Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Acute pulmonary embolism (PE) is a common cause of cardiovascular morbidity and mortality. Catheter-based therapies (CBT) are emerging technologies that provide reperfusion for patients with PE. However, the optimal timing of these interventions from initial presentation is unknown.
Aims: This study aimed to determine whether the timing of CBT affects outcomes among patients with acute PE managed with CBT.
Methods: This was a retrospective cohort study of patients with PE who underwent CBT and were included in the Nationwide Readmissions Database between January 2017 and December 2020. Patients who underwent early CBT (≤1 day from admission) were compared with those who underwent delayed CBT (>1 day). The primary outcome was death at 90 days, and secondary outcomes included 90-day readmissions. Propensity scores were estimated using logistic regression, and propensity score weighting (PSW) was utilised to compare outcomes between early and delayed CBT. Cox proportional hazards modelling was used to estimate the risk of primary and readmission outcomes.
Results: A total of 12,137 patients were included: 1,992 (16.4%) had high-risk PE, and 1,856 (15.3%) were treated with delayed CBT. After PSW, early CBT was associated with a lower risk of 90-day death in both intermediate-risk (hazard ratio [HR] 0.55, 95% confidence interval [CI]: 0.46-0.66) and high-risk (HR 0.89, 95% CI: 0.80-0.99) PE. Early CBT was associated with lower rates of all-cause readmission in patients with intermediate-risk PE (HR 0.86, 95% CI: 0.78-0.95) and in those with high-risk PE (HR 0.84, 95% CI: 0.69-1.05).
Conclusions: Among patients with intermediate- or high-risk PE, early CBT was associated with a lower risk of 90-day death and readmission. A further prospective study on the optimal timing for reperfusion using CBT is needed.
Pulmonary embolism (PE) is a leading cause of morbidity and mortality, particularly in patients with haemodynamic compromise. Catheter-based therapies (CBT), including catheter-directed thrombolysis (CDT) and mechanical thrombectomy (MT), have been developed for the treatment of PE and to provide rapid reperfusion1. Patients with PE may have rapid deterioration due to progressive right ventricular (RV) dysfunction, ischaemia and pressure overload2. In other acute cardiovascular disorders, including acute myocardial infarction (AMI) and ischaemic stroke, percutaneous, time-sensitive treatments are instituted to prevent tissue death34. However, the optimal timing of CBT in acute PE remains an unanswered question5. Smaller studies have suggested that early CBT in acute PE may improve echocardiographic and haemodynamic parameters, as well as post-procedure length of stay (LOS)567. However, the impact of early CBT on mortality and hospital readmissions has not been examined. Therefore, we aimed to investigate short-term (within 90 days) mortality, readmission rates and LOS in patients with PE treated with early (within 1 day of admission) versus delayed (after first day of admission) CBT.
Methods
Study design and population
We conducted a retrospective, observational cohort study using the Nationwide Readmissions Database (NRD), part of the Healthcare Cost and Utilization Project (HCUP), from 1 January 2017 to 31 December 2020. The NRD assigns unique identifiers to individual patients to track readmissions within a given calendar year. It captures approximately 50% of hospitalisations in the United States. Patients with a primary diagnosis of PE were identified using International Classification of Diseases, 10th Revision (ICD-10) codes (Supplementary Table 1). The first admission for PE in a given calendar year was considered the index PE hospitalisation. Intermediate-risk PE was defined as PE complicated by cor pulmonale; type 2 myocardial infarction (MI), as a surrogate for cardiac biomarkers; right heart failure (RHF), as a surrogate for right heart strain; and tachycardia without cardiogenic shock, vasopressor use, or cardiac arrest8. High-risk PE was defined as patients with concomitant diagnoses of cardiogenic shock, vasopressor use, or cardiac arrest. Procedures − including CBT, systemic thrombolysis, mechanical ventilation, transfusion, and extracorporeal membrane oxygenation (ECMO) − were identified using ICD-10 procedure codes. Comorbidities were captured using ICD-10 codes (Supplementary Table 1). Patients were deemed to be treated with early CBT if the first CBT procedure was performed on the day of admission (day 0) or day 1, and with delayed CBT if the first CBT procedure was performed after day 1 of admission. Patients with an unknown day of CBT were excluded (Supplementary Figure 1). This study was deemed exempt from review by the New York University Grossman School of Medicine’s Institutional Review Board because the data used are publicly available and deidentified.
Outcomes
Outcomes were identified using ICD-10 codes (Supplementary Table 1). The primary outcome was 90-day death − defined as death during the index hospitalisation or a readmission that resulted in death within 90 days of discharge. Secondary outcomes included 90-day readmissions, major bleeding during the index hospitalisation (defined as intracranial, gastrointestinal, or postprocedural bleeding or transfusion of blood products), and index hospitalisation LOS.
Statistical analysis
Propensity scores (PS) using a non-parsimonious multivariable logistic regression included age, sex, month of admission, year of admission, weekend admission, elective admission, history of cancer, smoking status, hypertension, prior venous thromboembolism, heart failure, diabetes, coronary artery disease, chronic kidney disease, liver disease, anaemia, thrombocytopaenia, long-term anticoagulation use, long-term antiplatelet use, prior stroke, home oxygen use, chronic lung disease, do not resuscitate (DNR) status, palliative care, obesity, cardiogenic shock, presence of other types of shock, use of vasopressors, deep vein thrombosis (DVT), respiratory failure during index hospitalisation, mechanical ventilation, systemic thrombolysis use, hospital size and location, insurance and zip code median income. PS were used to perform PS weighting (PSW) analysis with 1/PS being assigned to patients treated with delayed CBT and 1/(1−PS) for patients treated with early CBT9. Standardised mean differences (SMD) were calculated to assess for intergroup imbalances. Variables were considered imbalanced if the SMD was greater than or equal to 0.10.
Categorical outcomes are presented as frequency and percentages, and comparisons between groups were performed using chi-square tests before and after PSW. PSW time-to-event Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for 90-day death and readmission outcomes. The risk of major bleeding during the index hospitalisation was assessed using PSW logistic regression. Given the NRD does not track readmissions across calendar years, we excluded patients admitted after September for time-to-event analyses. The length of stay between groups was compared using the Student’s t-test.
A sensitivity analysis was performed comparing outcomes (primary outcome and 90-day readmission) of patients with intermediate- or high-risk PE who underwent CBT on day 0, day 1 and after day 1. Multivariable Cox proportional hazards regression modelling was performed, adjusting for age, sex, type of CBT, cancer, chronic lung disease, anaemia, thrombocytopaenia, DNR status, palliative care, respiratory failure, mechanical ventilation, and systemic thrombolysis.
All tests were two-tailed, and a p-value of <0.05 was considered significant. Statistical analyses were performed using SPSS version 29.0 (IBM) and Stata version 15 (StataCorp).
Results
Baseline characteristics
A total of 10,145 patients with intermediate-risk PE were included, with a mean age of 61.1 years; 4,835 (47.7%) were female, and 1,554 (15.3%) were managed with delayed CBT. The majority of CBT was CDT (78.2%). Prior to PSW, those managed with delayed CBT were more likely to be older (mean 62.5 years vs 60.8 years; SMD=0.110), be treated with MT (27.3% vs 20.8%; SMD=0.153), and have respiratory failure (53.0% vs 43.7%; SMD=0.187). After PSW, all variables were balanced (Table 1).
A total of 1,992 patients with high-risk PE were included, with an average age of 63.4 years; of these, 1,042 (52.3%) were female, 302 (15.2%) were treated with delayed CBT, and 1,258 (63.2%) were treated with CDT. Prior to PSW, patients managed with delayed CBT were more likely to be younger (mean 61.4 years vs 63.7 years; SMD=0.163), be treated with MT (44.0% vs 35.6%; SMD=0.172), have heart failure (49.0% vs 34.2%; SMD=0.304), chronic lung disease (26.5% vs 17.6%; SMD=0.216), present with cardiogenic shock (72.8% vs 66.9%; SMD=0.129), and have Medicaid insurance (18.2% vs 10.1%; SMD=0.234) but had lower rates of cardiac arrest (28.5% vs 37.2%; SMD=0.186) compared with patients treated with early CBT. After PSW, all variables were well balanced between the early and delayed CBT groups (Table 2).
Table 1. Baseline characteristics of patients with intermediate-risk PE treated with delayed versus early CBT before and after propensity score weighting.
| Unweighted | Propensity score weighted | ||||||
|---|---|---|---|---|---|---|---|
| All patients N=10,145 | Delayed CBT N=1,554 | Early CBT N=8,591 | SMD | Delayed CBT | Early CBT | SMD | |
| Age, years | 61.1±14.9 | 62.5±14.6 | 60.8±14.9 | 0.110 | 61.4±15.1 | 61.1±14.9 | 0.017 |
| Female sex | 4,835 (47.7) | 769 (49.5) | 4,066 (47.3) | 0.044 | 49.3% | 47.8% | 0.030 |
| CBT type | |||||||
| CDT | 7,933 (78.2) | 1,129 (72.7) | 6,804 (79.2) | 0.153 | 78.5% | 78.2% | 0.007 |
| MT | 2,212 (21.8) | 425 (27.3) | 1,787 (20.8) | 0.153 | 21.5% | 21.8% | 0.007 |
| Patient characteristics | |||||||
| Cancer | 898 (8.8) | 144 (9.3) | 752 (8.8) | 0.017 | 9.3% | 8.8% | 0.017 |
| Hypertension | 6,461 (63.7) | 1,064 (68.5) | 5,397 (62.8) | 0.120 | 63.6% | 63.6% | <0.001 |
| Prior VTE | 1,735 (17.1) | 263 (16.9) | 1,470 (17.1) | 0.005 | 16.6% | 17.1% | 0.013 |
| CHF | 2,292 (22.6) | 513 (33.0) | 1,779 (20.7) | 0.280 | 22.7% | 22.7% | <0.001 |
| DM | 2,728 (26.9) | 452 (29.1) | 2,276 (26.5) | 0.058 | 26.2% | 26.7% | 0.011 |
| AF | 1,099 (10.8) | 209 (13.4) | 890 (10.4) | 0.093 | 10.7% | 10.8% | 0.003 |
| CAD | 3,045 (30.0) | 548 (35.3) | 2,497 (29.1) | 0.133 | 30.4% | 30.1% | 0.007 |
| Smokers | 3,484 (34.3) | 539 (34.7) | 2,945 (34.3) | 0.008 | 33.8% | 34.4% | 0.013 |
| PAD | 229 (2.3) | 49 (3.2) | 180 (2.1) | 0.069 | 2.2% | 2.3% | 0.007 |
| CKD | 1,126 (11.1) | 250 (16.1) | 876 (10.2) | 0.175 | 11.8% | 11.2% | 0.019 |
| Chronic lung disease | 1,916 (18.9) | 398 (25.6) | 1,518 (17.7) | 0.193 | 18.7% | 18.9% | 0.005 |
| Home oxygen | 238 (2.3) | 59 (3.8) | 179 (2.1) | 0.101 | 2.3% | 2.3% | <0.001 |
| Prior stroke | 395 (3.9) | 89 (5.7) | 306 (3.6) | 0.100 | 3.8% | 3.9% | 0.005 |
| Liver disease | 510 (5.0) | 104 (6.7) | 406 (4.7) | 0.086 | 4.9% | 5.0% | 0.005 |
| Anaemia | 2,355 (23.2) | 427 (27.5) | 1,928 (22.4) | 0.118 | 23.9% | 23.2% | 0.016 |
| Thrombocytopaenia | 1,018 (10.0) | 152 (9.8) | 866 (10.1) | 0.010 | 10.5% | 10.0% | 0.016 |
| Long-term anticoagulation | 1,357 (13.4) | 217 (14.0) | 1,140 (13.3) | 0.020 | 13.0% | 13.3% | 0.009 |
| Long-term antiplatelet | 1,438 (14.2) | 204 (13.1) | 1,234 (14.4) | 0.038 | 13.5% | 14.1% | 0.017 |
| DNR status | 479 (4.7) | 89 (5.7) | 390 (4.5) | 0.055 | 5.0% | 4.8% | 0.009 |
| Palliative care | 132 (1.3) | 27 (1.7) | 105 (1.2) | 0.042 | 1.4% | 1.3% | 0.009 |
| Obesity | 4,164 (41.0) | 686 (44.1) | 3,478 (40.5) | 0.073 | 39.7% | 40.9% | 0.024 |
| Hospitalisation characteristics | |||||||
| Other shock | 238 (2.3) | 48 (3.1) | 190 (2.2) | 0.056 | 2.4% | 2.4% | <0.001 |
| Cor pulmonale | 7,475 (73.7) | 1,103 (71.0) | 6,372 (74.2) | 0.072 | 73.5% | 73.6% | 0.002 |
| Type 2 MI | 1,117 (11.0) | 209 (13.4) | 908 (10.6) | 0.086 | 11.0% | 11.1% | 0.003 |
| DVT | 6,080 (59.9) | 997 (64.2) | 5,083 (59.2) | 0.103 | 60.0% | 60.0% | <0.001 |
| Respiratory failure | 4,574 (45.1) | 823 (53.0) | 3,751 (43.7) | 0.187 | 44.8% | 45.1% | 0.006 |
| Mechanical ventilation | 258 (2.5) | 58 (3.7) | 200 (2.3) | 0.082 | 2.7% | 2.6% | 0.006 |
| Systemic thrombolysis | 540 (5.3) | 114 (7.3) | 426 (5.0) | 0.096 | 5.9% | 5.2% | 0.031 |
| Large or medium hospital | 8,981 (88.5) | 1,365 (87.8) | 7,616 (88.7) | 0.028 | 88.6% | 88.5% | 0.003 |
| Urban teaching hospital | 7,751 (76.4) | 1,183 (76.1) | 6,568 (76.5) | 0.009 | 76.8% | 76.5% | 0.007 |
| Medicare | 4,802 (47.3) | 772 (49.7) | 4,030 (46.9) | 0.056 | 48.2% | 47.5% | 0.014 |
| Medicaid | 851 (8.4) | 151 (9.7) | 700 (8.1) | 0.056 | 8.3% | 8.4% | 0.004 |
| Private insurance | 3,631 (35.8) | 505 (32.5) | 3,126 (36.4) | 0.082 | 35.6% | 35.8% | 0.004 |
| Lowest quartile zip code for income | 2,807 (27.7) | 476 (30.6) | 2,331 (27.1) | 0.077 | 27.6% | 27.7% | 0.002 |
| Data are presented as mean±SD or n (%), unless indicated otherwise. AF: atrial fibrillation; CAD: coronary artery disease; CBT: catheter-based therapy; CDT: catheter-directed thrombolysis; CHF: congestive heart failure; CKD: chronic kidney disease; DM: diabetes mellitus; DNR: do not resuscitate; DVT: deep vein thrombosis; MI: myocardial infarction; MT: mechanical thrombectomy; PAD: peripheral arterial disease; PE: pulmonary embolism; SD: standard deviation; SMD: standardised mean difference; VTE: venous thromboembolism | |||||||
Table 2. Baseline characteristics of patients with high-risk PE treated with delayed versus early CBT before and after propensity score weighting.
| Unweighted | Propensity score weighted | ||||||
|---|---|---|---|---|---|---|---|
| All patients N=1,992 | Delayed CBT N=302 | Early CBT N=1,690 | SMD | Delayed CBT | Early CBT | SMD | |
| Age, years | 63.4±14.3 | 61.4±15.1 | 63.7±14.2 | 0.163 | 63.0±14.7 | 63.3±14.4 | 0.018 |
| Female sex | 1,042 (52.3) | 144 (47.7) | 898 (53.1) | 0.108 | 54.7% | 52.4% | 0.046 |
| CBT type | |||||||
| CDT | 1,258 (63.2) | 169 (56.0) | 1,089 (64.4) | 0.172 | 58.9% | 63.1% | 0.086 |
| MT | 734 (36.8) | 133 (44.0) | 601 (35.6) | 0.172 | 41.1% | 36.9% | 0.086 |
| Patient characteristics | |||||||
| Cancer | 273 (13.7) | 39 (12.9) | 234 (13.8) | 0.026 | 12.3% | 14.1% | 0.053 |
| Hypertension | 1,236 (62.0) | 195 (64.6) | 1,041 (61.6) | 0.062 | 63.2% | 62.1% | 0.023 |
| Prior VTE | 256 (12.9) | 41 (13.6) | 215 (12.7) | 0.027 | 14.0% | 13.1% | 0.026 |
| CHF | 726 (36.4) | 148 (49.0) | 478 (34.2) | 0.304 | 39.6% | 36.8% | 0.058 |
| DM | 569 (28.6) | 87 (28.8) | 482 (28.5) | 0.007 | 28.6% | 28.7% | 0.002 |
| AF | 428 (21.5) | 80 (26.5) | 348 (20.6) | 0.139 | 21.0% | 21.5% | 0.012 |
| CAD | 637 (32.0) | 103 (34.1) | 534 (31.6) | 0.053 | 34.4% | 32.2% | 0.047 |
| Smokers | 581 (29.2) | 93 (30.8) | 488 (28.9) | 0.042 | 29.1% | 29.2% | 0.002 |
| PAD | 70 (3.5) | 15 (5.0) | 55 (3.3) | 0.085 | 2.6% | 3.3% | 0.041 |
| CKD | 288 (14.5) | 53 (17.5) | 235 (13.9) | 0.099 | 16.0% | 14.8% | 0.033 |
| Chronic lung disease | 378 (19.0) | 80 (26.5) | 298 (17.6) | 0.216 | 19.8% | 19.0% | 0.020 |
| Home oxygen | 45 (2.3) | 13 (4.3) | 32 (1.9) | 0.139 | 2.5% | 2.3% | 0.013 |
| Prior stroke | 98 (4.9) | 17 (5.6) | 81 (4.8) | 0.036 | 4.0% | 4.9% | 0.044 |
| Liver disease | 407 (20.4) | 76 (25.2) | 331 (19.6) | 0.135 | 20.4% | 20.6% | 0.005 |
| Anaemia | 864 (43.4) | 149 (49.3) | 715 (42.3) | 0.141 | 46.1% | 43.5% | 0.052 |
| Thrombocytopaenia | 373 (18.7) | 68 (22.5) | 305 (18.0) | 0.112 | 19.0% | 18.7% | 0.008 |
| Long-term anticoagulation | 216 (10.8) | 37 (12.3) | 179 (10.6) | 0.053 | 10.9% | 10.9% | <0.001 |
| Long-term antiplatelet | 232 (11.6) | 37 (12.3) | 195 (11.5) | 0.025 | 14.0% | 11.8% | 0.066 |
| DNR status | 406 (20.4) | 66 (21.9) | 340 (20.1) | 0.044 | 20.8% | 20.3% | 0.012 |
| Palliative care | 268 (13.5) | 47 (15.6) | 221 (13.1) | 0.071 | 14.9% | 13.6% | 0.037 |
| Obesity | 639 (32.1) | 116 (38.4) | 523 (30.9) | 0.158 | 33.5% | 32.1% | 0.030 |
| Hospitalisation characteristics | |||||||
| Cardiogenic shock | 1,351 (67.8) | 220 (72.8) | 1,131 (66.9) | 0.129 | 71.3% | 67.8% | 0.076 |
| Cardiac arrest | 715 (35.9) | 86 (28.5) | 629 (37.2) | 0.186 | 34.2% | 36.0% | 0.038 |
| Vasopressor | 447 (22.4) | 71 (23.5) | 376 (22.2) | 0.031 | 25.2% | 22.7% | 0.059 |
| DVT | 987 (49.5) | 185 (61.3) | 802 (47.5) | 0.280 | 52.3% | 49.6% | 0.054 |
| Respiratory failure | 1,582 (79.4) | 246 (81.5) | 1,336 (79.1) | 0.060 | 81.6% | 79.7% | 0.048 |
| Mechanical ventilation | 1,004 (50.4) | 146 (48.3) | 858 (50.8) | 0.050 | 50.4% | 50.6% | 0.004 |
| ECMO | 33 (1.7) | 9 (3.0) | 24 (1.4) | 0.109 | 1.8% | 1.8% | <0.001 |
| Systemic thrombolysis | 302 (15.2) | 55 (18.2) | 247 (14.6) | 0.097 | 18.1% | 15.1% | 0.081 |
| Large or medium hospital | 1,816 (91.2) | 276 (91.4) | 1,540 (91.1) | 0.011 | 91.5% | 91.1% | 0.014 |
| Urban teaching hospital | 1,615 (81.1) | 252 (83.4) | 1,363 (80.7) | 0.070 | 79.8% | 81.1% | 0.033 |
| Medicare | 1,078 (54.1) | 152 (50.3) | 926 (54.8) | 0.090 | 54.4% | 54.0% | 0.008 |
| Medicaid | 226 (11.3) | 55 (18.2) | 171 (10.1) | 0.234 | 11.8% | 11.1% | 0.022 |
| Private insurance | 550 (27.6) | 87 (28.8) | 463 (27.4) | 0.031 | 27.7% | 27.9% | 0.004 |
| Lowest quartile zip code for income | 567 (28.5) | 87 (28.8) | 480 (28.4) | 0.009 | 29.1% | 28.2% | 0.020 |
| Data are presented as mean±SD or n (%), unless indicated otherwise. AF: atrial fibrillation; CAD: coronary artery disease; CBT: catheter-based therapy; CDT: catheter-directed thrombolysis; CHF: congestive heart failure; CKD: chronic kidney disease; DM: diabetes mellitus; DNR: do not resuscitate; DVT: deep vein thrombosis; ECMO: extracorporeal membrane oxygenation; MT: mechanical thrombectomy; PAD: peripheral arterial disease; PE: pulmonary embolism; SD: standard deviation; SMD: standardised mean difference; VTE: venous thromboembolism | |||||||
Primary and secondary outcomes
In the intermediate-risk PE group, early CBT was associated with a lower risk of 90-day death (HR 0.55, 95% CI: 0.46-0.66) compared with delayed CBT. Moreover, early CBT was associated with lower risks of 90-day readmission (HR 0.86, 95% CI: 0.78-0.95), major bleeding during index hospitalisation (OR 0.79, 95% CI: 0.73-0.87) (Table 3, Figure 1), and shorter LOS (5.1 days vs 7.3 days; p<0.001) (Supplementary Table 2). After stratifying by early CBT type, early CDT was associated with a lower risk of 90-day death (HR 0.48, 95% CI: 0.39-0.59) but not early MT (HR 0.80, 95% CI: 0.60-1.18).
In the high-risk PE group, early CBT was associated with a lower risk of 90-day death (HR 0.89, 95% CI: 0.80-0.99) compared with delayed CBT. Moreover, early CBT was associated with numerically lower risks of 90-day all-cause readmission (HR 0.84, 95% CI: 0.69-1.05), major bleeding during index hospitalisation (OR 0.85, 95% CI: 0.75-0.97) (Table 3, Figure 1) and shorter LOS (mean 8.9 days vs 13.4 days; p<0.001) compared with delayed CBT. After stratifying by early CBT type, early CDT was associated with a lower risk of 90-day death (HR 0.82, 95% CI: 0.72-0.93) but not early MT (HR 1.02, 95% CI: 0.88-1.18).
Table 3. Logistic and Cox proportional hazards regression outcomes of patients with early versus delayed CBT before and after propensity score weighting.
| Unweighted HR or OR (95% CI) | Weighted HR or OR (95% CI) | |
|---|---|---|
| Intermediate-risk PE | ||
| Death at 90 days | 0.52 (0.38-0.73) | 0.55 (0.46-0.66) |
| 90-day readmission | 0.78 (0.65-0.94) | 0.86 (0.78-0.95) |
| Index hospitalisation major bleeding | 0.61 (0.52-0.72) | 0.79 (0.73-0.87) |
| High-risk PE | ||
| Death at 90 days | 0.98 (0.79-1.21) | 0.89 (0.80-0.99) |
| 90-day readmission | 1.04 (0.65-1.65) | 0.84 (0.69-1.05) |
| Index hospitalisation major bleeding | 0.74 (0.57-0.95) | 0.85 (0.75-0.97) |
| CI: confidence interval; HR: hazard ratio; OR: odds ratio; PE: pulmonary embolism | ||
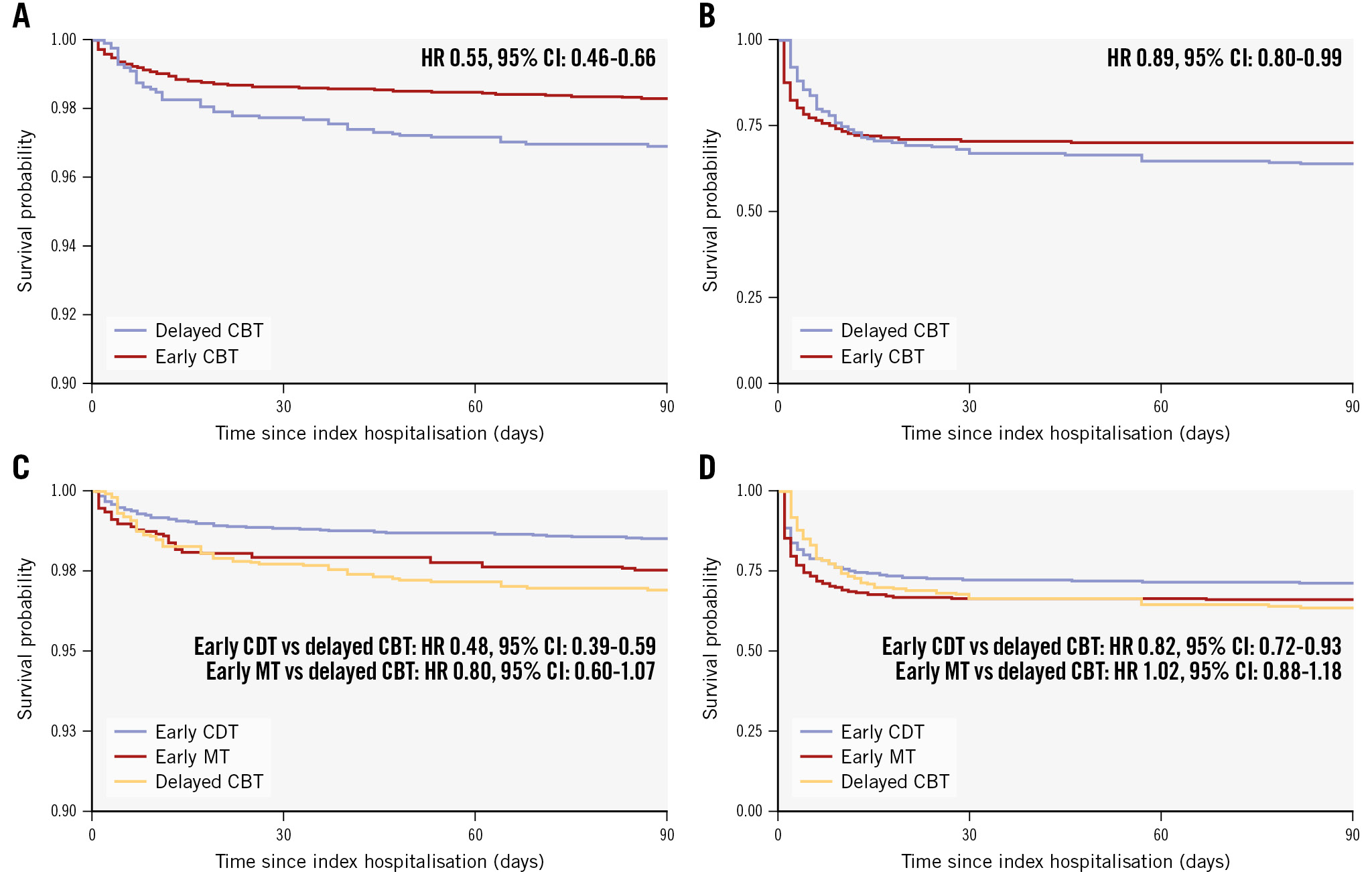
Figure 1. Survival at 90 days among patients managed with early versus delayed CBT after propensity score weighting. Kaplan-Meier survival curves of 90-day death in all patients with intermediate-risk PE (A) and high-risk PE (B) among patients treated with early versus delayed CBT after propensity score weighting. Kaplan-Meier survival curves of 90-day death in patients with intermediate-risk PE (C) and high-risk PE (D) stratified by early CBT type versus delayed CBT. CBT: catheter-based therapy; CDT: catheter-directed thrombolysis; CI: confidence interval; HR: hazard ratio; MT: mechanical thrombectomy
Sensitivity analysis: outcomes by day of CBT
Among patients with intermediate-risk PE, 5,121 (50.5%) underwent CBT on day 0 of admission, 3,470 (34.2%) on day 1, and 1,554 (15.3%) underwent CBT after day 1 of admission (Supplementary Table 3). The primary outcome occurred in 1.9%, 1.8%, and 3.1%, and 90-day readmission in 9.6%, 10.0%, and 12.3% of patients who underwent CBT on day 0, day 1 and after day 1, respectively (Supplementary Table 4). After multivariable Cox proportional hazards regression, CBT after day 1 (adjusted HR 1.61, 95% CI: 1.11-2.34), but not CBT on day 1 (adjusted HR 1.34, 95% CI: 0.96-1.88), was associated with a higher risk of the primary outcome compared to patients who underwent CBT on day 0. After multivariable Cox proportional hazards regression, CBT after day 1 (adjusted HR 1.22, 95% CI: 1.00-1.49), but not CBT on day 1 (adjusted HR 1.01, 95% CI: 0.86-1.19), was associated with a higher risk of 90-day readmission compared with CBT on day 0 (Supplementary Table 5).
Among patients with high-risk PE, 1,170 (58.7%) underwent CBT on day 0 of admission, 520 (26.1%) on day 1, and 302 (15.2%) underwent CBT after day 1 of admission (Supplementary Table 3). The primary outcome occurred in 38.2%, 35.6%, and 34.1%, and 90-day readmission in 15.3%, 15.8%, and 15.0% of patients who underwent CBT on day 0, day 1 and after day 1, respectively (Supplementary Table 4). After multivariable Cox proportional hazards regression, CBT on day 1 (adjusted HR 1.28, 95% CI: 1.06-1.54), but not CBT after day 1 (adjusted HR 1.00, 95% CI: 0.79-1.25), was associated with a higher risk of the primary outcome compared to patients who underwent CBT on day 0. After multivariable Cox proportional hazards regression, there was no difference in 90-day readmissions with CBT on day 1 (adjusted HR 1.00, 95% CI: 0.69-1.47) and CBT after day 1 (adjusted HR 1.00, 95% CI: 0.62-1.62) compared with CBT on day 0 (Supplementary Table 5).
Discussion
In this nationwide, retrospective, observational cohort study, among patients hospitalised for intermediate- or high-risk PE and treated with CBT, early CBT was associated with lower risks of 90-day death, 90-day readmission, and in-hospital major bleeding (Central illustration). Moreover, early CBT was associated with a significantly shorter index hospitalisation LOS.
Small studies have suggested a potential benefit of early CBT in patients with PE. In one review, early intervention (within 48 hours of presentation) was associated with improved echocardiographic parameters5. In another study of 41 patients with intermediate-risk PE, early CDT (defined as within 24 hours of presentation) was associated with improved invasive haemodynamics and postprocedural LOS6. Another small, single-centre study of 64 patients with acute PE managed with CBT showed that patients treated with early CBT (within 24 hours of admission to the emergency department) had a shorter hospital LOS and a larger difference in pulmonary arterial systolic pressure as measured with right heart catheterisation, though no differences in in-hospital mortality or readmissions within 3 months were noted7. However, these studies have been small, and the effect of CBT timing on in-hospital and short-term mortality and readmissions has not been investigated in larger cohorts. After stratifying by CBT type, early CDT, but not early MT, was associated with a lower risk of 90-day death compared with delayed CBT in both intermediate-risk and high-risk PE. Prior studies have showed no difference in outcomes between patients treated with CDT and MT101112. However, whether CBT modality impacts outcomes among patients undergoing early or delayed CBT is unclear and beyond the scope of our current study; further investigation is warranted.
Unlike other thrombotic cardiovascular diseases, such as AMI or ischaemic stroke, there is a lack of data on the benefit of early intervention in PE. Risk assessment of PE is not as clear-cut as it is for other conditions, including AMI. While guidelines exist for door-to-balloon times for patients with ST-elevation myocardial infarction (STEMI), no such guidelines exist for PE13. Indeed, our study suggests that, much like in AMI, early reperfusion may be beneficial among patients with intermediate- and high-risk PE. Recent studies have suggested that novel risk assessment scores, including the composite pulmonary embolism shock (CPES) score, may identify patients with PE who are relatively haemodynamically stable but in normotensive shock. The CPES score incorporates elevated biomarkers, moderately or severely reduced RV function on echocardiography, the presence of saddle PE, concomitant DVT, and tachycardia, and may help identify patients with PE who may benefit from earlier intervention1415. However, randomised clinical trials on the efficacy of CBT on hard endpoints, such as mortality, and on the timing of CBT should be conducted to confirm our findings.
Our study has limitations that should be considered when interpreting our results. The data available in the NRD are gathered via ICD-10 codes; therefore, granular details, including vital signs, symptom onset, laboratory data, use of anticoagulation and other medications cannot be obtained. Additionally, the precise timing of CBT in hours is unavailable; therefore, assessment of CBT timing to the resolution of hours is not possible. Apart from discharge or death, the temporal association of in-hospital outcomes (including bleeding) cannot be ascertained in the NRD; therefore, it is not possible to discern whether these outcomes occurred before or after CBT. Because the NRD only captures 50% of hospitalisations nationwide and does not record deaths that occur outside of a hospitalisation, it is not possible to ascertain overall mortality using this database, and the effect of CBT timing on this outcome remains unclear. While some socioeconomic variables, including zip code income and insurance status, are available in the NRD, the NRD does not record race or ethnicity, and therefore, their impact on outcomes is unclear and is a source of potential residual confounding.
Our classification of intermediate- and high-risk PE is based on ICD-10 codes, given that laboratory values, including cardiac biomarkers, and imaging findings of right ventricular strain are unavailable. It is possible that some patients may have been misclassified given this limitation. Additionally, we are also unable to differentiate intermediate-risk patients into intermediate-low or intermediate-high risk categories using this database. Moreover, the mortality rates in patients who did not undergo CBT were as follows: high in the high-risk PE cohort at 50.6%; 2.7% among the intermediate-risk PE patients; and 1.3% in those with neither intermediate- nor high-risk PE; these were consistent with prior data. Furthermore, the temporality of variables used to define high-risk PE cannot be defined; therefore, it is not possible to discern whether these characteristics were present at presentation or developed after CBT. This may, at least in part, explain the greater treatment effect of early CBT in intermediate-risk PE compared with high-risk PE. It is possible that early CBT in intermediate-risk PE may have prevented progression to high-risk PE. Prospective studies with more granular data are needed to test this hypothesis.
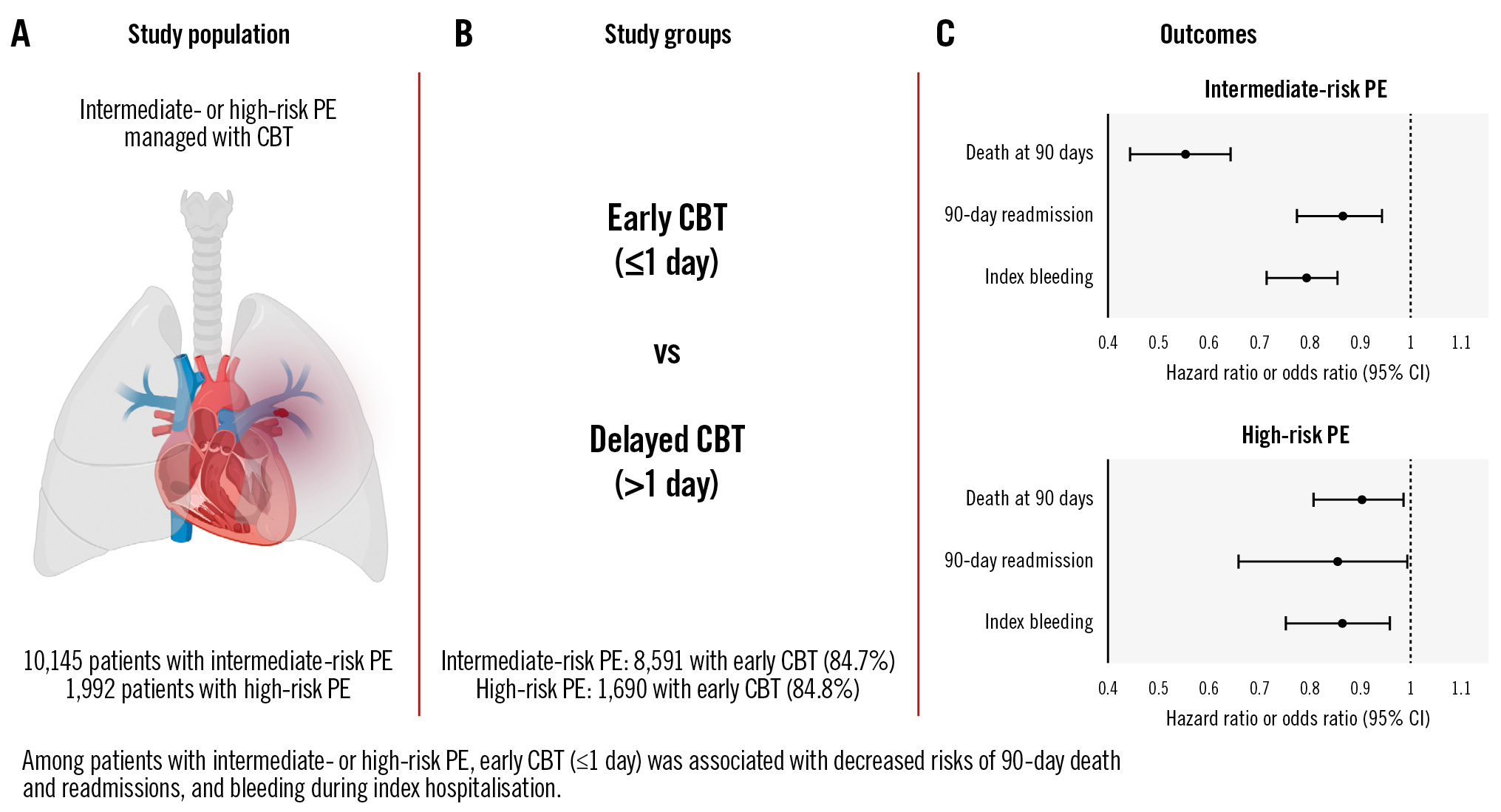
Central illustration. Early CBT associated with improved outcomes. A) The study population included patients with intermediate- or high-risk PE who underwent CBT. B) Patients with early CBT were compared with those with delayed CBT. C) Forest plot of outcomes of early versus delayed CBT. CBT: catheter-based therapy; CI: confidence interval; PE: pulmonary embolism
Conclusions
Pulmonary embolism is a common cardiovascular disease, and CBT may improve outcomes in this patient population, although the optimal timing of intervention is not yet well characterised. Our study suggests that, among patients with intermediate- or high-risk PE, early CBT may be associated with a lower risk of in-hospital death or readmission leading to death within 90 days, lower 90-day readmission, and reduced LOS. Our results are hypothesis-generating, and randomised trials are needed to better delineate the optimal timing for CBT.
Impact on daily practice
This large, nationwide, real-world cohort study of patients with intermediate- and high-risk pulmonary embolism and catheter-based therapy (CBT) suggests that early CBT may be associated with improved outcomes. Prospective studies are needed to confirm our findings.
Conflict of interest statement
R.P. Rosovsky receives consulting fees from Abbott, BMS, Dova, Inari, Janssen, and Penumbra, all for work outside the submitted research. S. Bangalore reports ad hoc consulting and speaker fees for Abbott, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, Inari, and Truvic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

