Cory:
Unlock Your AI Assistant Now!
Patients after an acute myocardial ischaemic syndrome1 (AMIS; formerly known as acute coronary syndrome [ACS]) are ideally treated with percutaneous coronary intervention (PCI) and drug-eluting stent (DES) implantation, which require the rapid introduction of dual antiplatelet therapy (DAPT; usually with aspirin plus prasugrel or ticagrelor − two P2Y12 inhibitors with more favourable pharmacokinetics than clopidogrel), traditionally for up to 12 months12. The most recent European Society of Cardiology (ESC)1 and American College of Cardiology/American Heart Association (ACC/AHA)3 guidelines suggest the subsequent discontinuation of the P2Y12 inhibitor to continue aspirin lifelong for most patients (“default strategy”). While the need for DAPT early on in this setting became clear after the completion of pivotal trials34, its optimal duration has never been crystal clear, and the traditional 12-month duration has been challenged by new evidence. The rapid decline in ischaemic risk after AMIS in most patients who are now properly treated with optimal medical therapy, including extensive use of cholesterol-lowering agents, and the decreased incidence of stent thrombosis with less thrombogenic DES, along with increased awareness of the negative prognostic impact of bleeding, have now enabled a safe reduction in DAPT duration in high bleeding risk patients. Recently, in an attempt to improve safety and maintain an optimal antithrombotic efficacy, a generalised strategy based on early aspirin discontinuation while continuing ticagrelor monotherapy has been proposed5. We argue that the choice of which drug to maintain after early DAPT discontinuation is still uncertain, and the position of specifically preferring ticagrelor monotherapy over aspirin monotherapy is not based on solid objective evidence, as it lacks direct comparison within randomised clinical trials (RCTs).
In attempts to minimise bleeding without compromising efficacy, several RCTs evaluated the possibility of progressively shorter DAPT regimens, followed either by aspirin monotherapy after interruption of the P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel) or by P2Y12 monotherapy, compared against the standard 12 months in patients undergoing PCI for either AMIS or non-AMIS. These RCTs were included in a systematic review and meta-analysis6. Eight trials (15,020 patients) compared 12-month DAPT with 6-month DAPT followed by aspirin monotherapy, and 4 trials (7,514 patients) compared 12-month with <6-month DAPT followed by aspirin monotherapy. Compared with 12-month DAPT, both of the shortened DAPT regimens were associated with similar relative risks (RR) for cardiovascular events (RR 1.11, 95% confidence interval [CI]: 0.86-1.45; RR 1.24, 95% CI: 0.89-1.72, respectively) and, close to statistical significance, a lower risk of major bleeding (RR 0.85, 95% CI: 0.56-1.28; RR 0.67, 95% CI: 0.43-1.04, respectively). Compared with 12-month DAPT, short-term DAPT followed by P2Y12 inhibitor monotherapy was also associated with a similar RR for cardiovascular events (RR 0.97, 95% CI: 0.78-1.22) but a significantly lower RR for major bleeding (RR 0.69, 95% CI: 0.50-0.96), likely due to the higher potency of the RCT. Similar results were obtained in a subgroup analysis restricted to patients with AMIS: compared with 12-month DAPT, (a) the RR of aspirin monotherapy after both shortened DAPT regimens was 1.32 (95% CI: 0.83-2.09) and 1.17 (95% CI: 0.63-2.18) for cardiovascular events; and 0.70 (95% CI: 0.35-1.38) and 0.82 (95% CI: 0.47-1.42) for major bleeding, respectively; (b) the RR of P2Y12 inhibitor monotherapy was 0.60 (95% CI: 0.32-1.14) for cardiovascular events and 0.64 (95% CI: 0.46-0.90) for major bleeding, respectively. An analysis of the hierarchy of treatment effectiveness and safety indicated that strategies based on short-term DAPT followed by aspirin monotherapy are the least effective to prevent ischaemic events but the most effective to prevent major bleeding.
RCTs published after the above-cited meta-analysis provided additional evidence with somewhat conflicting results. In the STOPDAPT-2 ACS RCT in patients with AMIS treated with successful PCI, clopidogrel monotherapy after 1-2 months of DAPT failed to achieve non-inferiority compared with 12-month DAPT for the net clinical benefit endpoint, primarily due to a numerical increase in cardiovascular events exceeding the reduction in bleeding7. However, in SMART-CHOICE8, monotherapy with a P2Y12 inhibitor (clopidogrel in 77% of cases) started after 3 months of DAPT was associated with non-inferior incidence of major adverse cardiac and cerebrovascular events (MACCE) and lower rates of bleeding at 12 months compared with continued DAPT. Therefore, the available evidence is inconclusive regarding whether P2Y12 inhibition by clopidogrel monotherapy after early DAPT discontinuation is non-inferior in efficacy to standard 12-month DAPT. Lesser bleeding and lack of clear evidence of non-increased ischaemic events could be consequent to an inadequate pharmacological response to clopidogrel in about 30% of patients, due to its impaired biotransformation to the active metabolite9.
More recently, the effects of monotherapy with one of the more efficient P2Y12 inhibitors, ticagrelor or prasugrel, after early DAPT discontinuation have been tested. Results of the more relevant RCTs exploring de-escalations to ticagrelor monotherapy were synthesised in a patient-level meta-analysis including a total of 24,407 patients from 6 RCTs. This meta-analysis showed that, compared with standard 12-month DAPT, de-escalation to ticagrelor monotherapy was non-inferior for MACCE (a composite of all-cause death, myocardial infarction, or stroke; hazard ratio [HR] 0.91, 95% CI: 0.78-1.07; p=0.004 for non-inferiority), while significantly reducing Bleeding Academic Research Consortium (BARC) Type 3 or 5 bleeding (HR 0.43, 95% CI: 0.34-0.54; p<0.001 for superiority) and all-cause mortality (HR 0.76, 95% CI: 0.59-0.98; p=0.034 for superiority)5. Trial sequential analyses confirmed the evidence for MACCE non-inferiority and bleeding superiority but not for mortality5. The beneficial effect with respect to bleeding was particularly pronounced among patients with AMIS at baseline (p for interaction=0.022)5. The authors argued for the use of ticagrelor monotherapy after early DAPT discontinuation as a strategy to be adopted for most patients5. We believe that the preference of ticagrelor over aspirin monotherapy in the setting of shortened DAPT is questionable because of the lack of RCTs directly comparing the two drug regimens.
With the progressive waning of ischaemic risk after AMIS, seen in recent times12NaN, it is conceivable that the intensity of antiplatelet therapy could be mitigated to improve safety by reducing bleeding events. Indeed, the above-cited meta-analysis5 shows that extensive blocking of platelet function by DAPT may not be necessary 1 or 3 months after AMIS. The same “default” 12-month duration of DAPT, so widely adopted and still recommended by current guidelines, is not actually written in stone, since it is based on a disputable inference from the CURE study. In this study, the maximum duration of aspirin plus clopidogrel, compared to aspirin monotherapy, for patients with non-ST-elevation ACS was 12 months, while the actual mean duration was 9 months3. Patients initially treated with aspirin plus ticagrelor are likely to bleed more10 than those treated with aspirin plus clopidogrel (4.5% vs 3.8%; p=0.03 for non-coronary bypass-related bleeding); the latter is associated with more bleeding than aspirin alone (3.7% vs 2.7%; p=0.001)3. Any de-escalation strategy is expected to be safer than 12-month DAPT and probably acceptable for efficacy given a decreasing ischaemic risk. However, this may not necessarily be true for all post-AMIS patients. The PEGASUS study, for instance, showed that some patients at high ischaemic risk and low bleeding risk who had survived 12-month DAPT without major bleeds may actually derive a net clinical benefit by prolonging DAPT with aspirin and ticagrelor (60 mg twice daily) beyond 12 months11. But, once we accept that some patients with a lower risk of recurrences can benefit from early de-escalation, how can we be reassured that the aspirin, instead of the ticagrelor component, of DAPT should be withheld?
Aspirin has the advantage of being a well-known, extensively studied agent, with fairly reproducible antiplatelet efficacy and bleeding similar to that of clopidogrel when used at the usual once-daily low doses (75-150 mg), deriving solely from its antihaemostatic effects rather than its gastrotoxicity12. Ticagrelor, however, is likely to be associated with more bleeding than aspirin (as shown by the higher rates of bleeding in the PLATO study compared with clopidogrel10). Consequently, is ticagrelor better than aspirin based on efficacy/safety considerations at that point? And, do we really need a “more effective” therapy with ticagrelor monotherapy instead of aspirin monotherapy, even in patients at low thrombotic risk, and for how long? The only RCT that has directly compared the antithrombotic efficacy of ticagrelor monotherapy and aspirin monotherapy failed to demonstrate superiority of ticagrelor in preventing stroke, myocardial infarction or death within 90 days in patients with acute non-severe ischaemic stroke or high-risk transient ischaemic attack13. Although the pathogenesis of thrombi in the coronary and cerebrovascular circulations partially differs, it must be noted that RCTs of antithrombotic drugs in patients with AMIS include the occurrence of stroke among the primary clinical endpoints (MACCE)5.
To solve the question of the best efficacy/safety compromise after early interruption of DAPT, we need a head-to-head RCT comparing the interruption of aspirin with that of ticagrelor or prasugrel, as summarised in Figure 1. This RCT needs to enrol around 20,000 patients, will be expensive, and is unlikely to be funded by the industry, given that tested drugs are now all off patent. But before then, we cannot rely on the only meta-analysis-based evidence produced so far, which showed the superiority of ticagrelor monotherapy over DAPT only in terms of safety; we need a head-to-head comparison of the two (or three) single antiplatelet therapies under scrutiny. It is notable that both the 2023 ESC1 and the 2025 ACC/AHA2 ACS guidelines still recommend 12-month DAPT as the default treatment for most AMIS patients.
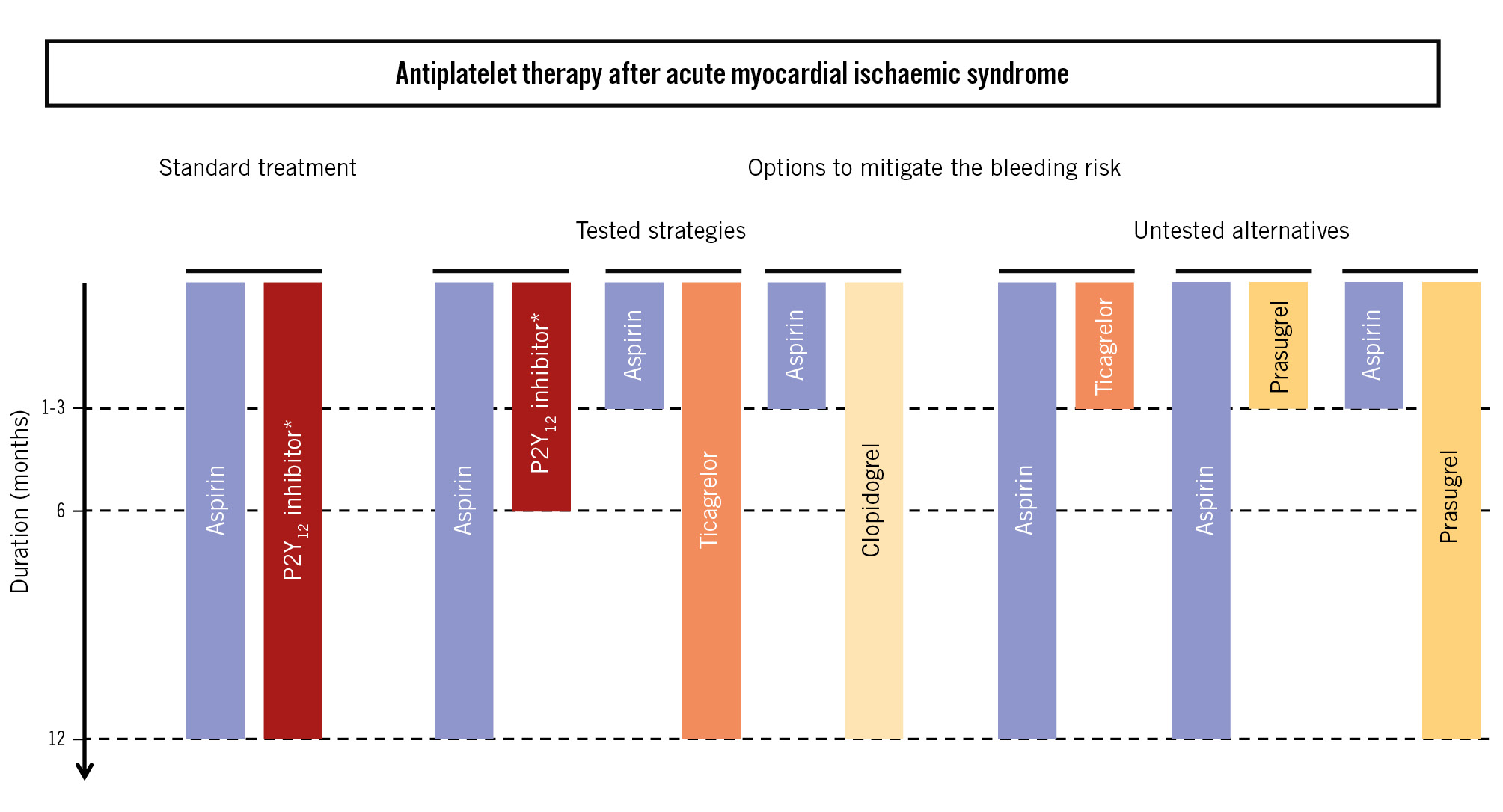
Figure 1. Tested and untested alternatives for standard and shortened durations of dual antiplatelet therapies after an acute myocardial ischaemic syndrome. *Ticagrelor, prasugrel or clopidogrel
Conflict of interest statement
R. De Caterina: consulting fees, honoraria for lecturing, and support to congress participation from Sanofi, Milestone, Daiichi Sankyo, Janssen, Pfizer, Bristol-Myers Squibb, Menarini, and Amarin, all unrelated to the topic under discussion. The other authors have no conflicts of interest to declare.

