Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Intracoronary imaging-guided percutaneous coronary intervention (PCI) has demonstrated clinical benefit over angiography-guided PCI for left main coronary artery (LM) disease. However, the optimal minimal stent area (MSA) thresholds to predict cardiovascular outcomes remain incompletely defined.
Aims: This study aimed to evaluate intravascular ultrasound (IVUS)-measured segmental MSA after LM crossover stenting.
Methods: We identified 829 consecutive patients who underwent IVUS-guided PCI for unprotected LM disease using a single-stent crossover technique. The final MSA was measured at the proximal LM, distal LM, and left anterior descending artery (LAD) ostium. The primary outcome was 5-year major adverse cardiac events (MACE), including all-cause death, myocardial infarction, and target lesion revascularisation.
Results: The MSA cutoff values best predicting 5-year MACE were 11.4 mm² for the proximal LM (area under the curve [AUC] 0.62), 8.4 mm² for the distal LM (AUC 0.58), and 8.1 mm² for the LAD ostium (AUC 0.57). Based on these cutoff values, stent underexpansion in the proximal LM was significantly associated with increased risk of 5-year MACE (adjusted hazard ratio [HR] 2.34; p<0.001). Additionally, patients with simultaneous stent underexpansion in both the distal LM and LAD ostium exhibited a significantly higher risk of 5-year MACE compared with those having adequate expansion or only single-site underexpansion (adjusted HR 2.57; p<0.001).
Conclusions: Achieving sufficient stent expansion in the proximal LM and preventing underexpansion in both the distal LM and LAD ostium are critical for improving long-term clinical outcomes. The identified MSA thresholds may serve as practical benchmarks for stent optimisation during LM PCI.
The advantages of using intracoronary imaging guidance during percutaneous coronary intervention (PCI) are most evident when treating patients with high-risk lesions123, particularly those with unprotected left main coronary artery (LM) disease, for which accumulating data suggest a mortality benefit over angiography guidance alone456. Current guidelines recommend the use of intravascular ultrasound (IVUS) during LM stenting to optimise PCI results by ensuring well-apposed and adequately expanded stents789. Although there is no standardised consensus on the definition of stent underexpansion10, the minimal stent area (MSA) assessed via IVUS is considered the most reliable predictor of future adverse events in post-PCI patients111213. However, the relationship between the MSA and cardiovascular outcomes in patients undergoing IVUS-guided PCI for unprotected LM disease has not been fully elucidated in the literature.
Previously, we proposed the “5-6-7-8” criteria for stent expansion in patients undergoing LM stenting to predict the risk of angiographic restenosis (i.e., soft endpoints)14. The study included a non-Western population that underwent either a single-stent (72%) or an upfront two-stent (28%) procedure. Recently, we revised these MSA criteria based on the 5-year clinical outcomes in patients undergoing upfront LM two-stenting using the crush technique15. The revised criteria suggested larger areas than previously proposed and showed similar MSA values to those from the Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial16. However, a knowledge gap remains regarding the optimal MSA threshold levels for LM PCI using a provisional one-stent strategy. Here, we investigated IVUS-derived segmental MSA cutoffs in patients who underwent LM crossover stenting to predict 5-year major adverse cardiac events (MACE).
Methods
Study population
The study included all consecutive patients with unprotected LM disease – regardless of bifurcation involvement or lesion location – who underwent IVUS-guided PCI using a single-stent crossover technique from the LM to the left anterior descending artery (LAD) with drug-eluting stent (DES) implantation, at Asan Medical Center, Seoul, Republic of Korea, between March 2005 and December 2022. The exclusion criteria were as follows: (1) patients who required a second stent at the left circumflex artery (LCx) ostium; (2) patients who underwent crossover stenting from the LM to the LCx; (3) patients with a history of coronary artery bypass grafting (CABG); and (4) patients with in-stent restenosis lesions in the LM. All study participants underwent a final post-stenting IVUS pullback from the LAD. The study protocol was approved by the Ethics Committee of Asan Medical Center, and all patients provided their written informed consent to participate in the study.
Study procedure
Coronary lesion severity was evaluated by visual assessment performed by two experienced interventional cardiologists. Significant stenosis was defined as a diameter narrowing of ≥50%. The extent of disease at the LAD and LCx ostia was assessed using the Medina classification system.
PCI was performed according to current clinical practice guidelines. The use of adjunctive devices and pharmacological agents, such as cutting balloons and rotational atherectomy, was left to the operator’s discretion. IVUS assessments prior to stent implantation were recommended. During the procedure, IVUS measurements guided the sizing of stents and the selection of post-dilation balloons. Additionally, repeated IVUS assessments during adjunctive post-dilation were recommended to ensure complete stent apposition and optimal expansion.
Intravascular ultrasound analysis
The final post-stenting IVUS imaging and offline IVUS analyses were performed as previously described1415. The final MSA within the prespecified segments was assessed, including the LAD ostium (5 mm distal to the carina), distal LM segment (5 mm proximal to the carina), and proximal LM (proximal segment of the stent). The cross-sectional area of the external elastic membrane at the MSA site was measured using two-dimensional planimetry and defined as the vessel area. The stent expansion index was defined as the MSA divided by the vessel area10.
Study outcomes
The primary outcome was 5-year MACE, defined as a composite of all-cause death, target lesion-related myocardial infarction (MI), and clinically driven target lesion revascularisation (TLR). Cardiovascular death and the individual components of the primary outcome comprised the secondary outcomes. Unless an incontrovertible non-cardiovascular cause was identified, all deaths were classified as cardiovascular deaths. MI was defined as elevated cardiac biomarker levels with concomitant ischaemic symptoms or signs and was supported by documentation from non-invasive (electrocardiography or imaging) or invasive (coronary angiography) examinations. Events not related to the index PCI but attributable to the target lesion (i.e., the LM ostial, shaft, or bifurcation segments) were classified as target lesion-related MI. LM-related TLR was defined as revascularisation for LM restenosis, involving the proximal or distal segments (within 5 mm) adjacent to the LM-to-LAD stent and the LCx ostium (within 5 mm distal to the carina). Isolated in-stent restenosis in the distal segments without ostial LAD involvement was not considered LM-related TLR. Any surgical revascularisation for LM restenosis was also classified as TLR.
Follow-up evaluations were performed at 1, 6, and 12 months post-PCI, and then annually through in-office visits or telephone calls. Clinical data were gathered from the prospective ASAN-MAIN registry by independent personnel at the Clinical Research Center, Asan Medical Center, Seoul, Republic of Korea, using a prespecified electronic case report form. All clinical outcomes of interest were validated using the collected source documentation and adjudicated by an independent group of clinicians who were blinded to both the initial PCI procedures and post-stenting IVUS images.
Statistical analysis
Categorical data are shown as counts and percentages, whereas continuous variables are presented as means and standard deviations or medians and interquartile ranges (IQRs), as deemed suitable. Group comparisons were conducted using either a parametric unpaired t-test or a non-parametric Mann-Whitney U test for continuous variables. Categorical variables were compared using either the χ2 test or Fisher’s exact test. The optimal cutoff values for the final MSA that accurately predicted the primary outcome were obtained by examining time-dependent receiver operating characteristic curves. A restricted cubic spline curve was generated to analyse the correlation between the MSA within each segment, treated as a continuous variable, and the unadjusted risk of the primary outcome. Cumulative occurrences were calculated using the Kaplan-Meier method and compared using log-rank tests.
Additionally, a Cox proportional hazards model analysis was performed to obtain the hazard ratio (HR) and 95% confidence intervals (CIs) for each study outcome. Patients were censored either at the time of the incident or on the date of the last follow-up, up to 5 years after the index PCI. The Schoenfeld residuals test validated the proportional hazards assumption, with no significant violations detected. Model 1 was adjusted for age, body mass index, body surface area, diabetes mellitus, chronic kidney disease, peripheral artery disease, and a left ventricular ejection fraction (LVEF) ≤50%. Model 2 included all covariates from model 1, with simultaneous adjustment for both MSA and the stent expansion index within each specific segment separately. Model 3 included all covariates from model 1, with concurrent adjustment for MSA from all three segments together, without considering the stent expansion index. Model 4 included all covariates from model 1, with additional adjustment for underexpansion in the proximal LM and underexpansion in both the distal LM and LAD ostium. Continuous variables (age, body mass index, body surface area, and MSA measurements) were standardised using Z-score transformation to calculate standardised HRs, representing the effect of a 1-standard deviation increase in each variable. None of these variables exhibited multicollinearity in the variance inflation factor analysis. Statistical analyses were conducted using R statistical software, version 4.4.2 (R Foundation for Statistical Computing). Two-sided results were considered statistically significant at a significance level of p<0.05.
Results
The data supporting the findings of this study are available from the corresponding author upon request.
Study population
A total of 879 patients underwent IVUS-guided PCI for unprotected LM disease using a provisional one-stent strategy at Asan Medical Center between March 2005 and December 2022. Of these, 50 patients who required a second stent in the LCx ostium were excluded. Consequently, 829 patients who underwent a single-stent LM-to-LAD crossover and had complete post-stenting IVUS images from the LAD pullback were included in the final analysis (Supplementary Figure 1).
The clinical characteristics of the study population are summarised in Table 1. The mean age of the overall population was 64.2±10.2 years. Among the patients, 79.0% were male, and 37.9% had acute coronary syndrome as the clinical indication for the index PCI. The mean LVEF was 60.0±7.7%, with 7.6% of patients having an LVEF ≤50%. Coronary angiography revealed the extent of disease as follows: 3.4% LM only, 35.5% LM with 1-vessel disease, 34.9% LM with 2-vessel disease, and 26.3% LM with 3-vessel disease. The LM lesion was located in the ostium or midshaft in 26.9% of cases and at the distal bifurcation in 73.1%. The majority of patients (75.9%) had Medina 1,1,0 lesions, while angiographically significant LCx ostial involvement was identified in 19.9% of cases. Right coronary artery disease was present in 45.5% of patients.
When comparing patients with and without MACE at 5-year follow-up, significant differences were noted in several parameters. Patients with MACE were older, had a lower body mass index, and had a lower body surface area. They also had a higher incidence of comorbidities, including heart failure, cerebrovascular accidents, peripheral artery disease, chronic kidney disease, and atrial fibrillation. Additionally, the mean LVEF was lower in patients with MACE, with a higher proportion exhibiting an LVEF ≤50%.
Table 1. Clinical characteristics.
| Characteristics | Overall population (N=829) | Major adverse cardiac events | p-value | |
|---|---|---|---|---|
| No (N=722) | Yes (N=107) | |||
| Demographics | ||||
| Age, years | 64.2±10.2 | 63.4±9.9 | 69.8±10.3 | <0.001 |
| Male sex | 655 (79.0) | 574 (79.5) | 81 (75.7) | 0.439 |
| BMI, kg/m2 | 24.5±3.0 | 24.6±3.0 | 23.6±2.6 | 0.001 |
| BSA*, m2 | 1.72±0.2 | 1.72±0.2 | 1.67±0.2 | 0.001 |
| Acute coronary syndrome | 306 (37.9) | 256 (36.6) | 50 (46.7) | 0.056 |
| Medical history | ||||
| Current smoker | 196 (23.6) | 179 (24.8) | 17 (15.9) | 0.098 |
| Hypertension | 577 (69.6) | 492 (68.1) | 85 (79.4) | 0.024 |
| Diabetes | 295 (35.6) | 248 (34.3) | 47 (43.9) | 0.068 |
| Dyslipidaemia | 627 (75.6) | 549 (76.0) | 78 (72.9) | 0.558 |
| History of MI | 54 (6.5) | 42 (5.8) | 12 (11.2) | 0.057 |
| History of PCI | 137 (16.5) | 112 (15.5) | 25 (23.4) | 0.057 |
| History of HF | 18 (2.2) | 9 (1.2) | 9 (8.4) | <0.001 |
| History of CVA | 59 (7.1) | 41 (5.7) | 18 (16.8) | <0.001 |
| History of PAD | 41 (4.9) | 24 (3.3) | 17 (15.9) | <0.001 |
| Chronic kidney disease | 31 (3.7) | 11 (1.5) | 20 (18.7) | <0.001 |
| Chronic lung disease | 14 (1.7) | 12 (1.7) | 2 (1.9) | 1.000 |
| Atrial fibrillation | 11 (1.3) | 7 (1.0) | 4 (3.7) | 0.058 |
| Echocardiography | ||||
| LVEF, % | 60.0±7.7 | 60.7±6.8 | 55.2±11.3 | <0.001 |
| LVEF ≤50% | 63 (7.6) | 42 (5.8) | 21 (19.6) | <0.001 |
| Coronary angiography | ||||
| Disease extent | 0.016 | |||
| LM only | 28 (3.4) | 27 (3.7) | 1 (0.9) | |
| LM with 1-vessel disease | 294 (35.5) | 265 (36.7) | 29 (27.1) | |
| LM with 2-vessel disease | 289 (34.9) | 252 (34.9) | 37 (34.6) | |
| LM with 3-vessel disease | 218 (26.3) | 178 (24.7) | 40 (37.4) | |
| LM lesion location | 0.182 | |||
| Ostium or midshaft | 223 (26.9) | 188 (26.0) | 35 (32.7) | |
| Distal bifurcation | 606 (73.1) | 534 (74.0) | 72 (67.3) | |
| Medina classification | 0.332 | |||
| 1,1,1 | 165 (19.9) | 137 (19.0) | 28 (26.2) | |
| 1,1,0 | 629 (75.9) | 554 (76.7) | 75 (70.1) | |
| 1,0,0 | 32 (3.9) | 28 (3.9) | 4 (3.7) | |
| 0,1,0 | 3 (0.4) | 3 (0.4) | 0 (0) | |
| Right CAD | 377 (45.5) | 315 (43.6) | 62 (57.9) | 0.008 |
| Values are presented as numbers (percentages) or means±standard deviation. *BSA was calculated using the Mosteller formula. BMI: body mass index; BSA: body surface area; CAD: coronary artery disease; CVA: cerebrovascular accident; HF: heart failure; LM: left main coronary artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PAD: peripheral artery disease; PCI: percutaneous coronary intervention | ||||
Procedural characteristics
The procedural characteristics of the study population are summarised in Table 2. An intra-aortic balloon pump or extracorporeal membrane oxygenation was used in 2.3% of the study population. Direct stenting was performed in 21.4% of cases. The mean total number of stents used per patient was 2.0±1.2, and the mean total length of stents was 50.8±29.6 mm. For LM stenting, the mean stent diameter was 3.6±0.4 mm, and the mean stent length was 27.2±7.3 mm. Final kissing balloon inflation was performed in 11.4% of procedures. Regarding the type of DES used, 15.2% were first-generation stents, and 84.8% were second- or newer-generation stents.
Table 2. Procedural characteristics.
| Characteristics (N=829) | Overall population | Major adverse cardiac events | p-value | |
|---|---|---|---|---|
| No (N=722) | Yes (N=107) | |||
| Use of IABP or ECMO | 19 (2.3) | 12 (1.7) | 7 (6.5) | 0.005 |
| Direct stenting | 141 (21.4) | 124 (21.5) | 17 (20.5) | 0.947 |
| Total stent number (per patient) | 2.04±1.2 | 2.04±1.1 | 2.07±1.2 | 0.756 |
| Total length of stents, mm | 50.8±29.6 | 50.8±29.5 | 51.2±30.3 | 0.898 |
| LM-to-LAD crossover stent | ||||
| Stent diameter, mm | 3.62±0.4 | 3.63±0.4 | 3.58±0.3 | 0.217 |
| Length of stents, mm | 27.2±7.3 | 27.1±7.4 | 27.6±7.0 | 0.535 |
| Final kissing balloon inflation | 94 (11.4) | 86 (12.0) | 8 (7.5) | 0.229 |
| Drug-eluting stent type | 0.826 | |||
| First-generation | 126 (15.2) | 111 (15.4) | 15 (14.0) | |
| Second- or newer-generation | 703 (84.8) | 611 (84.6) | 92 (86.0) | |
| Values are presented as numbers (percentages) or means±standard deviation. ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; LAD: left anterior descending artery; LM: left main coronary artery | ||||
Post-stenting minimal stent area and clinical outcomes
The mean MSA was 11.9±2.5 mm² in the proximal LM, 10.1±2.2 mm² in the distal LM, and 8.7±1.9 mm² at the LAD ostium in the overall population (Supplementary Table 1). Supplementary Figure 2 illustrates the final MSA distribution within each segment, along with the corresponding median values and IQRs. To predict 5-year MACE, the MSA cutoff value for each segment was 11.4 mm2 for the proximal LM (area under the curve [AUC] 0.62), 8.4 mm2 for the distal LM (AUC 0.58), and 8.1 mm2 for the LAD ostium (AUC 0.57) (Supplementary Figure 3). Using these MSA criteria, 46.2%, 19.2%, and 41.1% of the patients had stent underexpansion in the proximal LM (<11.4 mm2), distal LM (<8.4 mm2), and LAD ostium (<8.1 mm2), respectively.
The primary and secondary outcomes at 5 years are summarised in Table 3 and Supplementary Table 2. The median follow-up was 5.7 years (IQR 4.2-9.3 years). The primary outcome, MACE at 5 years, was observed in 107 patients when only the first event was counted in patients with multiple events. A gradual linear relationship between the unadjusted risk of 5-year MACE and the MSA within each segment was evident using the spline regression model (Supplementary Figure 4). Figure 1 illustrates the cumulative incidence of MACE and all-cause death according to stent underexpansion within each segment. Compared with patients with adequate stent expansion, those with underexpansion in the proximal LM showed increased risks of 5-year MACE (log-rank p<0.001) and all-cause death (log-rank p=0.013).
Patients with stent underexpansion in both the distal LM and LAD ostium (group 2) showed the highest rate of 5-year MACE (24.2%) compared with those who had stent underexpansion in either the distal LM or LAD ostium (group 1) and those who had no underexpanded segments in either the distal LM or LAD ostium (group 0) (Central illustration). Compared with group 0, group 2 demonstrated significantly increased risks of 5-year MACE (adjusted HR 2.34; p<0.001) (Figure 2A), all-cause death (adjusted HR 1.81; p=0.04) (Figure 2B), and clinically driven TLR (adjusted HR 4.30; p<0.001) (Figure 2C).
Of the 33 patients who underwent clinically driven TLR (at a median of 450 days), 2 patients required CABG, while the remaining 31 patients underwent PCI with DES implantation (n=22), drug-coated balloon (DCB; n=8), or thrombus aspiration for acute stent thrombosis in the LM shaft (n=1). Among these TLR cases, 26 involved ostial LCx stenosis. Of these, 19 presented as isolated LCx stenosis with a patent crossover stent. In this subset, 5 were treated with a DCB, and 14 received DES implantation in the LCx ostium using two-stent techniques (reverse crush, n=9; T and protrusion, n=5).
The multivariable-adjusted independent predictors of the primary outcome are shown in Table 4. Model 1 represents values adjusted individually for each variable while controlling for clinical covariates. Model 2 included three segment-specific models (for proximal LM, distal LM, and LAD ostium, respectively), each incorporating both MSA and stent expansion index. In all three models, the stent expansion index failed to show independent prognostic significance. Model 3, which adjusted concurrently for MSA values from all three segments, identified only MSA within the proximal LM as a significant prognostic factor (p=0.003); the MSAs within the distal LM and LAD ostium were not significant. In Model 4, underexpansion in the proximal LM and underexpansion in both the distal LM and LAD ostium were simultaneously included. Both remained independent predictors of 5-year MACE (adjusted HRs 1.93 and 1.94, respectively).
Table 3. Clinical outcomes at 5 years according to stent underexpansion in the proximal LM.
| Overall population (N=829) | Proximal LM MSA ≥11.4 mm2 (N=446) | Proximal LM MSA <11.4 mm2 (N=383) | p-value | |
|---|---|---|---|---|
| Primary outcome: MACE† | 107 (12.9) | 39 (8.7) | 68 (17.8) | <0.001 |
| Secondary outcomes | ||||
| All-cause death | 75 (9.0) | 30 (6.7) | 45 (11.7) | 0.017 |
| Cardiovascular death | 53 (6.4) | 20 (4.5) | 33 (8.6) | 0.022 |
| LM-related MI | 3 (0.4) | 0 (0) | 3 (0.8) | 0.196 |
| LM-related TLR | 33 (4.0) | 9 (2.0) | 24 (6.3) | 0.003 |
| Values are presented as numbers (percentage). The percentages presented in the table may differ from cumulative incidence estimates derived by the Kaplan-Meier method. †MACE was defined as a composite of all-cause death, LM-related MI, and clinically driven LM-related TLR. LM: left main coronary artery; MACE: major adverse cardiac events; MI: myocardial infarction; MSA: minimal stent area; TLR: target lesion revascularisation | ||||
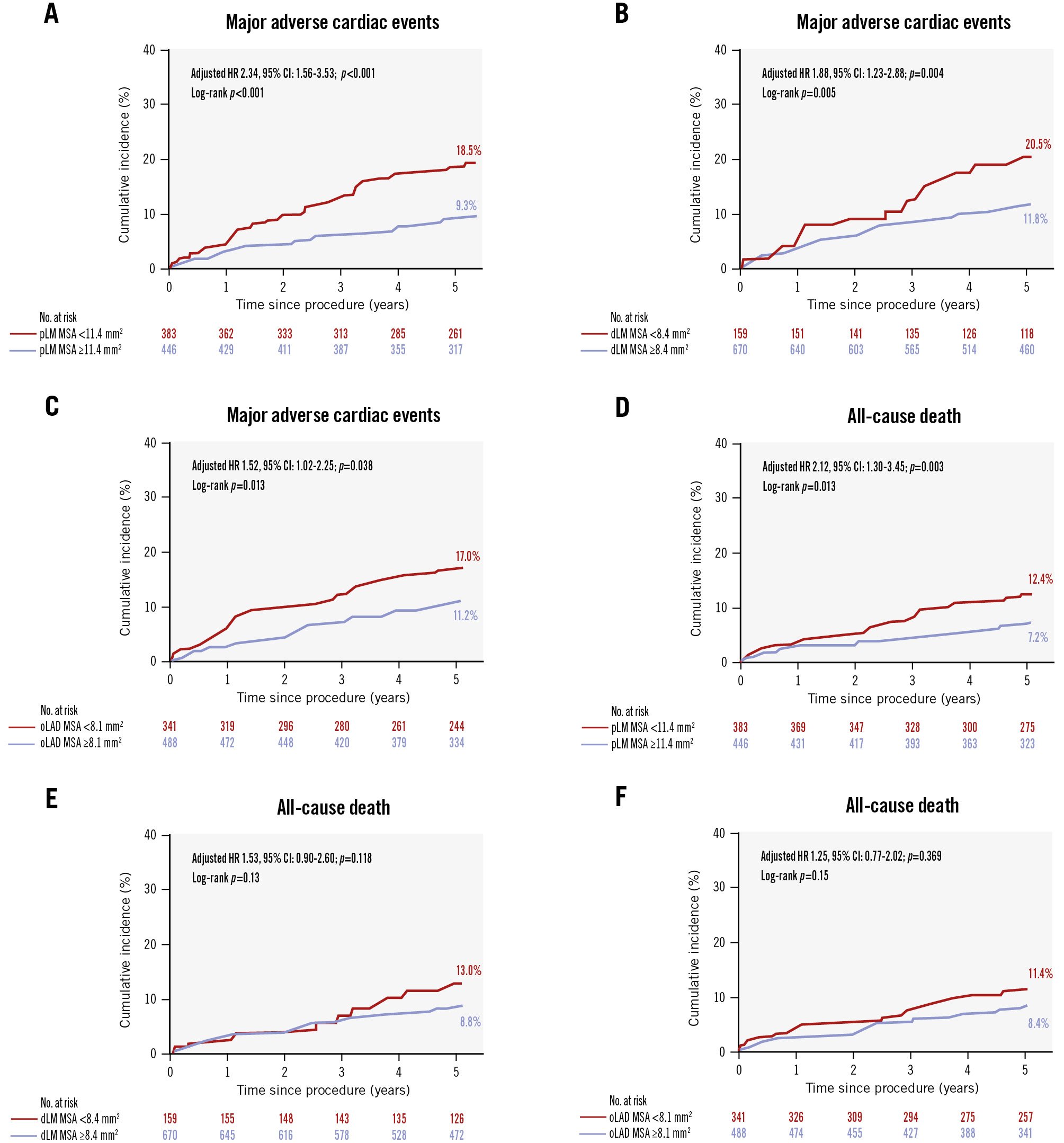
Figure 1. Cumulative incidences of major adverse cardiac events and all-cause death. The cumulative incidences of 5-year MACE according to the optimal MSA cutoff within the proximal LM (A), distal LM (B), and LAD ostium (C) are shown. The cumulative incidences of 5-year all-cause death according to the optimal MSA cutoff within the proximal LM (D), distal LM (E), and LAD ostium (F) are shown. CI: confidence interval; dLM: distal left main coronary artery; HR: hazard ratio; LAD: left anterior descending artery; LM: left main coronary artery; MACE: major adverse cardiac events; MSA: minimal stent area; oLAD: ostial left anterior descending artery; pLM: proximal left main coronary artery
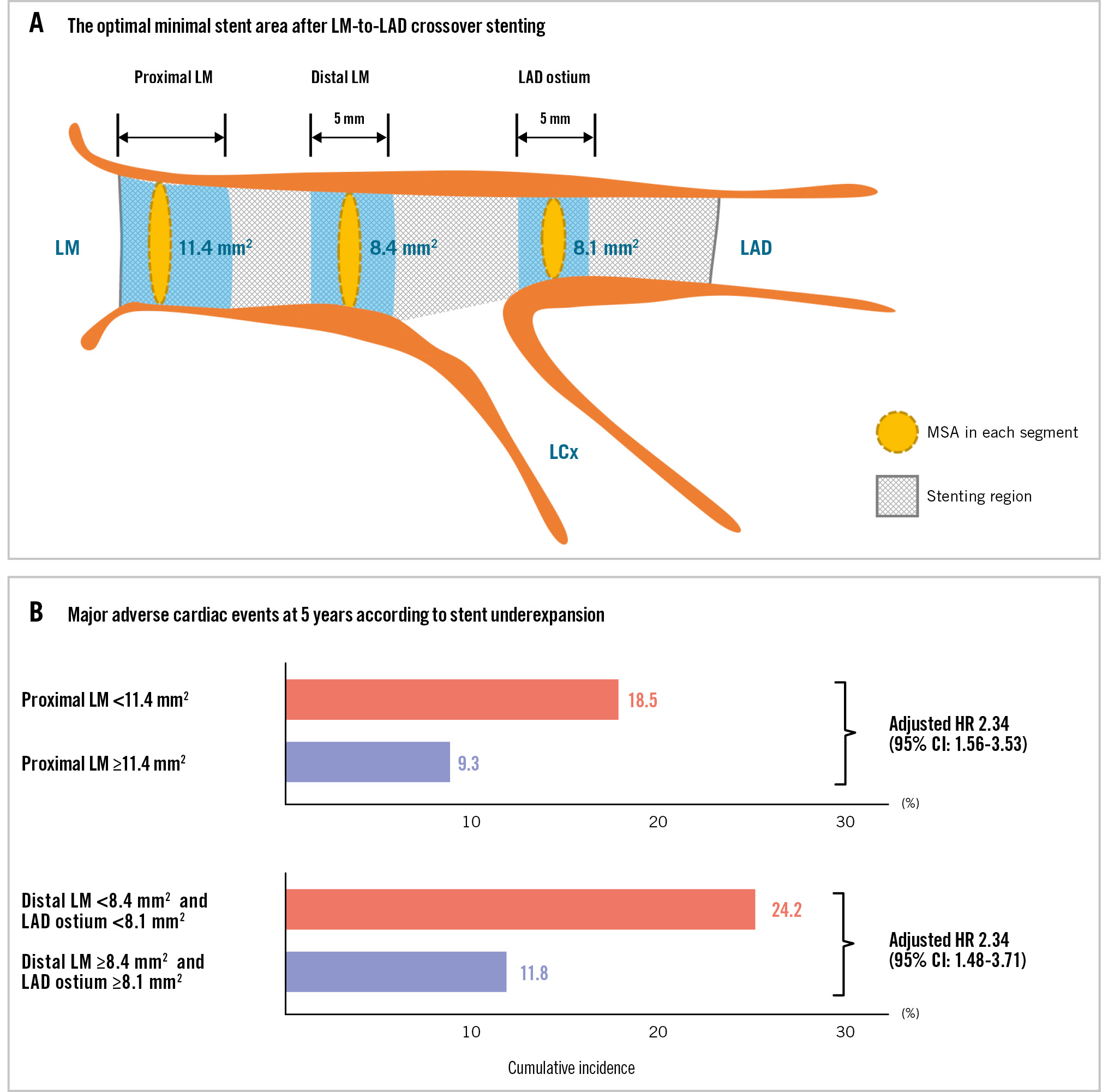
Central illustration. Optimal minimal stent area after left main crossover stenting and 5-year MACE. A) The optimal minimal stent area cutoff values for each segment were 11.4 mm2 for the proximal LM, 8.4 mm2 for the distal LM, and 8.1 mm2 for the LAD ostium. B) The cumulative incidences of 5-year major adverse cardiac events (MACE) according to stent underexpansion in the proximal LM, distal LM, and LAD ostium are shown. The hazard ratio was adjusted for age, body mass index, body surface area, diabetes mellitus, chronic kidney disease, peripheral artery disease, and a left ventricular ejection fraction ≤50%. CI: confidence interval; HR: hazard ratio; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery; MSA: minimal stent area
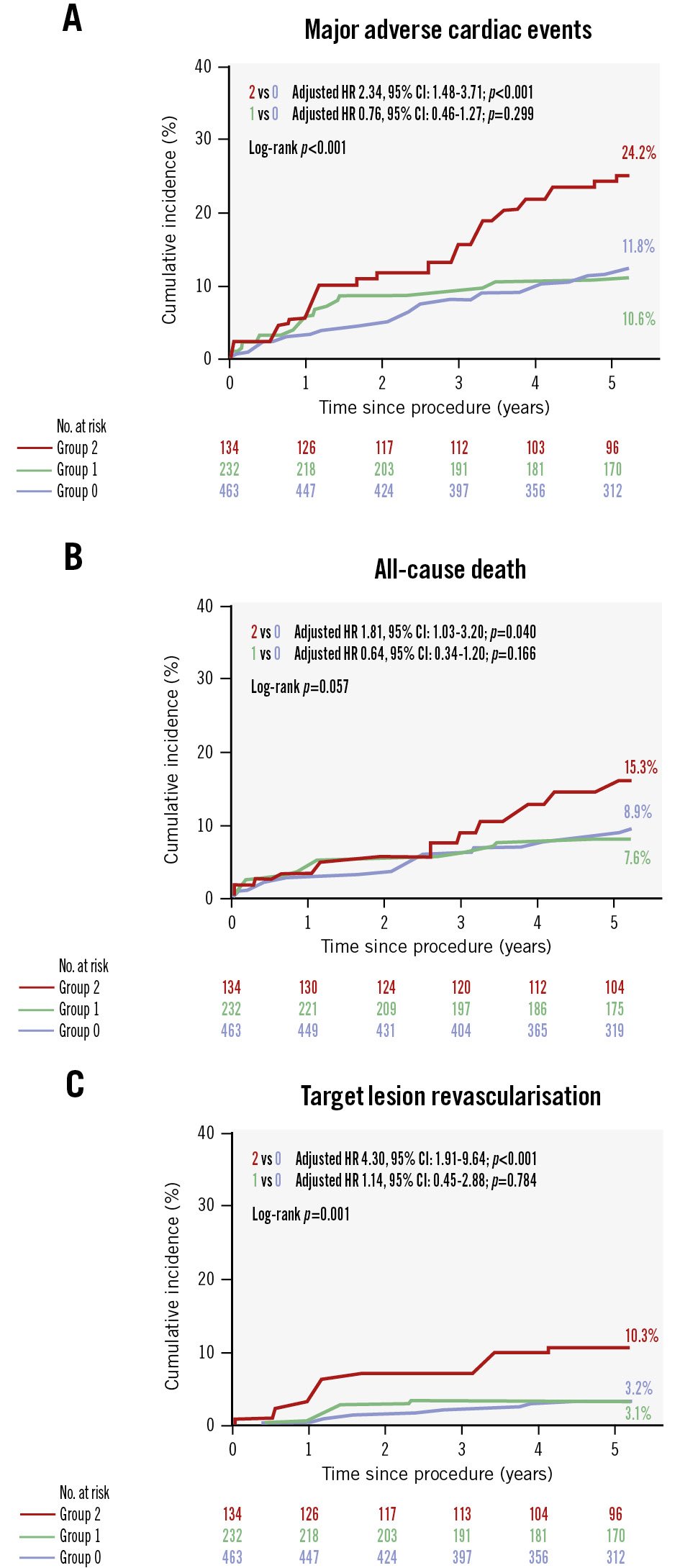
Figure 2. Cumulative incidences of major adverse cardiac events and its components. The cumulative incidences of 5-year MACE (A), all-cause death (B), and clinically driven target lesion revascularisation (C) in patients with stent underexpansion in both the distal LM and LAD ostium (group 2) are shown compared with those who had stent underexpansion in either the distal LM or LAD ostium (group 1) and those who had no underexpanded segments in either the distal LM or LAD ostium (group 0). CI: confidence interval; HR: hazard ratio; LAD: left anterior descending artery; LM: left main coronary artery; MACE: major adverse cardiac events
Table 4. Multivariable Cox proportional hazards model analysis for 5-year major adverse cardiac events.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Variables | Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
| Proximal LM, MSA* | 0.61 (0.49-0.77) | <0.001 | 0.68 (0.52-0.88) | 0.004 | 0.60 (0.43-0.84) | 0.003 | ||
| Proximal LM, stent expansion index* | 0.68 (0.55-0.85) | <0.001 | 0.83 (0.65-1.07) | 0.154 | ||||
| Proximal LM, underexpansion | 2.34 (1.56-3.53) | <0.001 | 1.93 (1.24-2.99) | 0.003 | ||||
| Distal LM, MSA* | 0.73 (0.59-0.91) | 0.006 | 0.71 (0.55-0.93) | 0.011 | 1.03 (0.74-1.44) | 0.859 | ||
| Distal LM, stent expansion index* | 0.87 (0.70-1.08) | 0.210 | 1.05 (0.82-1.35) | 0.700 | ||||
| Distal LM, underexpansion | 1.88 (1.23-2.88) | 0.004 | ||||||
| LAD ostium, MSA* | 0.79 (0.64-0.98) | 0.030 | 0.78 (0.61-1.00) | 0.052 | 0.99 (0.76-1.30) | 0.954 | ||
| LAD ostium, stent expansion index* | 0.90 (0.74-1.09) | 0.297 | 1.02 (0.81-1.29) | 0.841 | ||||
| LAD ostium, underexpansion | 1.52 (1.02-2.25) | 0.038 | ||||||
| Underexpansion in both the distal LM and LAD ostium | 2.57 (1.67-3.95) | <0.001 | 1.94 (1.22-3.09) | 0.005 | ||||
| *Continuous variables were standardised using Z-score transformation, resulting in standardised hazard ratios that represent the effect of a 1-standard deviation increase in each variable. Model 1 was adjusted for age, body mass index, body surface area, diabetes mellitus, chronic kidney disease, peripheral artery disease, and an LVEF ≤50%. Model 2 included all covariates from model 1, with simultaneous adjustment for both the MSA and stent expansion index within each specific segment separately (proximal LM, distal LM, and LAD ostium). Model 3 included all covariates from model 1, with concurrent adjustment for the MSA from all three segments together in the same model, without considering the stent expansion index. Model 4 included all covariates from model 1, with additional adjustment for underexpansion in the proximal LM and underexpansion in both the distal LM and LAD ostium. Stent underexpansion was defined as a final MSA value of <11.4 mm2 in the proximal LM, <8.4 mm2 in the distal LM, and <8.1 mm2 in the LAD ostium. CI: confidence interval; HR: hazard ratio; LAD: left anterior descending artery; LM: left main coronary artery; LVEF: left ventricular ejection fraction; MSA: minimal stent area | ||||||||
Additional analysis
The cumulative incidence of 5-year MACE was similar between patients with second- or newer-generation (13.9%) and first-generation DES implantation (11.9%; log-rank p=0.53). Postprocedural IVUS analysis revealed comparable MSAs within the proximal and distal LM between DES subgroups (Supplementary Table 3). Among patients with second- or newer-generation DES implantation (n=703), the proximal LM MSA was significantly associated with 5-year MACE (p=0.003), while the MSA values within the distal LM and LAD ostium did not show significant associations (Supplementary Table 4). In Model 2, when both MSA and the stent expansion index were incorporated into the same statistical model, the stent expansion index showed no independent predictive value for 5-year MACE.
Preintervention IVUS imaging was available for 254 patients (30.6%), while a post-stenting IVUS pullback from the LCx was available in only 47 patients. The pre-stenting IVUS findings are described in Supplementary Table 5. The minimal luminal area in the LCx ostium was significantly smaller in patients with 5-year MACE compared with those without MACE (4.4±1.8 mm² vs 5.5±2.8 mm²; p=0.006). In addition, plaque burden in the LCx ostium was significantly greater in patients with MACE (58% vs 52%; p=0.027).
Discussion
This study evaluated IVUS-derived MSA criteria for optimal stent expansion based on 5-year adverse events in patients who underwent PCI using a single-stent crossover technique for unprotected LM disease. We found that the final MSA values within the proximal LM (<11.4 mm2), distal LM (<8.4 mm2), and LAD ostium (<8.1 mm2) were significantly associated with the risk of 5-year MACE. When concurrently adjusted for MSA values from all three segments, only the MSA within the proximal LM was independently associated with the adjusted risk of 5-year MACE, whereas the MSA values in the distal LM and LAD ostium were not predictive of long-term outcomes. Furthermore, patients with stent underexpansion in both the distal LM and LAD ostium exhibited a significantly higher incidence of 5-year MACE compared with those who had either no underexpanded segments or underexpansion in only one of these segments.
LM disease, characterised by a large, jeopardised myocardium, exhibits distinct anatomical and pathophysiological characteristics, including diffuse involvement and positive remodelling1718. Conventional angiographic assessment of LM lesions is fundamentally limited by its two-dimensional nature; therefore, current guidelines recommend IVUS for the evaluation of LM lesion severity78. IVUS guidance provides valuable anatomical information for preprocedural planning and enables detection of potential complications during and after stent deployment, including stent underexpansion, incomplete apposition, edge dissection, and significant residual disease1920. Several observational studies and randomised trials with limited sample sizes support the benefit of intracoronary imaging, especially IVUS, in improving clinical outcomes in LM stenting456212223. However, standardised IVUS-guided optimisation protocols and criteria for LM stenting have not yet been specified, and the prognostic significance of the LM MSA as a predictor of long-term cardiovascular outcomes remains unclear24.
Recently, an IVUS subgroup analysis of the Nordic-Baltic-British Left Main Revascularization (NOBLE) trial including 224 patients (single-stent crossover: 67.4%) showed that the final LM MSA (12.5±3.0 mm2) was negatively associated with the TLR rate at 5 years, but not with the harder clinical endpoints25. Subgroup analysis of the EXCEL trial comprising 504 patients showed that the final LM MSA was 9.9±2.3 mm2 and that the smallest tertile of the LM MSA was associated with a higher rate of the composite outcome (all-cause death, MI, and stroke at 3 years) than the largest tertile16. Similarly, another study proposed IVUS-guided LM optimisation criteria using relative stent expansion (MSA >90% of the reference lumen) and found that patients with a median LM MSA of 11.8 mm2 (n=124, single-stent crossover: 85.5%) exhibited a lower incidence of composite outcomes (cardiac death, MI, and TLR at 1 year) than those guided by angiography alone26.
Our study exclusively included patients (n=829) who underwent single-stent crossover with a complete final IVUS pullback from the LAD. The distribution of the proximal LM MSA (median 11.6 mm2) and distal LM MSA (median 9.9 mm2) in our study was comparable to those of previous studies1516252627. A smaller final MSA might reflect an anatomically smaller vessel size rather than stent underexpansion. However, when comparing patients who had MACE with those who did not, the vessel area was equivalent in both groups. The stent expansion index was much lower in patients who had MACE, indicating that the stented LM segment was not adequately expanded. Interestingly, when both the MSA and the stent expansion index were simultaneously adjusted for within each segment, only MSA remained a significant predictor of clinical outcomes (Table 4). Accordingly, stent underexpansion in our analysis was defined solely based on absolute MSA values within each segment, without incorporating relative expansion indices. Indeed, a lower stent expansion index does not necessarily indicate true underexpansion, particularly in vessels with significant plaque burden and positive remodelling. This highlights the value of absolute MSA as a practical procedural target in IVUS-guided LM PCI.
Due to the modest AUC values, the MSA in either the distal LM alone or LAD ostium alone was not predictive of 5-year MACE after adjustment with the proximal LM MSA (Table 4). The observed limitation in predictive accuracy likely reflects that MSA assessment of either the distal LM alone or the LAD ostium alone fails to encompass adverse events originating from the LCx ostium. However, underexpansion in both the distal LM and LAD ostium was a significant predictor, as was underexpansion in the proximal LM (Table 4). These findings suggest that avoiding stent underexpansion through IVUS guidance can directly improve clinical outcomes during LM PCI. In fact, the use of intracoronary imaging guidance has led to the selection of larger stent sizes and superior stent expansion, primarily due to the use of non-compliant balloons for postadjunctive dilatation with high-pressure inflation1528293031. In a prospective application of contemporary optimisation criteria for LM lesions (MSA >7 mm2 for the distal segment and >8 mm2 for the proximal segment)32, the intracoronary imaging-guided LM PCI group (60.1% of whom achieved optimisation) had a significantly lower risk of composite cardiovascular events than the angiography-guided LM PCI group5.
Limitations
This study has certain limitations. First, the prospective observational design may have led to a selection bias and unmeasured confounding factors. Although randomised controlled trials are considered the gold standard of evidence, they are only feasible for a selected subset of patients treated for LM disease; therefore, evidence from all-comers registries remains essential, and our findings should be interpreted with caution. Second, our study did not examine whether intracoronary imaging could identify distal LM lesions better suited to a two-stent approach rather than the standard provisional strategy. The necessity for bailout implantation of a second stent arises in up to 22% of LM bifurcation lesions initially treated with a stepwise provisional technique2733. Future research should focus on identifying lesion characteristics predictive of the need for LCx ostial stenting, thereby improving procedural planning and efficiency. Third, this analysis from a single tertiary centre, which performs a high volume of LM stenting procedures34, limits the generalisability of our findings. Additional randomised studies in diverse clinical settings are needed for validation.
Conclusions
This study evaluated the IVUS-derived segmental MSA cutoffs in patients undergoing LM-to-LAD crossover stenting for unprotected LM disease. Achieving optimal stent expansion in the proximal LM and preventing underexpansion in both the distal LM and LAD ostium are critical for improving long-term clinical outcomes. The optimal MSA thresholds identified herein may serve as practical benchmarks for stent optimisation during LM PCI.
Impact on daily practice
This study established optimal minimal stent area thresholds for single-stent crossover in patients with unprotected left main coronary artery (LM) disease (proximal LM ≥11.4 mm², distal LM ≥8.4 mm², left anterior descending artery [LAD] ostium ≥8.1 mm²). To ensure favourable long-term outcomes in this high-risk patient population, adequate stent expansion in the proximal LM and avoidance of simultaneous underexpansion in both the distal LM and LAD ostium segments are essential. These results provide practical intravascular ultrasound-based optimisation targets for interventional cardiologists performing LM crossover stenting.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the government of the Republic of Korea (the Ministry of Science and ICT; the Ministry of Trade, Industry and Energy; the Ministry of Health & Welfare; the Ministry of Food and Drug Safety; Project Number: 2710000054, RS-2022-00141289).
Conflict of interest statement
The authors have no conflicts of interest relevant to the contents of this study to declare.
Supplementary data
To read the full content of this article, please download the PDF.

