Cory:
Unlock Your AI Assistant Now!
The value of intravascular imaging (IVI) to improve clinical outcomes after left main (LM) revascularisation has been the subject of multiple studies, with some attempting to identify specific minimum stent area (MSA) targets123.
It is worth noting that it is not just a matter of using IVI but of using it in an actionable way and oriented towards achieving certain targets, since the observable long-term prognostic benefit will depend on this4.
The group of investigators behind the study discussed in this editorial pioneered in defining these optimisation criteria, identifying an easy numerical series 5-6-7-8 mm2 for the MSA cutoff values in the ostial left circumflex (LCx), ostial left anterior descending (LAD), LM polygon of confluence, and LM above the polygon of confluence, respectively1.
These threshold values for MSA, derived from an Asian population, were notably lower than those found in a trial conducted in a Western population: 6, 7, and 10 mm2 for the LCx, LAD, and LM, respectively2.
Recently, the same authors revised the previous MSA criteria based on the 5-year clinical outcomes in patients undergoing upfront LM two-stenting. The MSA cutoff values for predicting major adverse cardiovascular events (MACE) were upgraded to 5.7, 8.3, and 11.8 mm2 for the LCx, LAD, and distal LM, respectively3.
In this issue of EuroIntervention, Kim et al5 describe a study they conducted including 829 consecutive patients with LM disease who underwent intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) with a single-stent crossover technique from the LM to the LAD. The MSA cutoff values best predicting 5-year MACE were 11.4 mm2 for the proximal LM, 8.4 mm2 for the distal LM, and 8.1 mm2 for the LAD ostium. Based on these cutoff values, sten t underexpansion in the proximal LM was significantly associated with an increased risk of 5-year MACE. Additionally, patients with simultaneous stent underexpansion in both the distal LM and LAD ostium exhibited a significantly higher risk of MACE compared with those having adequate expansion or only single-site underexpansion. The study is well executed and properly presented, as is typical of this research group.
One of the aspects that should be highlighted is the modest predictive value of the identified MSA cutoff values, with an area under the curve (AUC) ranging from 0.57-0.62. This may be explained by their inability to predict events originating in the LCx artery. In fact, the LCx was involved in 78% of target lesion revascularisation cases, and the ostial LCx was the only site involved in 57%.
That said, it would have been very relevant to analyse the predictive value of the minimum lumen area and/or plaque burden at the level of the LCx ostium as predictors of events. This information could even lead to the identification of factors that could recommend the upfront use of two stents. However, in this study, preintervention IVUS was available in 30.6% of patients and a final IVUS run from the LCx was carried out in only 5.6%. Nevertheless, the minimum lumen area in the LCx was smaller and the plaque burden higher in patients with MACE.
Another aspect to consider is the definition of stent underexpansion. Obviously, stent expansion is a parameter subject to different definitions, sometimes not easy to establish at the level of the LM due to the absence of adequate luminal reference areas. In this study, the stent expansion index was defined as the MSA divided by the vessel area, and it failed to show any predictive value. This definition of stent expansion is highly conditioned by the degree of plaque burden and remodelling at the lesion level and does not consider the value of the luminal area in healthy or less diseased reference sites. Thus, a low expansion index does not necessarily mean that a stent is underexpanded, since a very large plaque burden at a point with positive remodelling will always be associated with a low expansion index, even if the stent size is well selected and the stent is fully expanded.
Overall, MSA cutoff values have a certain, though modest, predictive value. Nevertheless, these have a much more limited value at the individual level since there is variability in the size of the coronary tree and in the distribution of disease that can condition the feasibility of reaching certain MSA cutoff points. Thus, I consider it most convenient to pursue a range of acceptable MSA values rather than a precise value and maybe combine stent expansion, which is a relative criterion, with MSA, which is an absolute criterion, to achieve a balance with the individualised anatomy of the patient (Figure 1).
The OPTIMAL trial (ClinicalTrials.gov: NCT04111770), currently in clinical follow-up, will be the first properly designed trial to provide definitive evidence on the clinical impact of IVUS guidance during LM PCI, as well as valuable information on optimisation targets6.
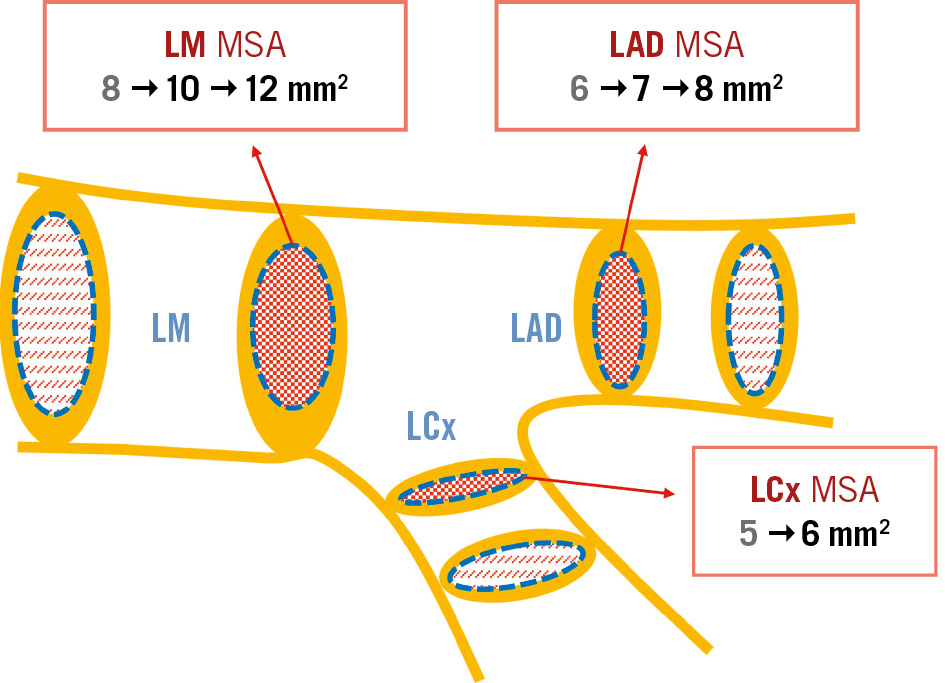
Figure 1. Evolution in the cutoff values for MSA identified as predictive of MACE after LM stenting. The most supported values are in bold. LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery; MACE: major adverse cardiovascular events; MSA: minimum stent area
Conflict of interest statement
J.M. de la Torre Hernandez discloses grants from Abbott and Biotronik; consulting fees from Medtronic, Philips, Abbott, and Daiichi Sankyo; and honoraria from Boston Scientific, Abbott, and Medtronic.

