Cory:
Unlock Your AI Assistant Now!
For three decades, dual antiplatelet therapy (DAPT), comprising aspirin and a P2Y12 inhibitor, has been the gold standard for the secondary prevention of atherothrombosis after an acute coronary syndrome (ACS). The limitations of the second-generation P2Y12 inhibitor clopidogrel – variable metabolism, slow onset of action, and relatively weak antiplatelet effect – led to the development and testing of the more potent P2Y12 inhibitors prasugrel and ticagrelor. Yet, even as prasugrel and ticagrelor improved outcomes compared with clopidogrel, approximately 10% of patients treated with high-potency P2Y12 inhibitors in pivotal trials experienced cardiovascular death, myocardial infarction (MI), or stroke in the year after ACS. The incomplete efficacy of DAPT for the prevention of recurrent atherothrombotic events is unsurprising in light of the pathophysiology of atherothrombosis: though platelets initiate the formation of thrombus at the site of a ruptured atherosclerotic plaque, the thrombus is propagated and stabilised by thrombin and fibrin, products of the coagulation cascade.
Recognition of the central role of the coagulation cascade in the pathophysiology of atherothrombosis led to the advent of so-called dual pathway inhibition, with DAPT plus an anticoagulant. Dual pathway inhibition was tested in the ATLAS ACS 2–TIMI 51 randomised controlled trial, which compared very low-dose rivaroxaban versus placebo on a background of DAPT in 15,526 patients with recent ACS. Rivaroxaban significantly reduced the incidence of the composite of cardiovascular death, MI, and stroke compared with placebo (8.9% vs 10.7%), but also significantly increased the incidence of major bleeding (2.1% vs 0.6%) and intracranial bleeding (0.6% vs 0.2%), despite use of rivaroxaban at a dose much lower than that used for full anticoagulation1.
ATLAS ACS 2–TIMI 51 shows the promise of dual pathway inhibition but also its peril. Until now, all comercially approved antithrombotic agents that have successfully reduced the incidence of thrombotic events after ACS have also increased the incidence of bleeding (Figure 1)2. The development of factor XI/XIa inhibitors has the potential to change this paradigm and decouple physiological haemostasis from pathophysiological thrombosis3. Unlike vitamin K antagonists (warfarin), direct thrombin inhibitors (dabigatran, dalteparin) and factor Xa inhibitors (apixaban, rivaroxaban, edoxaban, low-molecular-weight heparin, fondaparinux), which act on coagulation factors in the common pathway, factor XI/XIa inhibitors affect only the intrinsic pathway. Haemostasis is an extravascular process mediated by the extrinsic pathway: upon vessel injury, high concentrations of tissue factor in the adventitia cause explosive thrombin generation to form a vessel plug. By contrast, thrombosis is largely mediated by the intrinsic pathway: lower concentrations of tissue factor at the site of a ruptured atherosclerotic plaque generate a small amount of thrombin, which activates coagulation factors in the intrinsic pathway to create more thrombin, creating a positive feedback loop that ultimately leads to thrombus expansion beyond the initial site of injury. Effectively, the intrinsic pathway amplifies thrombin generation and allows for propagation of thrombus in an environment with limited tissue factor. The theoretical promise of the factor XI/XIa inhibitors lies in the fact that by inhibiting only the intrinsic pathway, they allow for maintenance of haemostasis while preventing thrombosis. This theoretical promise is buttressed by evidence from epidemiology and Mendelian randomisation studies: patients with factor XI deficiency have only a mild bleeding diathesis but are less prone to stroke, venous thromboembolism, and possibly MI3.
To date, 9 factor XI/XIa inhibitors have been developed and tested in Phase 2 clinical trials, including oral small molecules taken once or twice daily and monoclonal antibodies and antisense oligonucleotides given subcutaneously weekly or monthly. In the only completed Phase 3 trial comparing a factor XI/XIa inhibitor to standard-of-care anticoagulation, OCEANIC-AF, investigators randomised 14,180 patients with atrial fibrillation to the small-molecule factor XIa inhibitor asundexian or standard-dose apixaban. The trial was stopped prematurely due to inferior efficacy at the recommendation of the data safety and monitoring committee, after 1.3% of patients randomised to asundexian had stroke or systemic embolism versus 0.4% of patients randomised to apixaban; major bleeding was less frequent with asundexian compared with apixaban (0.2% vs 0.6%)4. The results of OCEANIC-AF suggest that factor XI/XIa inhibitors may not truly decouple thrombosis and haemostasis, with the greater safety of these agents balanced by lower antithrombotic efficacy.
However, in the case of dual pathway inhibition for ACS, lower potency anticoagulation with a factor XI/XIa inhibitor on top of DAPT may find the right “sweet spot” between bleeding and ischaemic risk. Asundexian has also been tested as part of a dual pathway inhibition strategy in patients with ACS. In the PACIFIC-AMI Phase 2 trial, 1,601 patients hospitalised with ACS were randomised to placebo or one of three different doses of asundexian, on top of standard-of-care DAPT. In contrast to the results of ATLAS ACS 2–TIMI 51, the incidence of major or clinically relevant bleeding was not higher in patients randomised to asundexian 50, 20, or 10 mg daily compared with placebo (10.5% vs 8.1% vs 7.6% vs 9.0%, respectively); the trial was not powered for efficacy, as there were only 10 total ischaemic events5. Though asundexian’s manufacturer has opted not to continue the development of asundexian for ACS, the ongoing LIBREXIA-ACS trial (ClinicalTrials.gov: NCT05754957), which will compare the small-molecule factor XIa inhibitor milvexian with placebo on top of standard-of-care DAPT in 16,000 patients with ACS, will definitively test the safety and efficacy of dual pathway inhibition with factor XIa inhibitors.
By inhibiting only the intrinsic coagulation pathway, factor XI/XIa inhibitors have the potential to partially decouple haemostasis and thrombosis, leading to a safer yet effective option for anticoagulation in patients with ACS.
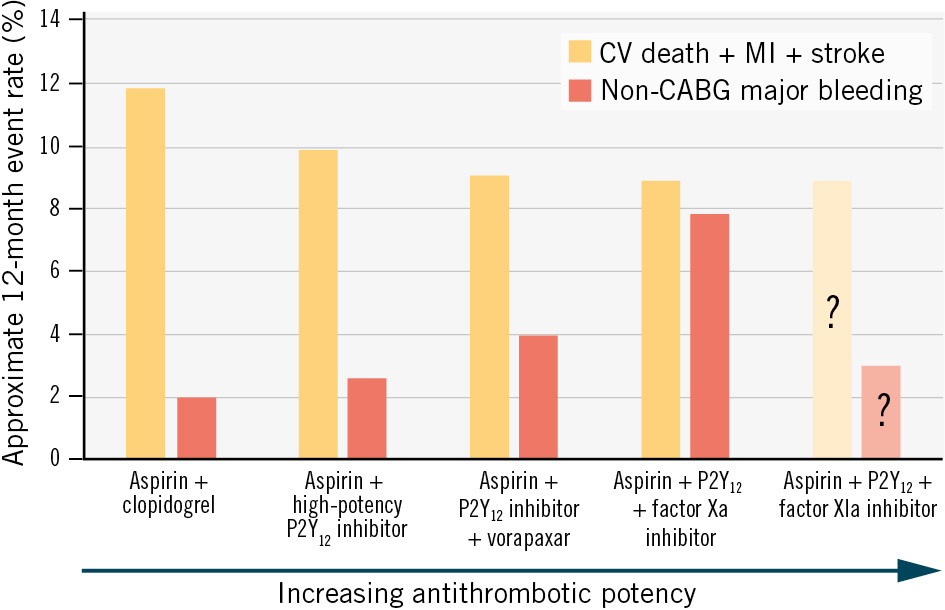
Figure 1. Coupling of bleeding and thrombotic risk leads to challenges in reducing residual thrombotic risk. The risk of cardiovascular death, myocardial infarction, or stroke remains approximately 10% in the 12 months after an acute coronary syndrome with standard-of-care dual antiplatelet therapy with aspirin+a high-potency P2Y12 inhibitor (prasugrel or ticagrelor). Attempts to reduce this residual thrombotic risk by adding the protease-1 receptor antagonist vorapaxar or the factor Xa inhibitor rivaroxaban have reduced the risk of cardiovascular events at the cost of unacceptably high bleeding. By inhibiting only the intrinsic pathway of the coagulation cascade, factor XIa inhibitors may be able to decouple thrombosis and haemostasis, reducing residual thrombotic risk without unacceptable increases in bleeding. The 12-month event rates shown are approximate and were derived using relative risks to allow for comparisons between clinical trials. CABG: coronary artery bypass grafting; CV: cardiovascular; MI: myocardial infarction
Conflict of interest statement
R.D. Lopes reports research grants or contracts from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi-Aventis; funding for educational activities or lectures from Pfizer, Daiichi Sankyo, and Novo Nordisk; and funding for consulting from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, and Novo Nordisk. A.C. Fanaroff has been a consultant for Novartis and Bristol-Myers Squibb/Johnson & Johnson; and consultant (unpaid) for the Centers for Medicare and Medicaid Services.

