Cory:
Unlock Your AI Assistant Now!
Drug-coated balloons have become a first-line treatment for femoropopliteal lesions in patients with lower limb peripheral artery disease (LLPAD), offering improved patency compared to plain old balloon angioplasty (POBA)1. Drug-coated balloons vary in drug composition, dosage, excipients, and coating techniques, influencing drug release kinetics and transfer to the target lesion. The COMPARE trial was the first randomised study comparing the long-term outcomes of low-dose (2.0 μg/mm²) versus high-dose (3.5 μg/mm²) paclitaxel-coated balloons (PCBs) in complex femoropopliteal lesions, reflecting real-world clinical scenarios. Non-inferiority was met for both primary efficacy and safety endpoints after 1 year, and comparable treatment effects were reported after 2 years23. Given ongoing concerns about the long-term mortality signal of PCBs, follow-up was extended to 5 years4.
The COMPARE trial was an investigator-initiated, prospective, multicentre trial that enrolled patients with symptomatic LLPAD across 15 sites in Germany (ClinicalTrials.gov: NCT02701543). The study protocol, population, endpoints, and statistical analyses have been described in depth in prior publications23. Briefly, patients with symptomatic lesions (Rutherford 2-4) of the native non-stented superficial femoral and/or proximal popliteal artery with a length of up to 30 cm and a stenosis of ≥70% were included. Participants were randomised in a 1:1 ratio to receive treatment either with the low-dose Ranger PCB (Boston Scientific) or the high-dose IN.PACT Admiral or Pacific PCB (Medtronic). Stratification by lesion length (≤10 cm, >10 and ≤20 cm, >20 cm and ≤30 cm) was applied to ensure a balanced allocation of short, intermediate, and long lesions between treatment arms. The primary efficacy endpoint was primary patency, defined as freedom from clinically driven target lesion revascularisation (CD-TLR) or binary restenosis at 12 months, and the primary safety endpoint included the absence of device- or procedure-related death within 30 days and the absence of major adverse events (target limb major amputation and CD-TLR) over 12 months. Extended follow-up endpoints assessed all-cause mortality, major target limb amputation, and CD-TLR. Patients were followed through in-person visits at 6, 12, and 24 months and via structured telephone interviews at 36, 48, and 60 months.
Out of 414 enrolled patients, vital status at 5 years was available for 130/207 (62.8%) patients in the high-dose group and 146/207 (70.5%) patients in the low-dose group. Lesion characteristics were similar across groups, with a mean lesion length of approximately 12.5 cm and over 40% classified as chronic total occlusions. At 5 years, Kaplan-Meier (KM) estimates showed no significant difference in freedom from CD-TLR, with 75.2±3.6% in the high-dose group and 67.1±3.7% in the low-dose group (log-rank p=0.1) (Figure 1). Stratification by lesion length showed consistent results, with the best patency observed for short lesions in both groups (Supplementary Figure 1). A total of 96 first target lesion revascularisations (TLRs) were performed across both groups. Subsequently, 27 second TLRs and 7 third TLRs were recorded. One patient in the low-dose group underwent a total of 6 TLR procedures. The median time to TLR was 677.3±442.5 days (high-dose group: 692.1±463.4 days vs low-dose group: 667.3±431.4 days; p=0.8), with reocclusions observed in 36.5% of target vessels (high-dose group: 38.5% vs low-dose group: 35.7%; p=0.5). Reinterventions were predominantly endovascular (96.8%). All-cause mortality was 13.8% (18/130) in the high-dose group and 15.1% (22/146) in the low-dose group (p=0.9), with no significant difference in KM survival estimates (87.1±2.9% vs 87.5±2.6%; p=0.8) (Supplementary Figure 2). One major target limb amputation was reported after 615 days in the high-dose group.
At 5 years, similar treatment effects between high-dose and low-dose PCB angioplasty were observed, indicating comparable long-term efficacy. Survival analysis revealed an early, non-significant separation of the curves between treatment arms up to 2 years, which remained stable over time. However, the patency curves remained almost overlapping during this period, indicating that the observed difference is likely attributable to chance, particularly given the low event rate. Despite the inclusion of long and complex lesions, including a high proportion of total occlusions, reintervention rates were generally moderate, and similar long-term patency rates after PCB treatment have been published previously5. The final results of the COMPARE trial demonstrate no evidence of increased mortality or major target limb amputation in either treatment arm.
Study limitations include that operator blinding was not feasible because of visible device differences. However, core laboratory personnel and members of the clinical events committee were blinded to the treatment assignments. Furthermore, extending the study’s follow-up after enrolment had begun may have impacted retention rates. Loss to follow-up rates were high, with a higher rate in the high-dose group, possibly introducing bias.
In conclusion, the 5-year results from the COMPARE trial suggest a comparable efficacy of low-dose PCB angioplasty to the high-dose alternative. Additionally, the trial demonstrated the safety of both PCBs, supporting their long-term viability as treatment options. These results reinforce the superior long-term patency of PCBs over POBA and provide valuable evidence for their continued use in managing challenging LLPAD cases.
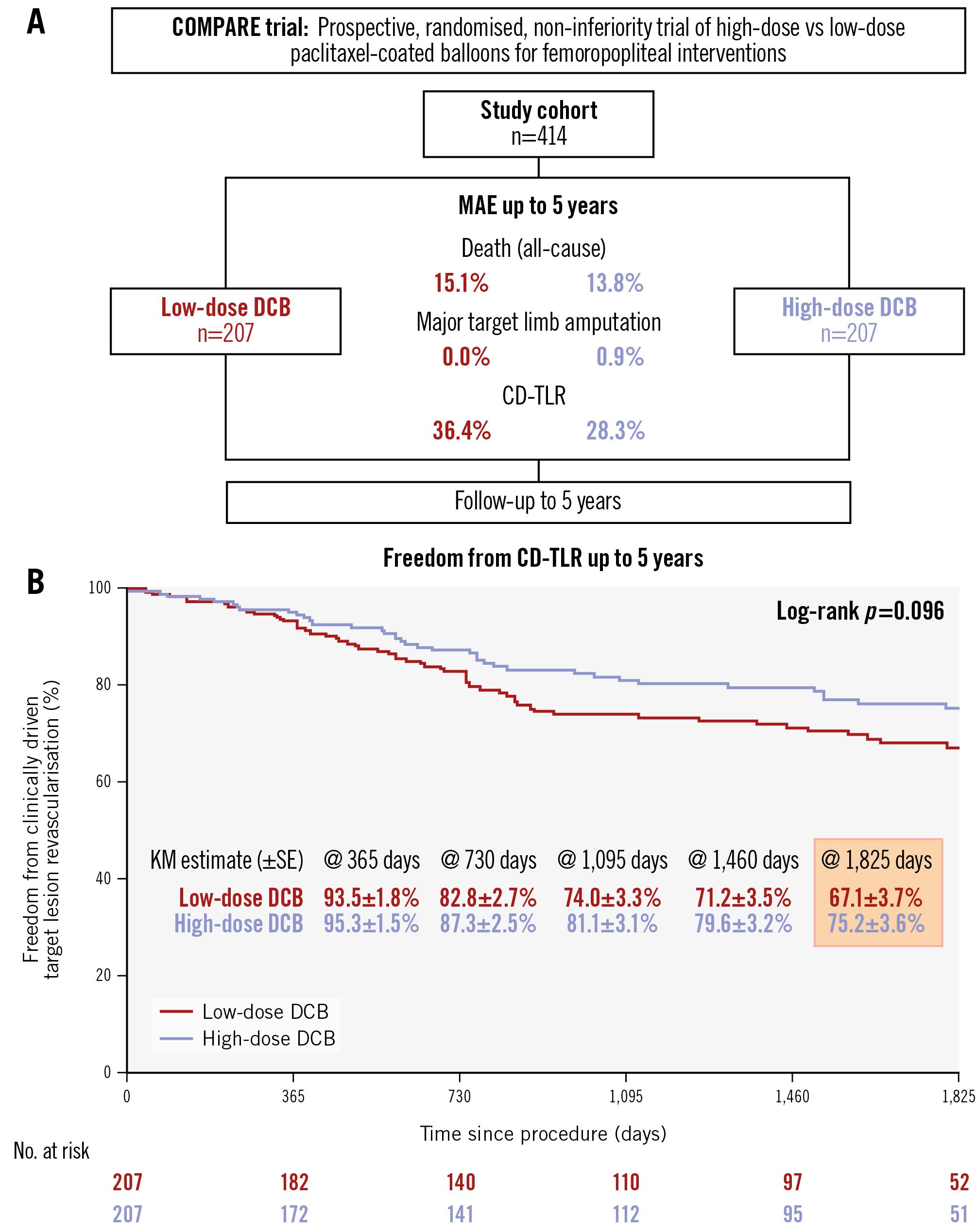
Figure 1. Study design and 5-year outcomes. A) Study design; (B) Kaplan-Meier estimates showing freedom from clinically driven target lesion revascularisation for low-dose (red curve) and high-dose (blue curve) paclitaxel-coated balloons, with the corresponding number of patients at risk. CD-TLR: clinically driven target lesion revascularisation; DCB: drug-coated balloon; KM: Kaplan-Meier; MAE: major adverse events; SE: standard error
Conflict of interest statement
S. Steiner has been a consultant or advisory board member for Angiodynamics, Biotronik, Boston Scientific, Cook Medical, and iThera Medical. A. Schmidt has been a consultant for Abbott, BD, Boston Scientific, Cook Medical, Reflow Medical, and Upstream Peripheral Technologies. T. Zeller has received consulting fees from Boston Scientific, W.L. Gore & Associates, Medtronic, Shockwave Medical, VentureMed, Veryan, and Reflow Medica; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Acotec, BD Bard, Biotronik, Boston Scientific, Cook Medical, Cordis, Medtronic, and Veryan; and has stock or stock options in ANT. G. Tepe is on the advisory board for Medtronic and Philips; and has received study support from Bard, Bayer, B. Braun, Biotronik, Boston Scientific, Cardiovascular Systems, Gore Medical, Veryan, and Shockwave Medical. E. Blessing has received honoraria from Abbott, B. Braun, Biotronik, Boston Scientific, Cook Medical, W. L. Gore & Associates, Medtronic, Philips-Spectranetics, and Shockwave Medical; and is a consultant for Boston Scientific, Medtronic, and Bayer. R. Langhoff has received consulting and speaker honoraria from Boston Scientific, Biotronik, Abbott, Contego Medical, Terumo, Cardinal Health, Alvimedica, B. Braun, and Kardionet; and has received speaker honoraria from Bard, and Bayer. N. Weiss has received speaker honoraria or research funding from Bard, Terumo, Optimed, Amgen, Bayer, Esperion, Pfizer, Pluristem, and TICEBA. D. Scheinert is a consultant for Abbott, Acotec, Boston Scientific, Concept Medical, Medtronic, Upstream Peripheral Technologies, Penumbra, Philips, and Reflow Medical. M. Thieme has received consulting and speaker honoraria from Reflow Medical, Bristol-Myers Squibb, and Pfizer. The other authors have no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

