxis is recommended for at least 6 months, or longer if significant residual shunting is present.
Management of problems and complications during follow-up
Recurrent cerebrovascular events
Recurrent stroke or transient ischaemic attack (TIA) after PFO closure in patients younger than 60 years of age is rare, with an annual incidence lower than 0.5% per year over a median follow-up of around 5 years4243. Residual shunting and device thrombosis are possible but infrequent causes, so any recurrence requires specialised neurovascular assessment. To prevent recurrence, upstream strict patient selection and lifelong control of associated vascular risk factors are crucial. Long-term single antiplatelet therapy has been suggested based on RCT design12, but the optimal duration of antithrombotic therapy after PFO closure has not been evaluated8182. European Stroke Organisation 2024 guidelines suggest an ILR in patients with a recurrent cerebrovascular accident after PFO closure to assess the cause or recurrence2.
Palpitations and arrhythmias
Based on anecdotal clinical experience, palpitations occur in 10-20% of patients following PFO closure, often as transient supraventricular extrasystoles or short runs of supraventricular tachycardia. If they cause significant and persistent symptoms, beta blockers can manage them, although some patients may need ECG or prolonged monitoring for AF screening. Standard-of-care therapy with rate control and anticoagulation is needed if AF is detected. Pharmacological or electrical cardioversion may be considered. Since procedure-related AF appears to occur only within 4 weeks after closure and is often transient183, the need for ongoing medication requires reassessment. Despite ILR allowing the detection of a higher incidence of arrhythmias after closure as compared to those detected in randomised trials80, its clinical benefits are controversial202122. Therefore, except in cases of recurrent cerebrovascular accident, an ILR after PFO closure is generally not warranted, especially if thorough AF screening has been performed preclosure2.
Residual shunts
Assessment of residual shunts after PFO closure is required for all devices and closure techniques. Endothelisation is a key mechanism of closure, taking up to 5 years, and may prolong assessment. Complete closure has been reported beyond 1 year in up to 98% of the patients with DDDs60, but PFO size, the presence of an ASA, the size and type of devices as well as the diagnostic technique influence the reported efficacy of long-term sealing6465848586. Suture closure is associated with long-term significant residual shunt in up to 21% of patients56. If follow-up shows large or significantly increased residual shunt, further investigation with TOE may be necessary to assess failure of closure or other shunting sites. A recent meta-analysis of observational, mainly retrospective, trials showed that residual shunting is associated with an increased risk of recurrent stroke in patients with previous PFO-related stroke/TIA87, although in randomised trials, trivial amounts of residual shunting were not associated with a risk of recurrent stroke from paradoxical embolism. Therefore, an individualised decision is needed before considering surgical closure, reintervention or medical therapy, taking into consideration the low level of certitude of these data and that the degree of risk needs to be better defined according to the severity of the shunt.
Chest pain
Chest pain after PFO closure may indicate serious complications such as device erosion, pericardial effusion, and pericarditis. A thorough evaluation is required, including ECG and echocardiographic assessment. Erosion is rare but requires surgery. Pericarditis can be managed with anti-inflammatory medications, but rarely, a nickel allergy may require device removal. Alternatively, mild atypical chest pain may be related to extrasystoles and is generally transient.
Device thrombosis
Usually, clinically evident device thrombosis, albeit uncommon, develops before complete endothelisation; therefore, late thrombosis is even rarer. In case of embolic manifestations or the potential for thrombus on a device, TOE is needed for characterisation. Short-term anticoagulation may be needed while a personalised management plan is being developed.
Device embolisation
Late device embolisation is a rare complication of PFO closure but often requires retrieval. If the device embolises to the pulmonary artery, it is often asymptomatic. If it embolises to a ventricle, it can produce symptomatic arrhythmias.
Conclusions
Percutaneous PFO closure is an established medical practice for secondary prevention of PFO-related stroke. While it may be reasonable to close a PFO in selected patients with other medical conditions, primary prevention is still not recommended in any clinical condition. A rigorous work-up is always necessary to determine PFO relatedness, and interdisciplinary collaboration is essential.
PFO closure procedures have been simplified and adapted since 1976 but must be tailored to individual clinical and anatomical characteristics, which sometimes may pose technical challenges. Solid evidence of efficacy and safety from double-disc devices after long-term follow-up exists. Percutaneous suture has the advantage of minimising the foreign bodies left behind, but its clinical effectiveness remains uncertain at the time of writing this article, and it is technically demanding. Bioresorbable devices are again under development after being interrupted for many years.
Guidelines suggest prolonged antiplatelet therapy after the procedure; however, the ideal duration and dosage are unclear. Complications are uncommon and usually temporary, nevertheless close monitoring is necessary to identify any potential issue.
Interventional PFO treatment has opened new possibilities, but further exploration is needed. Precision medicine with the support of artificial intelligence has the potential to improve personalised indications in such complex scenarios. More evidence is also needed in controversial indications such as cryptogenic stroke in patients >60 years old, migraine, and primary prevention, particularly preventing stroke during non-cardiac surgery. Newly available and forthcoming devices may improve outcomes, but efficacy and safety data are required. Scientific evidence is also necessary to determine how to select between existing devices and techniques. The same applies to new imaging modalities, such as the recently available three-dimensional (3D) matrix array ICE catheters, which are particularly helpful when TOE is challenging or when minimal sedation is preferred. While experience with 3D ICE is growing, ongoing refinement of techniques and workflows are needed to optimise its effectiveness88.
Lastly, research is required to determine the best drug regimen after closure and to treat complications like peri/postprocedural AF.
In conclusion, percutaneous PFO closure is a safe and user-friendly therapy that benefits many patients, but it must be tailored to those with PFO-related medical conditions. Ongoing research and technology advancements could improve its benefits and expand its indications.
Conflict of interest statement
C. Pristipino reports an institutional research grant from Mendes SA outside of this submitted work, in his previous affiliation. J. Carroll reports consultant fees, lecture honoraria, and institutional research grants from Abbott; and consultant fees from Holistick Medical. J.-L. Mas reports lecture honoraria from Abbott. N.C. Wunderlich reports consultant fees from Holistick Medical; and lecture honoraria from Lifetech Scientific, Boston Scientific, W. L. Gore & Associates, Siemens, GE Healthcare, Philips, Abbott, and Edwards Lifesciences. L. Sondergaard reports previous consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT; and, at present, being Chief Medical Officer and Divisional Vice President, Medical Affairs, at Abbott.
Supplementary data
To read the full content of this article, please download the PDF.
Moving images 1. Intracardiac echocardiography (1) and fluoroscopy recording (2) of the unsheathing of the left atrial disc of an Amplatzer device.
Moving images 2. Intracardiac echocardiography (1) and fluoroscopy recording (2) of the unsheathing of the left atrial disc of an Amplatzer device.
Moving images 3. Intracardiac echocardiography (3) and fluoroscopy recording (4) of the apposition of the left atrial disc of an Amplatzer device onto the left atrial aspect of the interatrial septum and the unsheathing of the right atrial disc.
Moving images 4. Intracardiac echocardiography (3) and fluoroscopy recording (4) of the apposition of the left atrial disc of an Amplatzer device onto the left atrial aspect of the interatrial septum and the unsheathing of the right atrial disc.
Moving images 5. Intracardiac echocardiography (5) and fluoroscopy recording (6) of the “wiggle” manoeuvre.
Moving images 6. Intracardiac echocardiography (5) and fluoroscopy recording (6) of the “wiggle” manoeuvre.
Moving images 7. Intracardiac echocardiography of the final assessment after the release of the device.
Moving images 8. Intracardiac echocardiography of the final assessment after the release of the device.

