Abstract
Aortic regurgitation (AR) is a common clinical disease associated with significant morbidity and mortality. Investigations based largely on non-invasive imaging are pivotal in discerning the severity of disease and its impact on the heart. Advances in technology have contributed to improved risk stratification and to our understanding of the pathophysiology of AR. Surgical aortic valve replacement is the predominant treatment. However, its use is limited to patients with an acceptable surgical risk profile. Transcatheter aortic valve implantation is an alternative treatment. However, this therapy remains in its infancy, and further data and experience are required. This review article on AR describes its prevalence, mechanisms, diagnosis and treatment.
Aortic regurgitation (AR) is an important and frequently encountered disease, associated with debilitating symptoms, heart failure and premature mortality. Treatment until recently was largely surgical aortic valve replacement (SAVR). With the advent of transcatheter aortic valve implantation (TAVI), the scope of treatment has been widened. We describe the epidemiology of AR, its assessment using multimodality imaging and appraise the treatment options available.
Epidemiology
In the Framingham study – which analysed a population-based cohort aged 28-62 years, attending routine clinical examination – 4.9% of patients had moderate AR, and 0.5% had severe AR. The prevalence of any AR was higher in male compared to in female patients (13.0% vs 8.5%, respectively) and increased with age (odds ratio for 10-year increase: 2.3, 95% confidence interval [CI]: 2.0-2.7)1. Among an older population (>65 years), a community-based study identified a 15% prevalence of any AR and a 1.6% prevalence of moderate/severe AR. AR is also a common finding in patients with mixed valvular heart disease – 81% of such patients had some degree of AR2.
Classification
The assessment of AR includes evaluation of its underlying mechanism and assessment of its severity. The mechanism of AR can be classified based on the geometry of the aortic annulus and the motion of the aortic cusps (Figure 1)3. Similar to the Carpentier classification of mitral regurgitation4, based on the motion of the aortic cusps, AR can be classified as type I when the motion is normal, type II when there is excessive motion (prolapse), and type III when there is restrictive motion3. Type I AR can be subclassified according to the dilatation of the components of the aortic annulus that causes malcoaptation of the aortic cusps: type Ia when there is dilatation of the sinotubular junction and ascending aorta, type Ib when the sinus of Valsalva and the sinotubular junction are dilatated, and type Ic when there is only dilatation of the ventriculo-aortic junction. In addition, perforation of the aortic cusps (i.e., in infective endocarditis) leads to AR that is classified as type Id. This classification is useful in planning the surgical approach (suitability for aortic valve repair and type of repair vs aortic valve replacement)3. However, some of these mechanisms may coexist, for example, in patients with a bicuspid aortic valve, which may show restrictive motion of the cusps due to the calcified fusion raphe and dilatation of the aortic root.
Because of its wide availability, echocardiography is the first-choice imaging technique to evaluate the pathophysiological mechanisms of AR. However, the dimensions of the aortic root are better assessed with three-dimensional (3D) imaging techniques such as magnetic resonance and computed tomography (CT)5.
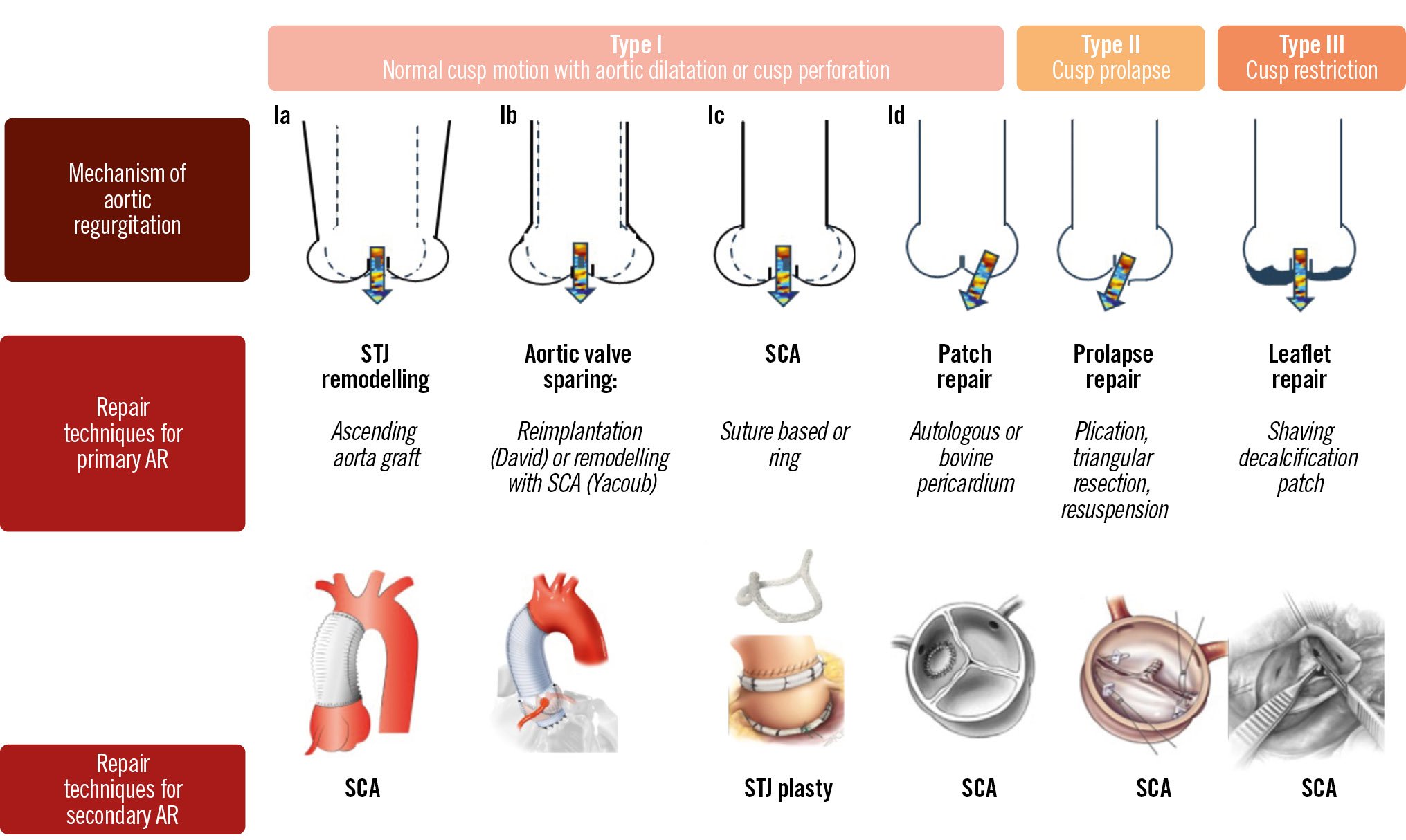
Figure 1. Mechanisms of aortic regurgitation. Adapted with permission from Boodhwani et al3. AR: aortic regurgitation; SCA: subcommissural annuloplasty; STJ: sinotubular junction
ASSESSMENT OF AORTIC VALVE ANATOMY
Assessment of the aortic valve anatomy (tricuspid vs bicuspid) is carried out from the parasternal short-axis view. A tricuspid valve has 3 commissures and 3 cusps, whereas a bicuspid valve has 2 commissures and 2 cusps, or 3 commissures and 2 functional cusps due to the presence of a fusion raphe between 2 of the 3 cusps (Figure 2). When the acoustic window is not appropriate, a transoesophageal echocardiography provides a more accurate assessment of the aortic valve anatomy. If the aortic valve is calcified, differentiation between a bicuspid and a tricuspid valve anatomy may be challenging. In those cases, CT provides the highest spatial resolution to assess the anatomy of the aortic valve (Figure 2). Cine images of the short axis of the aortic valve acquired with cardiac magnetic resonance imaging (CMR) can also provide an accurate evaluation of the aortic valve anatomy. In order to avoid artefacts when using 3D-imaging techniques, data acquisition should be performed with electrocardiogram (ECG)-gating. This is particularly important when assessing the dimensions of the aortic root and ascending aorta. When using transthoracic echocardiography, the aortic root and ascending aorta are visualised in the parasternal long-axis view, and dimensions are measured using the leading-to-leading edge method in diastole (Figure 3).
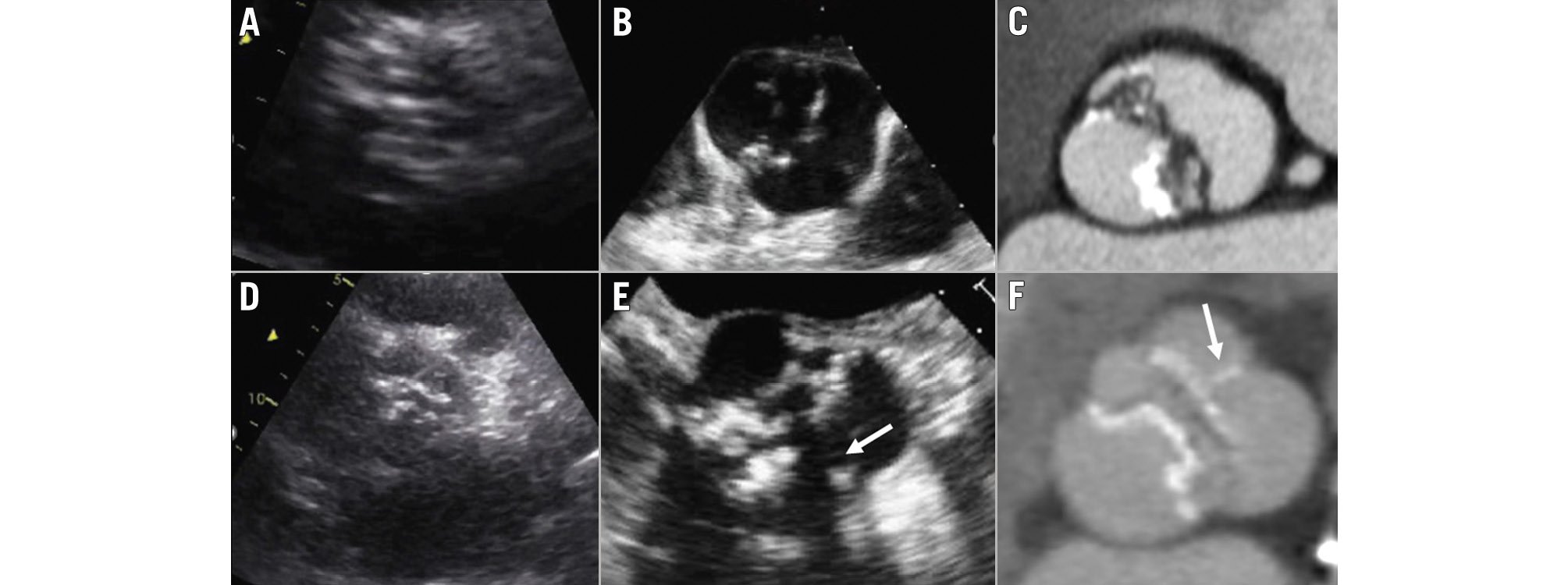
Figure 2. Anatomy of the aortic valve. Images (A), (B) and (C) show the transthoracic and transoesophageal echocardiograms and computed tomography of a patient with a bicuspid aortic valve with 2 commissures and 2 cusps. Images (D), (E) and (F) show the transthoracic and transoesophageal echocardiograms and computed tomography of a patient with a bicuspid aortic valve with 2 commissures and 3 cusps, 2 of which are fused by a fusion raphe (arrow).
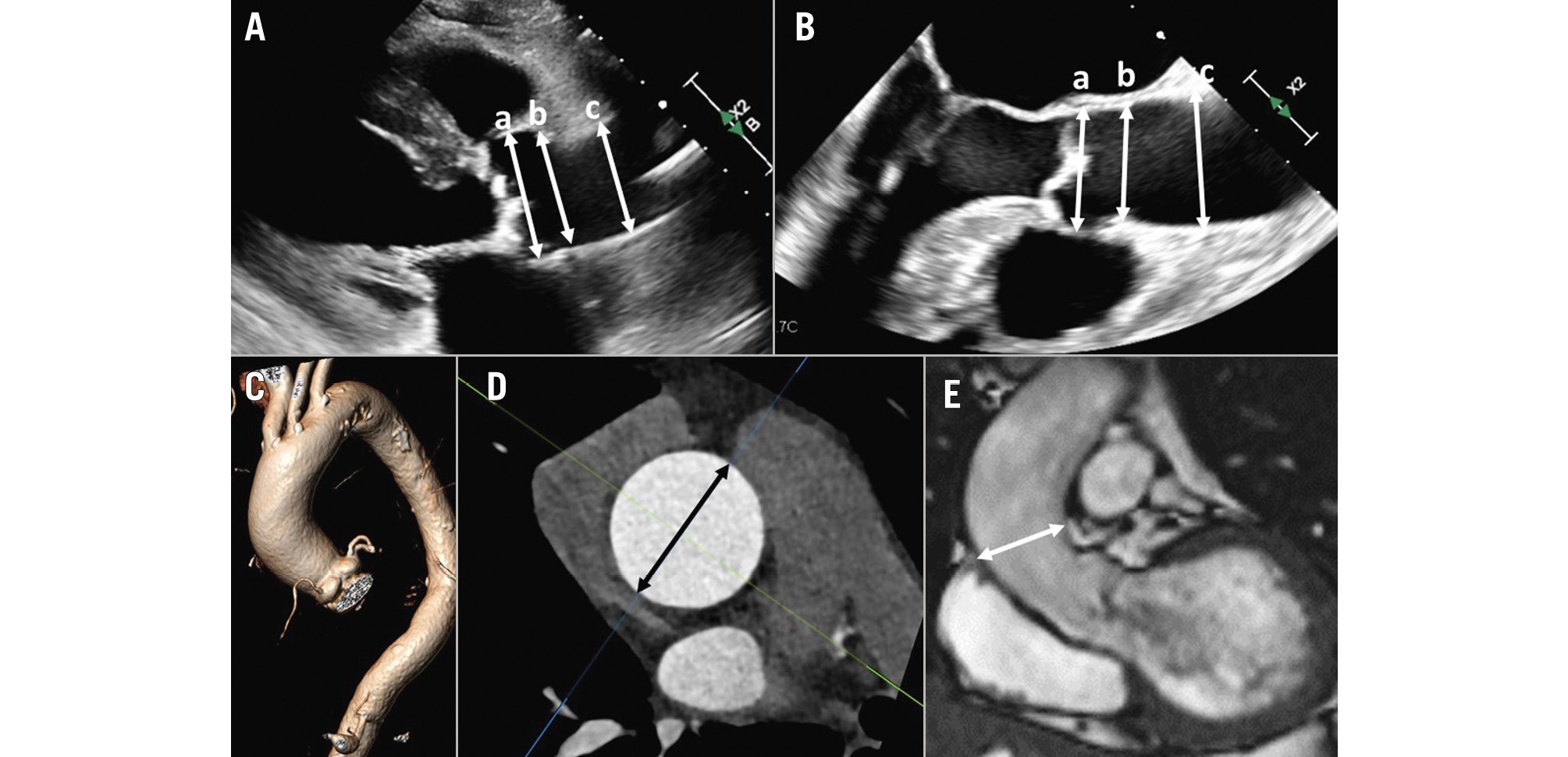
Figure 3. Assessment of aortic root and ascending aorta dimensions. Measurement of the diameter of the aortic root at the level of the sinus of Valsalva (a), sinotubular junction (b) and the ascending aorta (c) on a transthoracic echocardiogram (A) and a transoesophageal echocardiogram (B). On computed tomography angiography (C), from the 3-volume acquisition, the multiplanar reformation planes can be aligned to obtain the cross-sectional area of the ascending aorta (black double arrowhead in D). E) An example of a cine cardiac magnetic resonance acquisition and measurement (white double arrowhead) of the ascending aorta in a patient with severe aortic regurgitation.
ASSESSMENT OF THE AORTIC ROOT AND ASCENDING AORTA
Aortic root and ascending aortic dimensions are systematically underestimated when using inner-to-inner edge measurements and may be underestimated when using two-dimensional (2D) echocardiography, as the true long axis of these structures may not be shown (Figure 3). When using 3D techniques, the cross-sectional area of the ascending aorta and sinotubular junction can be reconstructed with multiplanar reformation planes, and the measurement of the dimensions of these structures is more accurate than when measuring them from the axial views (Figure 3). The sinus of Valsalva and the ventriculo-aortic junction (aortic annulus) do not show a circular shape, and therefore, the measurement is better performed from the true cross-sectional area obtained from 3D multiplanar reformation planes. The 2D echocardiographic measurements from the left parasternal long-axis view using the leading-to-leading edge correlate with the sinus-to-sinus inner-to-inner edge measurements obtained with CT or CMR. For the ventriculo-aortic junction (aortic annulus), the cross-sectional plane obtained with 3D-imaging techniques allows the measurement of the maximum and minimum diameters, the perimeter and the area, which are key for the selection of the prosthesis size (Figure 4)678. The 2D echocardiographic diameter of the aortic annulus is usually concordant with the minimum diameter obtained with 3D-imaging techniques (Figure 4).
Finally, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is a valuable technique to assess aortitis as an underlying cause of AR5. While echocardiography, CT and CMR show homogeneous circumferential thickening of the aortic wall, 18F-FDG PET/CT demonstrates a circumferential high-intensity signal that permits the differentiation between aortic atherosclerosis and intramural haematoma.
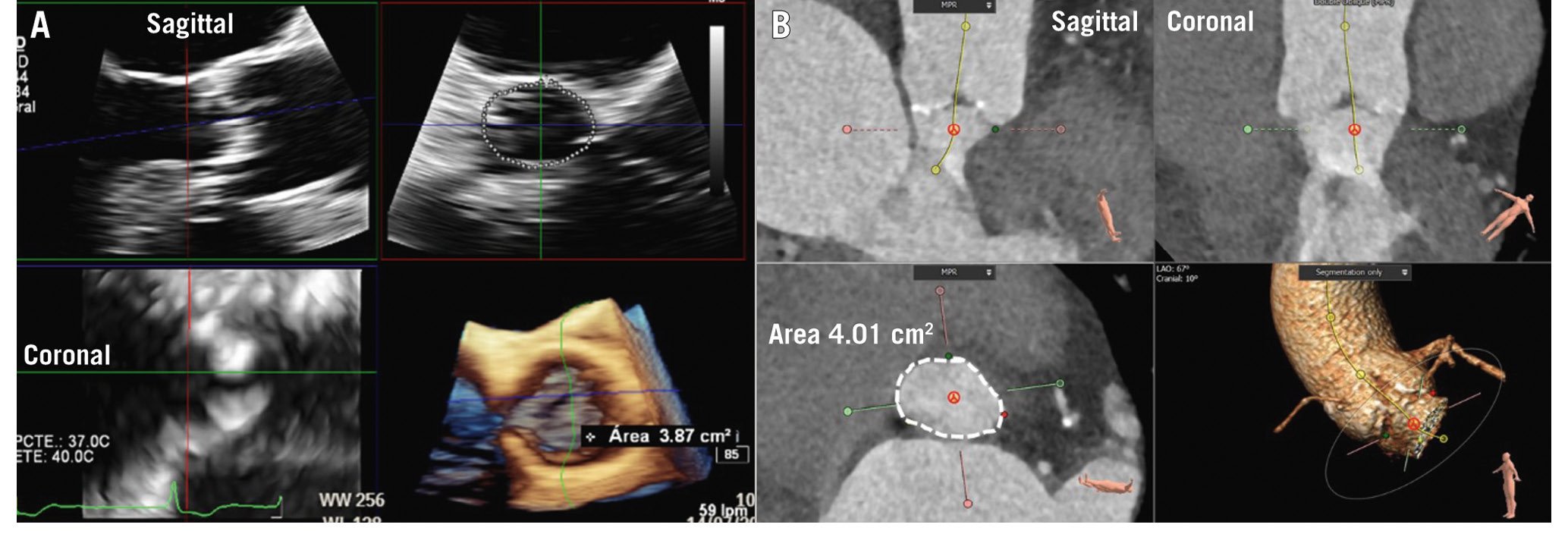
Figure 4. Measurement of the aortic annulus. Aortic annulus measurement with 3-dimensional echocardiography (A) and computed tomography (B). The multiplanar reformation planes are aligned across the aortic annulus bisecting the nadir points of the aortic cusps. The aortic annulus area is then planimetered (dotted line).
Quantification of aortic regurgitation
To assess the severity of AR, a multiparametric approach that includes qualitative, semiquantitative and quantitative parameters is frequently used (Table 1)910.
Qualitatively, severe AR is characterised by a large colour flow convergence zone, a dense signal of the continuous wave Doppler signal of the regurgitant jet and holodiastolic flow reversal in the descending aorta with an end-diastolic peak velocity of ≥20 cm/s (Figure 5). The presence of diastolic flow reversal in the abdominal aorta visualised from the echocardiographic subcostal view indicates acute, severe AR. Other methods to quantify AR include the measurement of the regurgitant volume by subtracting the pulmonic flow from the aortic forward flow, which requires a competent pulmonic valve, and the difference between the right and the left ventricular stroke volume obtained with planimetry, though it is an unreliable method when mitral or tricuspid regurgitation coexist.
Echocardiography is a versatile imaging technique that provides a comprehensive assessment of the severity and chronicity of AR (based on the haemodynamic consequences of the regurgitant volume) (Central illustration). CMR provides a more accurate assessment of left ventricular response to the AR as well as a more accurate assessment of the aortic valve regurgitant volume. Three-dimensional echocardiography provides values of the effective regurgitant orifice area and the regurgitant volume that are closer to those provided by CMR, as compared to the values measured with 2D echocardiography11. When using CMR, phase-contrast velocity mapping is performed in a plane perpendicular to the aortic root, just above the aortic valve. By integrating the velocity of each pixel into its area over the cardiac cycle, the forward and the reverse flow are derived to calculate the regurgitant fraction. Although the current cutoff value of the regurgitant fraction to define severe AR is set at 50%, a previous study has shown that a regurgitant fraction of >33% was associated with an increased incidence of cardiac events, including heart failure symptoms and the need for valve replacement12. However, these results need to be confirmed in additional, larger and prospective studies.
In addition, the haemodynamic consequences of the aortic regurgitant volume should be evaluated. Left ventricular volumes and ejection fraction are important parameters considered in decision-making. Chronic severe AR leads to left ventricular dilatation and hypertrophy due to both pressure and volume overload imposed on the left ventricle. Three-dimensional imaging techniques provide a more accurate assessment of left ventricular volumes than 2D echocardiography. In a multicentre, retrospective cohort study including 178 patients with chronic severe AR, patients with symptoms had larger left ventricular volumes measured with CMR as compared to asymptomatic patients (66 [interquartile range {IQR} 46-85] mL/m2 vs 42 [IQR 30-58] mL/m2, respectively; p<0.001), whereas there were no differences in left ventricular volumes measured with echocardiography (38 [IQR 30-58] mL/m2 vs 27 [IQR 20-42] mL/m2, respectively; p=0.07)13. These findings have important clinical implications since aortic valve intervention may be delayed unnecessarily if echocardiography demonstrates a non-dilatated left ventricle. Left ventricular systolic function assessed with deformation imaging has demonstrated that the measurement of global longitudinal strain detects systolic dysfunction earlier than left ventricular ejection fraction (LVEF) does14 and has incremental prognostic value over LVEF15. Left ventricular global longitudinal strain may reflect more accurately than LVEF the changes that occur at the myocardial level. Using tissue characterisation, CMR techniques have shown that severe AR is associated with increased interstitial myocardial fibrosis but to a lesser extent as compared to mitral regurgitation (25.3 [IQR 24.0-28.6]% vs 28.2 [IQR 26.3-30.1]%, respectively; p<0.001)16.
Table 1. Imaging criteria for severe aortic regurgitation.
| Echocardiography | Qualitative | |
|---|---|---|
| Aortic valve morphology | Abnormal/flail/large coaptation defect | |
| Colour flow AR jet width | Large in central jet, variable in eccentric jets | |
| Colour flow convergence | Large | |
| CW signal of AR jet | Dense | |
| Diastolic flow reversal in descending aorta | Holodiastolic flow reversal (end-diastolic velocity ≥20 cm/s) | |
| Diastolic flow reversal in abdominal aorta | Present | |
| Semiquantitative | ||
| Vena contracta width, mm | >6 | |
| Jet width/LVOT diameter, % |
≥65 | |
| Jet CSA/LVOT CSA, % | ≥60 | |
| Pressure half-time, ms | <200 | |
| Quantitative | ||
| EROA, mm2 | ≥30 | |
| Regurgitant volume, mL | ≥60 | |
| Regurgitant fraction, % | ≥50 | |
| Cardiac magnetic resonance | Regurgitant fraction, % | ≥50 |
| AR: aortic regurgitation; CSA: cross-sectional area; CW: continuous wave Doppler; EROA: effective regurgitant orifice area; LVOT: left ventricular outflow tract | ||
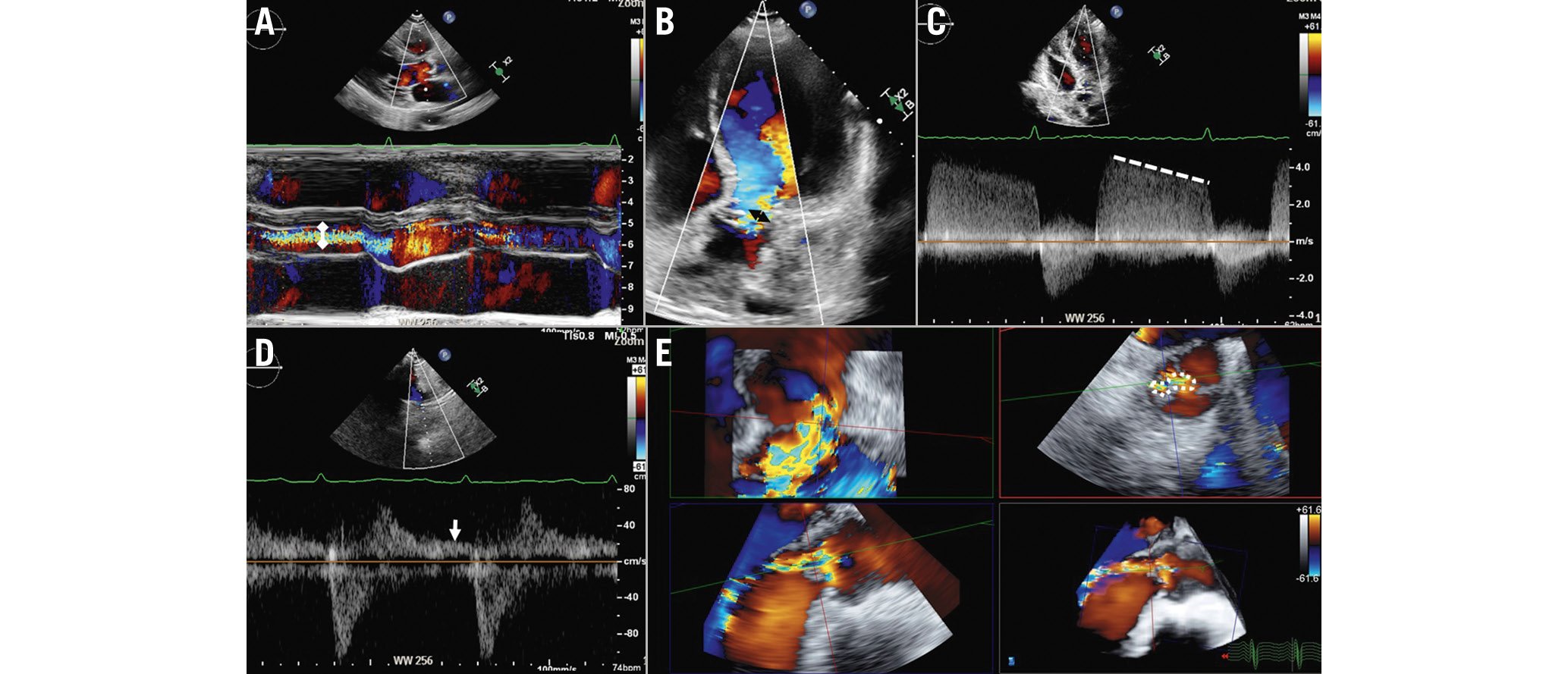
Figure 5. Assessment of aortic regurgitation with echocardiography. The following parameters should be taken into consideration to assess the severity of aortic regurgitation: the ratio between the width of the regurgitant jet (double white arrowhead) and the diameter of the left ventricular outflow tract on colour M-mode of the parasternal long-axis view (A), the vena contracta as measured on the colour Doppler image acquired from the apical 5-chamber view (double black arrowhead; B), and the dense signal of the regurgitant jet on a continuous wave Doppler image where the pressure half-time can be measured (C, dotted line). D) Diastolic flow reversal obtained with pulsed wave Doppler from the suprasternal view (white arrow). Three-dimensional colour Doppler transoesophageal echocardiography permits the measurement of the anatomical regurgitant orifice area (E, dotted encircling line) by aligning the multiplanar reformation planes across the vena contracta.
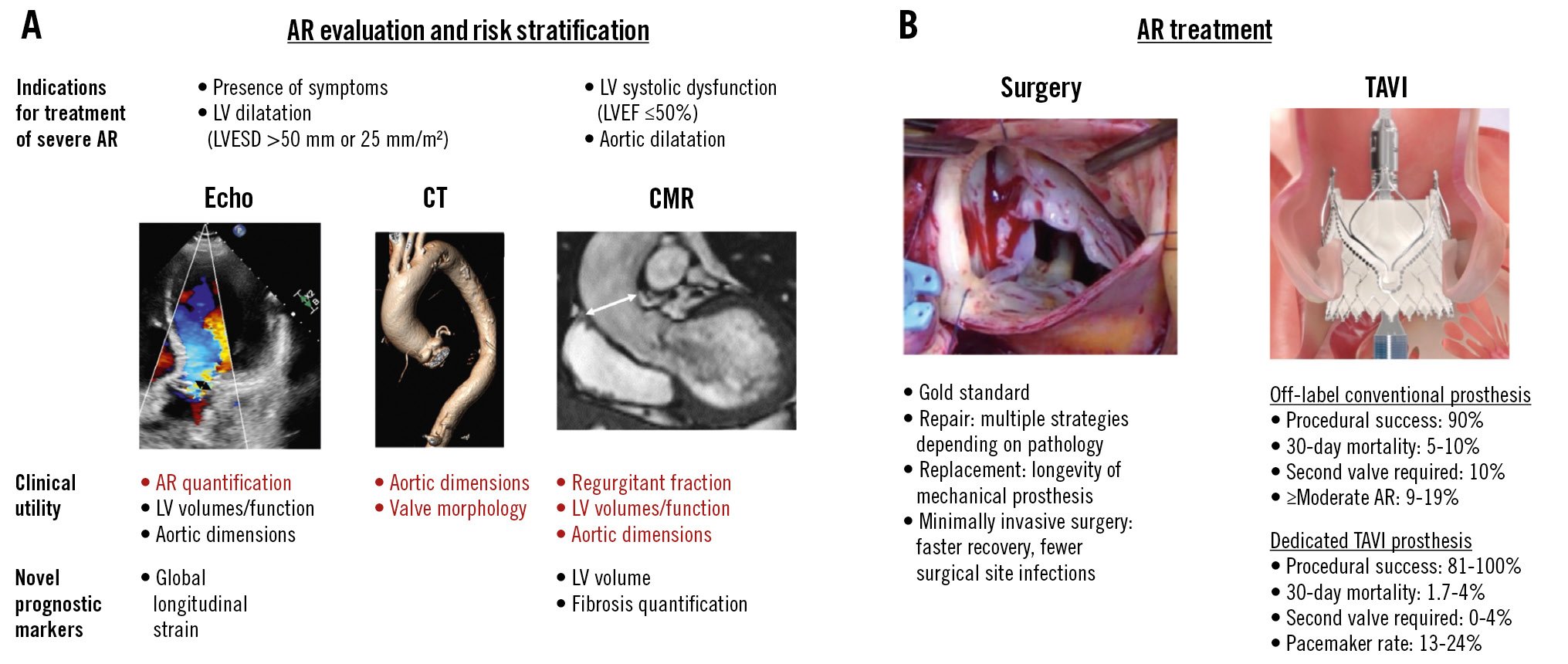
Central illustration. Aortic regurgitation – its evaluation, risk stratification and treatment. A) The evaluation and risk stratification of AR, with the key strengths of each imaging modality in red. B) The main treatment options. AR: aortic regurgitation; CMR: cardiac magnetic resonance imaging; CT: computed tomography; Echo: echocardiography; LV: left ventricle; LVESD: left ventricular end-systolic diameter; TAVI: transcatheter aortic valve implantation
Natural history
Patients with chronic severe AR are usually asymptomatic for a long time, even though chronic left ventricular volume and pressure overload lead to initially compensatory but eventually detrimental structural remodelling of the myocardium.
Despite being asymptomatic and still having preserved left ventricular ejection fraction, patients already exhibit a significantly reduced survival rate when AR is severe as compared to mild-moderate (69±9% vs 92±4%, respectively)17. Additionally, waiting for the LVEF to reduce is associated with increased operative and postoperative mortality rates and a higher risk of heart failure hospitalisation. Patients with preserved LVEF undergoing SAVR have better outcomes compared to those with depressed LVEF18.
There is a growing body of evidence that the LVEF is not sensitive enough to identify patients with already established detrimental structural myocardial changes resulting in worse outcome. Indexed left ventricular end-systolic dimensions, as well as left ventricular global longitudinal strain, seem to discriminate better with regard to risk of mortality192021 and should therefore be considered in clinical decision-making.
Vasodilator therapy as well as angiotensin-converting enzyme (ACE) inhibitors have been recommended in the past to delay surgical treatment. However, the supportive evidence is not sufficient to recommend the use of these drugs any longer for this indication22.
As soon as left ventricular function drops, patients develop heart failure symptoms. At this stage, the mortality rate of the patients increases significantly, depending on the severity of symptoms (9.4%/year in New York Heart Association [NYHA] Class II patients, 24.5%/year in NYHA Class III/IV patients)23.
Medical therapy in symptomatic patients based on ACE inhibitors and vasodilators is only supportive and might decrease heart failure symptoms; however, the course of the disease does not change unless patients undergo definitive aortic valve treatment.
SPECIAL POPULATIONS AND AORTIC REGURGITATION
AR IN PATIENTS WITH A LEFT VENTRICULAR ASSIST DEVICE
Up to 30% of patients with an implanted left ventricular assist device (LVAD) develop clinically significant moderate to severe AR within the first year due to haemodynamic and structural changes of the aortic root induced by their LVAD24. The recirculation of regurgitant blood volume between the ascending aorta and the pump results in a significant decrease of effective cardiac output leading to progressive heart failure despite regular LVAD function. The high perioperative risk in these patients, as well as their comorbidities, usually prohibits surgical aortic valve replacement. Transcatheter-based therapy with TAVI devices dedicated to treating aortic stenosis has been used to treat these high-risk patients; however, the yield of procedural efficacy and safety is not satisfactory because of the risk of valve migration and residual AR. Early reports of treatment with a dedicated device show promise based on the short-term results25. Whether this translates into improved outcomes for these patients needs to be elucidated.
SURGICAL TECHNIQUES AND OUTCOMES
Surgical aortic valve repair or replacement is considered the gold standard therapy for patients with severe symptomatic AR. In general, surgical intervention leads to an improved quality of life, symptom relief, improved cardiac function, a reduced risk of heart failure, and very good long-term survival. In a large study of 1,417 patients with preserved ejection fraction, the survival of patients who underwent aortic valve surgery was similar to that of an age- and sex-matched US population without a history of AR, while non-treated patients showed much worse survival at 10-year follow-up (13% mortality vs 29% mortality; log-rank p<0.001)26. Current European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines recommend aortic valve surgery in symptomatic patients or those with depressed LVEF as a Class I indication. They also recommend pre-emptive aortic valve surgery in asymptomatic patients with preserved LVEF in the setting of a dilatated left ventricle (left ventricular end-systolic diameter [LVESD] >50 mm [Class I indication] or indexed LVESD >20 mm/m2 [Class IIb indication])22.
In the past decade, the surgical treatment of AR has evolved from a strategy that was focused mainly on aortic valve replacement to one focused on aortic valve repair. In experienced centres, repair is preferred over replacement whenever the aortic valve can be salvaged. Nevertheless, it should be noted that complex aortic valve repair is much dependent on the experience and skills of the operator, and in the absence of such skills, aortic valve replacement should be preferred.
The aim of aortic valve repair is to preserve the native valve structure and to restore leaflet coaptation. Several surgical techniques can be used to achieve this aim. The choice of technique depends on the underlying cause and mechanism of regurgitation. In type I AR with normal cusp motion, ascending aorta dilatation or aortic root dilatation are the most common causes of disease. When the ascending aorta is dilatated and the sinotubular junction is preserved, grafting of the ascending aorta alone can be performed to increase leaflet coaptation. In cases of AR due to aortic root dilatation, valve-sparing root replacement (David procedure) or remodelling with or without subcommissural annuloplasty (Yacoub procedure) are the techniques of choice, with a tendency towards the David procedure due to its better long-term results27. Recently, subcommissural annuloplasty with specially designed rings for tricuspid and, even more frequently, for bicuspid valves has emerged as a new technique to stabilise the aortic valve annulus and to prevent further annular dilatation28. Patch repair using autologous or bovine pericardial tissue is used in cases of leaflet perforation.
In type II AR with excessive cusp motion, leaflet prolapse is the common cause of disease. Various techniques can be used and combined to restore leaflet function including free margin plication, free margin resuspension and even triangular leaflet resection in cases with excessive leaflet tissue; subcommissural annuloplasty can be considered to stabilise the repair.
In type III AR with restricted cusp motion, leaflet repair usually affords modification and mobilisation of the restricted parts of the cusps by removal of fibrotic tissue (shaving) or partial decalcification. Figure 1 gives an illustrative overview of the most commonly used aortic valve repair techniques according to the mechanisms of AR.
In patients with bicuspid aortic valves, the same techniques for repair are used, but operative complexity is increased. Phenotyping of the bicuspid valve is an important part of the operative strategy and helps to predict the long-term durability of the repair. In general, the repair of a symmetrical phenotype (type A) is easier and related to better long-term outcomes than the repair of a very asymmetrical phenotype (type C)29 (Figure 6). Bicuspidisation is the technique of choice in unicuspid valves but should be used in selected cases only because of the unproven long-term durability of the repair30.
It is important to note that individual patient outcomes after aortic valve repair can vary, and the long-term success depends on a variety of factors such as the underlying cause of the AR, the surgical technique employed, the skill of the surgical team, and the quality of the repair itself. In cases where the repair is successful and the valve is effectively restored to proper function, the recurrence rate of significant AR is low, ranging from 5% to 15% over the long term3132.
When the aortic valve shows an adverse phenotype for repair, or the leaflet tissue is damaged in a way that does not allow for repair, SAVR is the treatment of choice. Isolated SAVR for AR is an easy and straightforward operation that carries a very low procedural risk. Biological tissue valves are most commonly used, and mechanical prostheses are reserved for young patients with very long life expectancy. As for SAVR for aortic stenosis, it is of utmost importance that accurately sized valves with proven long-term durability are implanted to avoid patient-prosthesis mismatch (PPM) and to allow for future valve-in-valve procedures using TAVI devices. Usually, the aortic annulus in patients presenting with pure AR is larger, compared to in patients with aortic stenosis, and allows for sufficient prosthetic valve sizes. Nevertheless, in smaller patients, and women in particular, surgical aortic root enlargement should be part of the surgical armamentarium to avoid PPM. With SAVR, and in the absence of calcifications, particular care has to be taken when placing the stitches at the right and non-coronary sinuses to protect the conduction system and avoid the need for postoperative pacemaker implantation.
In recent years, minimally invasive surgical techniques have been developed for isolated aortic valve operations and can now be considered standard of care in the absence of contraindications. These approaches involve much smaller incisions, either through partial sternotomy or lateral minithoracotomy, and often result in quicker recovery times, faster wound healing, fewer access site infections and a reduced hospital stay, compared to a traditional full sternotomy33.
Despite the fact that surgical aortic valve repair or replacement is the gold standard treatment for severe symptomatic aortic stenosis, several factors can influence the decision to recommend surgery, and a careful patient-specific assessment of the potential risks and benefits is needed. A well-functioning Heart Team, including cardiac imaging specialists, interventional cardiologists and cardiac surgeons, provides the best possible care tailored to the individual patient’s needs. Early intervention before the onset of heart failure is crucial for the best possible long-term outcome.
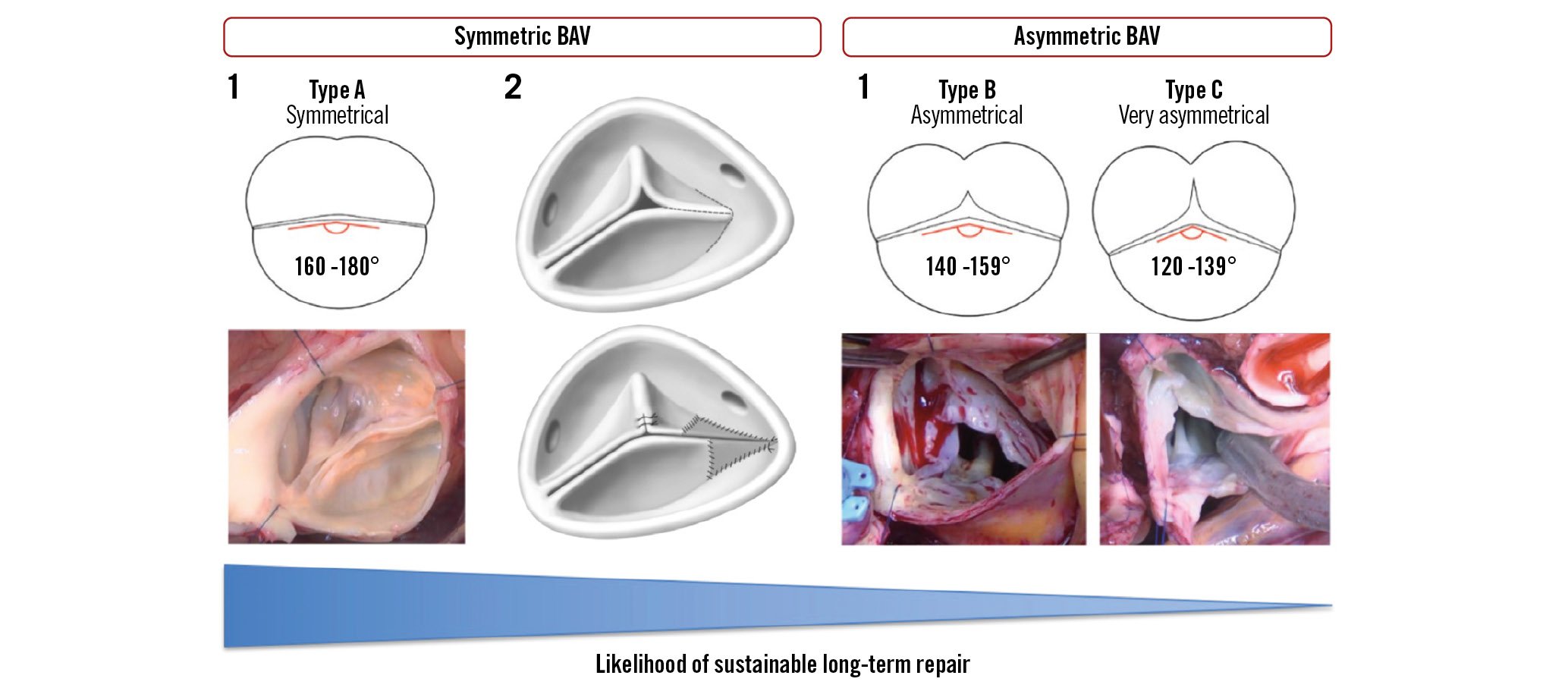
Figure 6. Phenotypes of bicuspid aortic valves and related repair techniques. BAV: bicuspid aortic valve
Transcatheter treatment and outcomes
Several studies have revealed an undertreatment of AR. Only 25-30% of patients with severe symptomatic AR underwent surgical treatment343536. The predominant reasons were advanced age, female sex, high surgical risk, and severe left ventricular systolic dysfunction. The undertreatment of severe AR needs to be targeted in order to offer surgical or interventional care to more patients.
TAVI provides an attractive alternative to surgery, but the current devices are commonly designed and indicated for the treatment of aortic stenosis (AS), as they rely on calcification of the native valve leaflets for anchoring. In these patients, TAVI has proven to be a standard treatment with high success and low complication rates3738. Table 2 shows a summary of studies using transcatheter heart valves (THV) for AR.
CT has become an indispensable tool for TAVI procedural planning. The measurement of annular size allows accurate selection of an appropriately sized prosthesis39. Additionally, CT enables the prediction of complications, such as coronary obstruction, and vascular access planning40.
Table 2. Characteristics and outcomes of transcatheter aortic valve implantation studies.
| Author (year) | Poletti, et al (2023) 49n=201 | Anwaruddin, et al (2019) 48n=230 | De Backer, et al (2018) 46n=254 | Yoon, et al (2017) 42n=331 | Sawaya, et al (2017) 47n=78 | ||||
|---|---|---|---|---|---|---|---|---|---|
| THV generations | New | New149 | Early81 | New145 | Early109 | New212 | Early119 | New41 | Early37 |
| Patient characteristics | |||||||||
| Mean age, years | 79 | 69 | 68 | 75 | 74 | 75 | 74 | 74 | |
| Female sex | 45 | 46 | 33 | 51 | 40 | 51 | 43 | 41 | |
| Mean STS Predicted Risk of Mortality | 5.1 | 8.6 | 8.6 | 6.2 | 6.9 | 6.2 | 7.6 | 6.7 | |
| Number and type of valves implanted | 132 SE,69 BE (incl. 21 JenaValve1 TA) | 149 SE | 81 SE | 133 SE,12 BE (incl. 34 JenaValve1 TA) | 103 SE, 6 BE | 171 SE,41 BE (incl. 64 JenaValve1 TA,1 J-Valve2 TA) | 110 SE, 9 BE | 40 SE,1 BE (incl. 23JenaValve1 TA) | 33 SE, 4 BE |
| Device success | 76 | 87 | 72 | 82 | 47 | 81 | 61 | 85 | 54 |
| Residual AR >moderate severity | 9.5 | 6.3 | 19.1 | 4 | 31 | 4.2 | 18.8 | 3 | 27 |
| Transcatheter migration or embolisation | 12.4 | 7 | 14 | 6 | 17 | n.a. | n.a. | n.a. | n.a. |
| Need for second valve | 10.5 | 10.1 | 33.3 | 8 | 33 | 12.7 | 24.4 | 10 | 24 |
| Conversion to surgery | 2.0 | n.a. | n.a. | n.a. | n.a. | 3.8 | 3.4 | n.a. | n.a. |
| Postprocedural mean gradient, mmHg | 6.7 | 6.8 | n.a. | n.a. | 10.2 | 7.7 | n.a. | n.a. | |
| New pacemaker implantation | 22.3 | 20 | 18 | n.a. | n.a. | 18.6 | 17.5 | 18 | |
| Stroke/transient ischaemic attack | 1.5 | 6 | 0 | 4 | 3 | 5.7 | 1.7 | 3 | 5 |
| Major vascular complication | 7.5 | 1 | 1 | 5 | 7 | 3.3 | 5.9 | 8 | 8 |
| Major bleeding | 10.6 | 8 | 14 | 1* | 5* | 6.1 | 10.1 | 3* | 3* |
| 30-day mortality | 5# | 10 | 19 | 8 | 17 | 9 | 13 | 8 | 22 |
| Data are presented as %, unless otherwise stated. #In-hospital mortality. *Life-threatening bleeding. 1JenaValve. 2JC Medical. AR: aortic regurgitation; BE: balloon-expandable; incl.: including; n.a.: not available; SE: self-expanding; STS: Society of Thoracic Surgeons; TA: transapical; THV: transcatheter heart valve | |||||||||
OFF-LABEL DEVICES FOR TAVI IN PURE AORTIC REGURGITATION
In patients with pure AR, there is often little or no valvular calcification. Furthermore, aortic root dilatation, large stroke volumes and left ventricular outflow tract (LVOT) shape can create challenges in positioning and anchoring the prosthesis. Oversizing of the valve is a routine technique to overcome these issues, but the procedure remains technically challenging and is associated with poor outcomes414243.
In particular, safety and efficacy parameters like the need for a second valve (10-33%), device success (47-77%) and residual AR ≥moderate (9-31%) are not acceptable compared to outcomes in AS patients4244.
Although new-generation TAVI devices are associated with better outcomes in patients with pure AR, results in off-label implantation are still not comparable to those in AS patients. Device success remains below 87%, with a rate of about 3% of conversion to open surgery and 8-13% of cases requiring a second valve implantation. Furthermore, studies report a 3-10% rate of moderate or greater paravalvular AR and a 30-day mortality rate of 5-10%4245464748. These results are confirmed in the very recent PANTHEON trial that presents the outcomes of patients with pure AR who were treated with the latest-generation off-label TAVI devices (90%) and JenaValve THV (10%), showing an overall device success rate of just 76.8%49. Consequently, TAVI for patients with AR has remained an off-label procedure, establishing a clear need to develop a dedicated THV that provides safe anchoring and, therefore, could improve results in patients with pure AR.
DEDICATED TRANSCATHETER HEART VALVES FOR PURE AORTIC REGURGITATION
To overcome the technical challenges and improve outcomes for patients with AR, dedicated TAVI systems using a transapical approach have been designed in the past: the JenaValve and the J-Valve (JC Medical). Both devices showed improved procedural success rates, with over 96%5051 and 93%52, respectively, in patients with pure AR, and reduced paravalvular regurgitation to mild or better at 30 days in all treated patients. Nevertheless, due to their “transapical only” access, these systems did not reach mainstream adoption and are no longer available to the market. Both systems have since been completely redesigned for transfemoral use.
TRANSFEMORAL TAVI DEVICES
THE TRILOGY SYSTEM
The first-in-human implantation of the novel JenaValve device was performed successfully in 2017 using a transfemoral approach in a patient with pure AR53. The newest iteration, the Trilogy valve (JenaValve), received European conformity (CE) marking for the treatment of AR and AS in 2021, and thus is the first commercially available dedicated TAVI system for the treatment of patients with pure AR.
The self-expanding bioprosthesis consists of a nitinol frame with 3 porcine pericardial valve leaflets (Figure 7). The unique feature of the Trilogy valve is the locator technology which provides perfect positioning in the native valve and safe anchoring in the annulus39. The 3 radial locators are positioned under fluoroscopy or transoesophageal echocardiography in each aortic root sinus, and thus, commissural alignment can be reliably achieved54. Coronary access is further facilitated by 3 large open cells at the top of the prosthesis. The technology also limits deep implantation and therefore decreases the risk of protrusion into the LVOT or migration into the left ventricle. Additionally, a “natural” paravalvular seal results during the final deployment step, with the locators clipping onto the native leaflets and pinching them to the stent frame (Figure 7).
The Trilogy device is advanced through an 18 Fr, 85 cm long preshaped introducer sheath that extends through the aortic arch. The delivery system includes a feature to rotate the valve to allow commissural alignment of the locators and engagement with each cusp (Figure 8). The prosthesis is available in 3 sizes (23 mm, 25 mm, and 27 mm) which allows treatment of annular diameters up to 28.6 mm39.
The first clinical results of the transfemoral Trilogy prosthesis in a German, multicentre, real-world cohort have just been published. The authors showed remarkable outcomes for the system in 58 patients with pure AR (36% female, mean age 76.5 years, European System for Cardiac Operative Risk Evaluation [EuroSCORE] II 6.1%), resulting in 98% device success and 96% of patients with no or trace paravalvular AR at 30 days55. Furthermore, the data showed a low 30-day mortality rate of only 1.7% and promising haemodynamic performance with a mean aortic valve gradient of 4.3 mmHg. In nearly 20% of patients, a new pacemaker was required following the procedure.
These encouraging results were confirmed in a recent conference presentation from the US clinical ALIGN-AR Pivotal Trial56. In 180 patients with severe AR (47% female, mean age 75.5 years, Society of Thoracic Surgeons [STS] score 4.1%), the predefined endpoints for safety at 30 days and efficacy at 12 months were met. Technical success was 95%, all-cause mortality at 30 days was 2.2%, and there was no or trace paravalvular regurgitation in 92.2% of the patients at 12 months. The pacemaker implantation rate was 24% at 30 days, and the mean gradient was 4.3 mmHg at 12 months with an effective orifice area of 2.8 cm257.
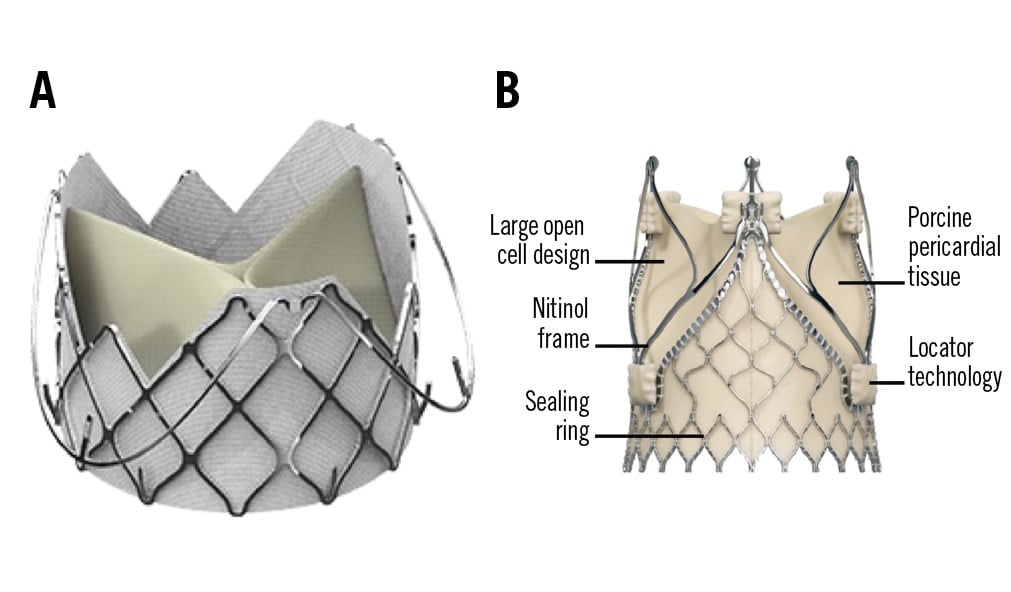
Figure 7. Transfemoral transcatheter heart valves. A) J-Valve prosthesis (JC Medical). B) Trilogy prosthesis (JenaValve).
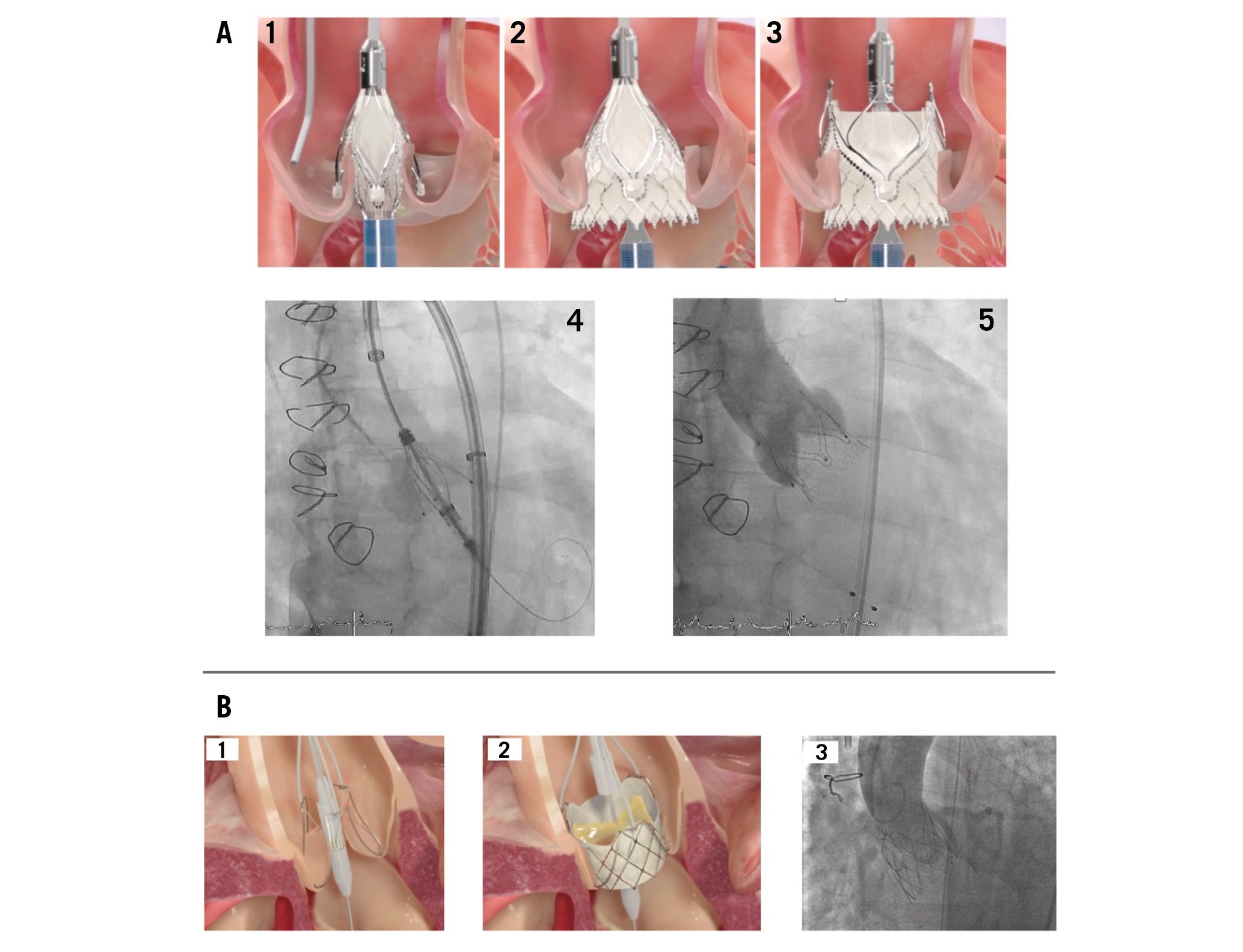
Figure 8. Deployment of transcatheter heart valves. A) Deployment of the Trilogy THV: 1. Locators align the THV with the native cusps; 2. Inflow is deployed, with limited protrusion; 3. Outflow is deployed: locators “clip” onto native leaflets, forming a seal and stable securement; 4. THV with locators spread during implantation, seating the locators in the sinuses; 5. Valve after implantation with secure anchoring and no paravalvular regurgitation. B) Deployment of the J-Valve THV: 1. Anchor rings grasp the native leaflets in correct anatomical alignment; 2. The THV is deployed, anchoring in the annulus; 3. The THV after implantation with full expansion and no aortic regurgitation. THV: transcatheter heart valve
THE J-VALVE
A similar anchoring concept to that used for the Trilogy prosthesis is used for the J-Valve. This prosthesis consists of 3 bovine pericardial leaflets in a self-expanding, low-profile nitinol frame with 3 U-shaped “anchor rings” (Figure 7). It is available in 5 sizes (22, 25, 28, 31, and 34 mm) to treat a wide range of anatomies, with an annular diameter of up to 33.1 mm.
The valve is advanced using an 18 Fr delivery system. The deployment is performed in 2 steps. First, the anchor rings are opened and advanced into the aortic sinuses for commissural alignment. Second, the THV frame is opened and cinched against the clasped native leaflets, leading to safe anchoring in the annulus58 (Figure 8).
The first transfemoral implantation was performed successfully in 201859. Just recently, data were published from a North American multicentre compassionate use registry with 27 pure AR patients (41% female, median age 81 years, STS score 4.3%), showing no moderate or severe AR at 30 days and overall good haemodynamic performance. General outcomes were satisfactory with a 30-day mortality rate of 4% and a stroke rate of 4%, as well as a need for new pacemaker implantation in 13% of the patients. Nevertheless, device success was just 81% in the whole series (1 patient with a second valve needed, 2 patients with conversion to surgery), which led to changes in valve design and improvement in later cases58. A recently announced early feasibility study will provide insights on safety and efficacy in larger patient cohorts.
Unmet needs/gaps in research
AR is significantly undertreated, with 1 report demonstrating that only 1 in 4 patients with severe symptomatic AR received SAVR within 1 year of diagnosis. Women and older patients were less likely to be treated and consequently had a high mortality rate at 1 year (24%)36. This disparity in treatment, along with its underutilisation, needs to be urgently addressed. The timing of valve replacement is key to preventing cardiac damage/dysfunction and balancing the benefits of treatment with the potential risks. Indications for valve replacement are based on symptoms, severity of AR, left ventricular dysfunction or dilatation22. However, further research needs to establish whether specific patient cohorts may benefit from aortic valve replacement, such as those with moderate AR and heart failure, different metrics of impaired left ventricular function (global longitudinal strain), and the presence of late gadolinium enhancement. In order to do so, our understanding of the disease and its interaction with the myocardium requires more research.
Given the invasive nature and recognised complications associated with SAVR, which restrict its use, and the suboptimal results with conventional TAVI, there is a need for a dedicated percutaneous technology for the treatment of AR. However, thus far, evidence of the safety and efficacy of this dedicated percutaneous technology is limited to small case series39586061, one larger series49 and a trial57. Although outcomes in both the larger series and the trial were excellent, there might be a selection bias due to only suitable anatomies being treated (e.g., cases with large annuli or extreme angulation of the aorta had to be excluded). Studies with longer follow-up are needed to confirm the safety and efficacy of the technology.
One debatable point is the high rate of permanent pacemaker implantation following TAVI with dedicated systems for pure AR (19.6% with Trilogy, 13% with J-Valve), compared to after TAVI for patients with AS. It seems that a class effect must be considered, since rates are comparable to those with off-label TAVI devices for pure AR, which range from 18-22%424749. Reasons for this outcome might include ventricular and LVOT dilatation, a higher prevalence of underlying conduction disturbances, as well as device-related and technical characteristics like oversizing or implantation depths. In fact, with changes in implant technique and oversizing strategy, the pacemaker implantation rate declined from an initial 30% in the first 60 patients to 14% in the last 60 patients, as shown in the Trilogy ALIGN-AR Pivotal Trial57. Permanent pacemaker implantation following SAVR is less common, at 3-11.5%6263, but still higher compared to rates after surgery for AS, underlining a combined effect of anatomical and procedural factors.
Additionally, long-term valve durability, rates of structural valve degeneration and hypoattenuated leaflet thrombosis (HALT) will be important to evaluate. If the transcatheter system is used in younger patients with longer lifespans, consideration will need to be given to lifetime management. Is it possible to perform a valve-in-valve TAVI procedure? If so, which prosthesis would provide optimal haemodynamics and reduce the risk of coronary obstruction?
Three-dimensional printing and patient-specific computer simulation may have a role to play in guiding treatment for AR by enabling the prediction of procedural complications and optimising prosthesis sizing. Largely used in patients with aortic stenosis, both technologies can be particularly useful in complex anatomies, such as patients with bicuspid aortic valves6465. Further studies are required to evaluate their role in patients with AR.
Conclusions
Aortic regurgitation is an increasingly common disease with a significant clinical burden. Advances in imaging have led to a better understanding of its natural history, the identification of novel prognostic markers and improved quantification of severity and cardiac remodelling. Surgical intervention is the gold standard for treatment and is appropriate for those at low and intermediate surgical risk. Patients at higher risk have the option of transcatheter aortic valve implantation. Although procedural success has been suboptimal with commonly available prostheses, dedicated devices are leading to improved safety and efficacy for patients and are necessary to provide a viable alternative to SAVR. However, the technology is in its infancy and used in a select subpopulation of patients. Further real-world evidence is required before its widespread adoption. A well-functioning Heart Team is mandatory to assess the patient’s clinical and anatomical characteristics in order to tailor their treatment options.
Conflict of interest statement
A. Baumbach has received speaker fees from Abbott, Medtronic, and MicroPort; and has been a proctor for JenaValve. K.P. Patel has received an unrestricted research grant from Edwards Lifesciences. V. Delgado has received speaker fees from Edwards Lifesciences, GE HealthCare, Novartis, and Philips; and has received consulting fees from Edwards Lifesciences, Novo Nordisk, and Merck & Co. T.K. Rudolph has been a proctor and medical advisor for JenaValve; she has received speaker fees from Edwards Lifesciences, Boston Scientific, Medtronic, and JenaValve. H. Treede has been a proctor and advisor for JenaValve. A.R. Tamm has been a proctor for the Trilogy system.

