Cory:
Unlock Your AI Assistant Now!
As risk stratification for acute pulmonary embolism (PE) has evolved over the past two decades, important parallels to the approach to myocardial infarction (MI) have emerged. The integration of clinical factors, cardiac biomarkers, electrocardiographic findings, and cardiac imaging characterise the assessment of both disorders. Furthermore, high- and intermediate-high-risk PE share similarities with those experiencing ST-elevation (STEMI) and non-ST-elevation myocardial infarction (NSTEMI). First, high- and intermediate-high-risk PE patients demonstrate a similarly elevated risk of in-hospital mortality compared with those presenting with STEMI and NSTEMI12. Second, the acute care of these life-threatening cardiovascular conditions is based on a similar team-based approach, represented by STEMI teams and pulmonary embolism response teams (PERTs), respectively3. In MI, early reperfusion therapy is aimed at restoring coronary artery blood flow and preventing myocardial necrosis and the decline of left ventricular systolic function12. Similarly, in high- and intermediate-high-risk PE patients with clinical deterioration, reperfusion therapy is aimed at restoring pulmonary blood flow, improving gas exchange, and alleviating right ventricular dysfunction (RVD)1. Additionally, the benefit of reperfusion appears to be time dependent in high-risk PE as well as in STEMI patients2. Conversely, in intermediate-high-risk PE patients, the optimal timing for reperfusion is less clear, as in NSTEMI patients12. While the use of systemic fibrinolysis has substantially changed the treatment of STEMI and PE, reducing relative mortality rates4, the associated risk of major bleeding events, especially intracranial haemorrhage, has limited its widespread utilisation for both disorders.
In contrast to STEMI, early reperfusion was not considered the mainstay of therapy in NSTEMI until MI transfer networks were developed to facilitate percutaneous coronary intervention as a replacement for systemic fibrinolysis. Likewise, the unfavourable risk-benefit ratio of systemic fibrinolysis in intermediate-high-risk PE has discouraged its routine use24. With the recent explosion in device therapy for PE, including low-dose catheter-based fibrinolysis and mechanical embolectomy, the door to early reperfusion in such patients seems to have opened. However, the lack of high-quality randomised controlled trial data supporting the use of catheter-directed therapies (CDT) in high- and intermediate-high-risk patients remains an important obstacle to an MI-like approach. A successful and timely reperfusion strategy resulting in the improvement of haemodynamic status and peripheral perfusion remains fundamental in these patients3. Conversely, in the event of treatment failure, defined as the lack of haemodynamic improvement within 2 to 4 hours after completing the local thrombolysis infusion in high-risk PE patients or as the persistence of compromised vital signs in intermediate-high-risk patients after 24-48 hours of therapeutic anticoagulation, escalation of therapy is warranted3.
Guidelines for the management of acute PE rely heavily on haemodynamic status as an indication for reperfusion via the peripheral intravenous administration of fibrinolytic drugs, CDT or surgical embolectomy1. Specifically, systemic fibrinolysis is recommended as the primary reperfusion therapy of choice in high-risk PE patients (Class I recommendation), defined as those with a systolic blood pressure <90 mmHg or cardiac arrest, as soon as possible13. Conversely, patients with a working diagnosis of STEMI require immediate reperfusion therapy using primary percutaneous coronary intervention (PPCI), or fibrinolysis if PPCI is not possible within 120 minutes of the diagnosis. Nevertheless, in high-risk PE, systemic fibrinolysis remains underused because of concerns regarding absolute and relative contraindications, major bleeding, especially in medically complex and frail elderly patients, loss of expertise in the use of fibrinolytic drugs, and tissue plasminogen activator shortage5. In intermediate-high-risk PE patients, defined as normotensive PE patients with evidence of both RVD on imaging and increased cardiac biomarkers, routine reperfusion is not recommended because of the risk of major haemorrhage, especially intracranial bleeding1. However, a subset of these normotensive PE patients, ranging between 5% and 10%, will suffer haemodynamic deterioration following diagnosis with associated increased mortality3. These subjects may be considered for reperfusion therapy if haemodynamic decompensation occurs1. However, in intermediate-high-risk PE patients, the optimal timing for the systemic or catheter-based reperfusion remains less clear.
The parallels in clinical outcomes, risk stratification, and reperfusion challenges between MI and PE suggest that a similar therapeutic approach may be advantageous for high- and select intermediate-high-risk PE patients. An MI-like approach to high-risk PE holds the promise of facilitating earlier access to reperfusion and reducing door-to-needle time for systemic fibrinolysis and door-to-catheter insertion time for CDT (Figure 1). Such efforts may reduce the early mortality associated with high-risk PE like the observed survival benefit of PPCI for STEMI achieved with reduced door-to-balloon time4. For intermediate-high-risk PE patients, transfer to a referral centre with experience in clinical monitoring and primary reperfusion for PE may offer the promise of reduced short-term mortality. Other benefits associated with earlier reperfusion could include the prevention of long-term complications such as chronic thromboembolic pulmonary hypertension, which often remains undiagnosed and has an estimated incidence ranging from 0.4% to 6.2%, post-PE syndrome, shortening the length of hospitalisation, and a faster return to home and work3. Finally, an MI-like approach for acute PE could facilitate a shift away from systemic fibrinolysis towards a catheter-based approach with an associated reduction in major bleeding complications, especially intracranial haemorrhage. Such a benefit has been realised in the primary management of MI3.
While the potential benefits of an MI-like approach to acute PE are certainly alluring, there are also important obstacles complicating the realisation of such a strategy (Table 1). In contrast to the literature supporting an early interventional approach for MI and stroke, there is a paucity of randomised controlled trials evaluating hard clinical, patient-centred, and long-term outcomes1. Because there is such a dearth in the evidence base for CDT in PE, clinical practice guidelines based on the current evidence have been unable to provide solid recommendations345. Furthermore, in contrast to MI and stroke, no studies have yet prospectively evaluated the optimal therapeutic windows for high-and intermediate-high-risk patients5. Similarly, the selection of a CDT approach, such as catheter-based fibrinolysis, versus mechanical embolectomy has not been the subject of any adequately powered clinical trials to date. Hopefully, such data are forthcoming from the HI-PEITHO (ClinicalTrials.gov: NCT04790370), PEERLESS (NCT05111613), PEERLESS II (NCT06055920), STORM-PE (NCT05684796), and PE-TRACT (NCT05591118) studies. Moreover, limited access to catheterisation and angiographic laboratories as well as scarce financial and personnel resources represent significant obstacles to an MI-like treatment model for acute PE5. Additionally, the use of CDT, especially in Europe, is limited by the lack of adequate logistical resources, device availability, and the absence of a widespread diffusion of specialised centres offering interventional treatments at night and at weekends5.
While the parallels between the clinical approach to and challenges of MI and PE are myriad, much more research is required to truly demonstrate whether the shift from systemic fibrinolysis to a catheter-based approach in MI could be feasible and beneficial in acute PE. Currently, PE does not have the wealth of data that MI had when it approached the crossroads for such a primary intervention approach. On the other hand, the widespread integration of multidisciplinary PERTs suggests a favourable pathway forward for such an approach. Given the potential for reduction in the heterogeneity in PE care and the potential lower risk of major bleeding events derived by the adoption of a MI-like treatment approach, randomised clinical trials assessing the effects of an early invasive approach in high- and intermediate-high-risk PE should be considered, much like those historically executed in MI. However, before such groundbreaking clinical trials can be realised, the evidence foundation for the benefits and safety of catheter-directed therapy in acute PE must be firmly established. Only then may guidelines provide a framework for the appropriate integration of interventional strategies.
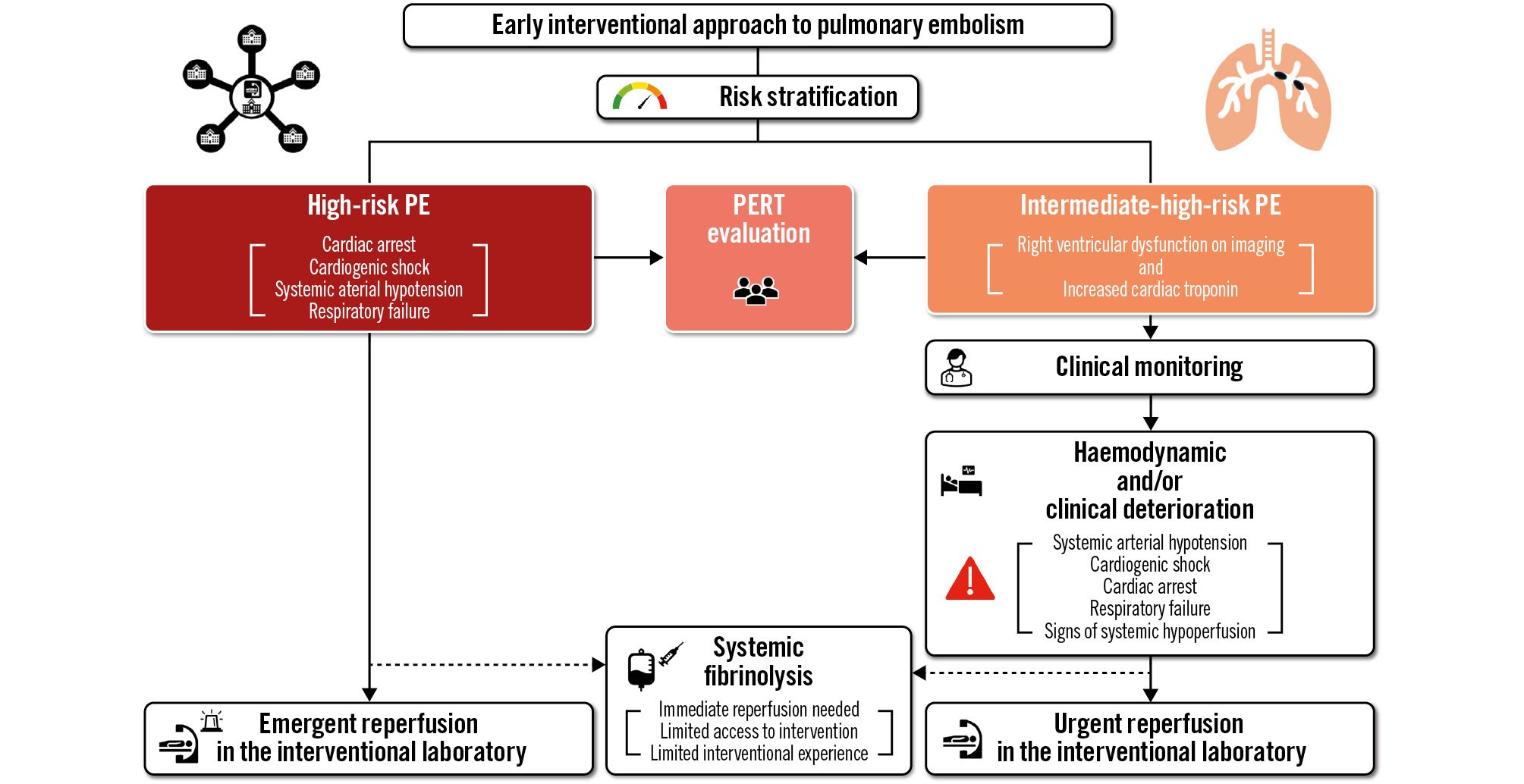
Figure 1. Algorithm for the application of a myocardial infarction-like therapeutic approach in high- and intermediate-high-risk pulmonary embolism patients. The dashed line indicates the current recommended reperfusion strategy by the European Society of Cardiology Guidelines. PE: pulmonary embolism; PERT: pulmonary embolism response team
Table 1. Potential obstacles and solutions regarding the application of a myocardial infarction-like approach for the treatment of high- and selected intermediate-high-risk PE patients.
| Obstacles | Potential solutions |
|---|---|
| Identification of intermediate-high-risk PE patients who would benefit from early reperfusion | New prediction tools |
| Specialised PE interventionalists | Creation of adequate fellowship/training programmes |
| Financial and logistical resources | Implementation of financial, logistical and personnel resources |
| Diffusion of PE response teams | |
| Underutilisation of advanced therapies, especially in high-risk PE patients | Involvement of guidelines committees |
| Lack of data regarding the impact of CDT on hard clinical outcomes (short- and long-term PE-related mortality and other complications) | Randomised and cohort/PE response team-related registries |
| Optimal door-to-catheter insertion time | |
| Optimal therapeutic windows for different CDT | |
| CDT: catheter-directed therapies; PE: pulmonary embolism | |
Conflict of interest statement
G. Piazza has received research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific; and consulting fees from Pfizer, Boston Scientific, Janssen, and Amgen. M. Zuin has no conflicts of interest to declare.

