Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Medium- and long-term outcomes after transcatheter paravalvular leak (PVL) closure remain poorly documented, with limited prospective data on predictors of morbidity and mortality.
Aims: This study aimed to assess medium-term outcomes and identify key predictive factors of mortality or surgical reintervention at 2 years after transcatheter PVL closure.
Methods: The prospective Fermeture de Fuite ParaProthétique (FFPP) Registry included consecutive symptomatic patients undergoing transcatheter PVL closure across 24 European centres between 2017 and 2019. Predictive factors for mortality and surgical reintervention were analysed over a 2-year follow-up.
Results: A total of 213 symptomatic patients underwent 237 procedures. The mean age was 68±11 years, with a median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II of 6 (interquartile range [IQR] 4-10). PVL involved the mitral valve in 64.6% of cases and mechanical prostheses in 53.3%. Heart failure and haemolytic anaemia were present in 89.5% and 49.8% of patients, respectively. The transapical approach was used in 6.8% of cases. Technical success was achieved in 87.3% of procedures, and clinical success at 1 month was achieved in 70.5% of patients. The median follow-up was 24.4 months (IQR 23.2-26.4). The survival rate at 2 years without the need for surgical reintervention was 66.1% (95% confidence interval [CI]: 60.1-72.7). Multivariate analysis identified mitral PVL, mechanical valves, and haemolytic anaemia as independent risk factors for adverse outcomes during follow-up. The absence of clinical success at 1 month was the strongest predictor of adverse outcomes (hazard ratio 5.00, 95% CI: 2.70-9.09; p=0.001).
Conclusions: Transcatheter PVL closure offers a durable therapeutic option for high-risk patients when early clinical success is achieved. Mitral valve involvement, mechanical prostheses, and haemolytic anaemia remain key predictors of poor outcomes over a 2-year follow-up.
Paravalvular leaks (PVLs) are a frequent complication following surgical valve replacement, occurring in 5-18% of prosthetic valves. The incidence varies according to valve position, ranging from 2-10% for aortic valves and from 7-17% for mitral valves123. While many PVLs remain asymptomatic and clinically insignificant4, others can lead to serious complications, such as heart failure and haemolytic anaemia, often requiring repeat intervention5.
In recent years, transcatheter PVL closure (PVLc) has emerged as a well-established, minimally invasive alternative to redo-surgical intervention, particularly for patients considered at high or prohibitive surgical risk678. This shift in management is supported by growing evidence of its efficacy and safety. Reflecting this evolution, both European and American guidelines910 now recommend transcatheter PVLc for symptomatic patients, especially those presenting with heart failure or haemolytic anaemia.
We previously reported encouraging early outcomes from our international multicentre cohort11, confirming that transcatheter PVLc is an effective and safe option1213. However, our findings also highlighted a lower clinical success rate in patients with haemolysis and/or mechanical prostheses. Despite these advances, data on mid- and long-term outcomes remain limited, with only a few studies addressing this critical aspect. Moreover, the role of cardiac morphological parameters as predictors of morbidity and mortality is still poorly defined in current international guidelines. This lack of comprehensive long-term data represents a significant gap in the management of PVLs. A better understanding of prognostic factors is essential to optimise patient selection, anticipate complications, and improve overall outcomes.
In this study, we aim to fill these gaps by providing a detailed analysis of medium-term outcomes and identifying predictors of mortality or surgical reintervention in a prospective, multicentre European cohort of patients undergoing transcatheter PVLc. Our findings are intended to guide clinical practice and contribute to refining future recommendations for this high-risk population.
Methods
Study population
We designed a prospective, multicentre, observational registry named Fermeture de Fuite ParaProthétique (FFPP, closure of PVLs). The study design and methods have been previously reported11. Briefly, 24 centres across France, Poland, Belgium, and Turkey each enrolled at least one patient, between 1 January 2017 and 31 December 2019. All participating centres included consecutive patients referred for transcatheter PVLc. Patients were selected for transcatheter PVLc instead of first-line surgical PVLc if they were considered at high or prohibitive surgical risk or if transcatheter PVLc was considered highly feasible as an alternative to surgery. Patients were selected by the Heart Team at each participating centre according to local practice. All participating centres had prior experience in PVLc and the most experienced operators frequently assisted in procedures in low-volume centres, thereby minimising the risk of learning curve bias. The study was coordinated by the clinical research unit of Marie-Lannelongue Hospital (Le Plessis-Robinson, France) and was approved by an independent ethics committee (CCTIRS, 23 November 2016, n°16.622bis). It was registered at ClinicalTrials.gov: NCT05089136 and complied with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent to participate during the index hospitalisation. The study was funded by Marie-Lannelongue Hospital.
Preprocedural data
For each patient, an electronic case report form (http://easy-crf.com [Easy-CRF SAS]) was completed. Data collected included medical history (particularly prior heart surgery), symptoms, physical examination findings, laboratory test results, and echocardiographic parameters. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II (http://euroscore.org) was systematically calculated. Renal failure was defined as creatinine clearance below 60 mL/min/1.73 m², while severe renal failure was defined as below 30 mL/min/1.73 m². Heart failure was defined according to the European Society of Cardiology guidelines14. Anaemia was defined as haemoglobin <13 g/dL in males and <12 g/dL in females, while haemolytic anaemia was defined as anaemia associated with elevated lactic dehydrogenase, the presence of schistocytes, and/or low haptoglobin, in the absence of other known causes of anaemia.
Periprocedural data
Technical success was defined as correct positioning of at least one device within the leak with a reduction in regurgitation of at least one severity grade and a final echocardiographic leak severity grade ≤2; successful access, delivery, and retrieval of the device delivery system; no valve dysfunction; and no need for surgical conversion. Echocardiographic assessment of PVL severity followed the expert consensus statement of the Valve Academic Research Consortium15. Clinical success was defined as survival at 1 month without rehospitalisation for heart failure, need for blood transfusion, or open-heart valve surgery.
Follow-up
Follow-up was prospectively conducted at 1 month, 1 year, and 2 years, assessing hospitalisation for heart failure, blood transfusion for haemolytic transfusion, surgical valve reintervention, and death (including cause if known). Follow-up was performed by the local investigator either through clinical evaluation or by telephone contact.
Statistical analysis
Continuous variables are described as mean±standard deviation (SD) if they follow a normal distribution (determined using the Shapiro-Wilk test). If non-normally distributed, they are reported as median (interquartile range [IQR]). Categorical variables are presented as counts (%). Comparisons of continuous variables were conducted using the Student’s t-test for normally distributed data and the Mann-Whitney U test otherwise. For categorical variables, Pearson’s χ² test was applied, with the Monte Carlo procedure used when any expected count was below 5. Associations between variables of interest and mortality or surgical reintervention were assessed by univariate logistic regression. Multivariate models were constructed by including variables with a p-value<0.1 in the univariate analysis, provided that at least 80% of values were available. All p-values are two-sided, with statistical significance set at p<0.05. Risk estimates are provided with 95% confidence intervals (95% CIs). Time-to-event curves were generated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed using R software, v3.5.1 (R Foundation for Statistical Computing).
Results
Between January 2017 and December 2019, a total of 213 patients underwent 237 transcatheter PVLc procedures. Of these, 194 patients had a single procedure, 15 patients underwent two procedures, 3 patients had three procedures, and 1 patient required four procedures. The baseline characteristics of the study population, categorised by the targeted valve, are summarised in Table 1. The characteristics of the 237 procedures, along with univariate and multivariate analyses assessing associations with mortality or surgical reintervention, are presented in Table 2.
Table 1. Baseline characteristics of the study population.
| Variable | All procedures (N=237) | Mitral (N=153) | Aortic (N=80) | Tricuspid (N=4) | p-value (mitral vs aortic) |
|---|---|---|---|---|---|
| Age at procedure, years | 68±11 | 68±10 | 67±12 | 62±14 | 0.518 |
| Male sex | 133 (56.8) | 72 (47.1) | 60 (75.0) | 1 (25.0) | <0.001 |
| BMI, kg/m² | 25±6 | 25±5 | 26±7 | 19±1 | 0.159 |
| Previous heart surgery | <0.001 | ||||
| 0 | 7 (2.9) | 3 (1.9) | 4 (5.0) | 0 (0) | |
| 1 | 107 (45.0) | 59 (38.6) | 47 (58.0) | 1 (25.0) | |
| 2 | 72 (30.4) | 45 (29.4) | 25 (31.3) | 2 (50.0) | |
| ≥3 | 52 (21.9) | 46 (30.0) | 5 (6.6) | 1 (25.0) | |
| Previous transcatheter PVLc procedure | 0.339 | ||||
| 1 | 28 (11.8) | 19 (12.4) | 9 (11.1) | 3 (75.0) | |
| 2 | 5 (2.1) | 5 (32.7) | 0 (0) | 0 (0) | |
| 3 | 2 (0.8) | 1 (0.7) | 0 (0) | 0 (0) | |
| Type of targeted valvea | 0.004 | ||||
| Biological prosthesis | 102 (45.3) | 68 (44.7) | 33 (47.8) | 1 (25.0) | |
| Mechanical prosthesis | 120 (53.3) | 84 (55.3) | 36 (52.2) | 0 (0) | |
| Annuloplasty | 1 (0.4) | 0 (0) | 0 (0) | 1 (25.0) | |
| Symptoms at inclusion | 0.001 | ||||
| Heart failure | 212 (89.5) | 138 (90.2) | 70 (87.5) | 4 (100) | |
| Haemolytic anaemia | 118 (49.8) | 90 (58.8) | 27 (33.8) | 0 (0) | |
| NYHA Class | 0.028 | ||||
| I | 1 (0.5) | 0 (0) | 1 (1.4) | 0 (0) | |
| II | 42 (19.8) | 22 (16.0) | 20 (28.6) | 0 (0) | |
| III | 135 (63.7) | 90 (65.2) | 42 (60.0) | 3 (75.0) | |
| IV | 34 (16.0) | 26 (18.8) | 7 (10.0) | 1 (25.0) | |
| CrCl <60 mL/min/1.73 m2 | 77 (32.4) | 50 (32.7) | 26 (32.1) | 1 (25.0) | 1 |
| LVEF, %b | 55 (45-60) | 55 (47-61) | 50 (42-60) | 55 (49-63) | 0.003 |
| sPAP, mmHgc | 49 (39-60) | 50 (42-64) | 40 (32-47) | 44 (38-45) | <0.001 |
| EuroSCORE IId | 6 (4-10) | 7 (4-11) | 5 (3-8) | 8 (6-14) | <0.001 |
| Data are presented as mean±SD, median (IQR), or n (%). a5% missing data; b3.7% missing data; c15.1% missing data; d13.9% missing data. BMI: body mass index; CrCl: creatinine clearance; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PVLc: paravalvular leak closure; SD: standard deviation; sPAP: systolic pulmonary artery pressure | |||||
Table 2. Procedural characteristics with univariate and multivariate analysis according to primary outcome.
| Variable | Procedures (n=237) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age, years | 68±11 | 1.00 (0.98-1.02) | 0.687 | ||
| Male sex | 133 (56.8) | 1.27 (0.81-1.98) | 0.299 | ||
| BMI, kg/m² | 25±6 | 1.00 (0.96-1.04) | 0.927 | ||
| Previous heart surgery (ref. 1) | |||||
| 2 | 72 (30.4) | 1.84 (1.02-3.34) | 0.043* | ||
| ≥3 | 52 (21.9) | 4.89 (2.83-8.46) | <0.001* | ||
| Previous transcatheter PVLc procedure (ref. 0) | |||||
| 1 | 28 (11.8) | 1.43 (0.77-2.64) | 0.260 | ||
| 2 | 5 (2.1) | 2.78 (0.67-11.56) | 0.161 | ||
| 3 | 2 (0.8) | 4.88 (1.18-20.19) | 0.029* | ||
| Mitral vs aortic valve | 153 (64.6) | 2 (1.19-3.33) | 0.009* | 1.92 (1.03-3.70) | 0.041* |
| Mechanical vs biological prosthesisa | 120 (53.3) | 2.04 (1.25-3.33) | 0.004* | 1.88 (1.12-3.22) | 0.017* |
| Heart failure | 212 (89.5) | 0.38 (0.21-0.68) | 0.001* | ||
| Haemolytic anaemia | 118 (49.8) | 2.07 (1.31-3.25) | 0.002* | 1.96 (1.17-3.29) | 0.010* |
| NYHA Class (ref. I-II) | |||||
| III | 135 (63.7) | 1.64 (0.80-3.38) | 0.179 | ||
| IV | 34 (16.0) | 2.51 (1.07-5.88) | 0.034* | ||
| CrCl <60 mL/min/1.73 m2 | 77 (32.4) | 1.88 (1.21-2.92) | 0.005* | ||
| LVEF, %b | 55 (45-60) | 1.03 (1.01-1.05) | 0.004* | ||
| sPAP, mmHgc | 49±15 | 1.03 (1.01-1.04) | <0.001* | ||
| EuroSCORE IId | 6 (4-10) | 1.04 (1.02-1.07) | <0.001* | ||
| Recent blood transfusion (<3 months)e | 87 (38.2) | 1.75 (1.11-2.77) | 0.016* | ||
| Number of PVLs treated (ref. 1) | |||||
| 2 | 63 (26.6) | 1.02 (0.62-1.69) | 0.933 | ||
| 3 | 9 (3.8) | 1.34 (0.42-4.29) | 0.623 | ||
| Transapical access | 16 (6.8) | 2.63 (1.38-5.00) | 0.003* | 1.91 (0.98-3.75) | 0.059 |
| Number of devices implanted | |||||
| 1 | 138 (58.2) | 2.58 (1.25-5.33) | 0.010* | ||
| 2 | 58 (24.4) | 1.64 (0.99-2.71) | 0.057 | ||
| 3 | 16 (6.8) | 0.84 (0.30-2.36) | 0.745 | ||
| 4-5 | 7 (2.9) | 1.94 (0.60-6.29) | 0.268 | ||
| Procedure duration, minf | 90 (60-143) | 1.01 (1.00-1.01) | <0.001* | ||
| Absence of technical success | 30 (12.7) | 2.32 (1.19-4.54) | 0.013* | ||
| Residual regurgitationg | 101 (42.6) | 3.59 (2.14-6.01) | <0.001* | ||
| Absence of clinical success at 1 month | 37 (29.5) | 1.25 (3.44-9.09) | <0.001* | ||
| Data are presented as mean±SD, median (IQR), or n (%). a5% missing data; b3.7% missing data; c15.1% missing data; d13.9% missing data; e3.8% missing data; f11.4% missing data; g10.1% missing data; *indicates statistical significance. BMI: body mass index; CI: confidence interval; CrCl: creatinine clearance; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HR: hazard ratio; IQR: interquartile range; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PVL: paravalvular leak; PVLc: paravalvular leak closure; ref.: reference; SD: standard deviation; sPAP: systolic pulmonary artery pressure | |||||
Study population
Among the 237 procedures, the median age was 68±11 years, with 56.8% being male, and all patients were symptomatic at baseline, with 89.5% presenting with heart failure and 49.8% with haemolytic anaemia. The mitral valve was targeted in 64.6% of procedures, and a mechanical valve was present in 53.3%. Among procedures with a mitral PVL, 50.3% exhibited both heart failure and haemolytic anaemia, compared to 27.5% in the aortic PVL group. A history of at least two prior heart surgeries was recorded in 52.3% of all procedures. Seven of the patients had not undergone any previous cardiac valve surgery. Of these patients, three underwent transcatheter mitral valve implantation and four underwent aortic valve implantation. The median EuroSCORE II was 6 (IQR 4-10), with higher scores observed in the mitral PVL group (7 [IQR 4-11]) compared to the aortic PVL group (5 [IQR 3-8]).
PVLc procedure
Treatment of multiple PVLs (≥2) was performed in 30.6% of cases, and at least two devices were implanted in 34.1% of procedures. Technical success was achieved in 87.3% of procedures, corresponding to 91.0% of patients with success rates of 85.0% for mitral PVLs and 91.4% for aortic PVLs. Clinical success at 1 month was observed in 70.5% of procedures.
Follow-up
The median follow-up of surviving patients was 24.4 months (IQR 23.2-26.4). Complete follow-up was achieved in all patients. At 2-year follow-up, a total of 80 events were recorded, comprising 44 surgical reinterventions and 36 deaths occurring without prior surgical reintervention. Among patients who underwent surgical reintervention, 9 deaths were reported during subsequent follow-up. Sixteen events of death or surgical reintervention occurred within the first month after the procedure. The Kaplan-Meier curve illustrating survival free from surgical reintervention is presented in Figure 1A. Survival without surgical reintervention was 75.8% (95% CI: 70.5-81.6) at 1 year and 66.1% (95% CI: 60.1-72.7) at 2 years.
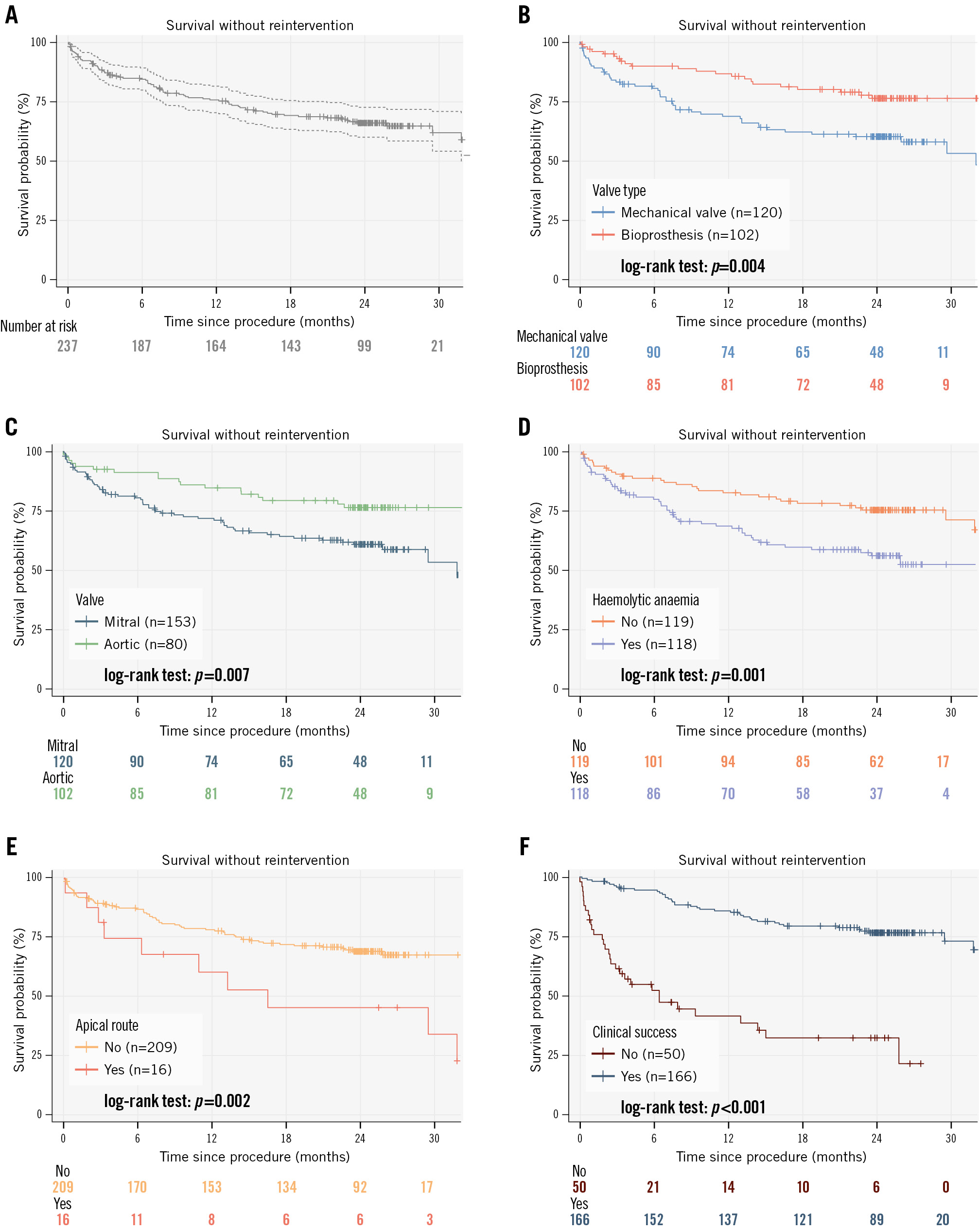
Figure 1. Kaplan–Meier curves for survival without surgical reintervention across clinical and procedural subgroups. A) Kaplan-Meier curve analysis according to survival without surgical reintervention. Cumulative incidences of survival without surgical reintervention among patients with a mechanical versus bioprosthetic valve (B); among patients with a mitral versus aortic valve (C); among patients with versus without haemolytic anaemia (D); among transapical versus non-transapical patients (E); and among patients with versus without clinical success (F).
Kaplan-Meier analysis
Kaplan-Meier analysis identified the following parameters as independent risk factors for death or surgical reintervention at 24-month follow-up (Figure 1B-Figure 1C-Figure 1D-Figure 1E-Figure 1F): survival without surgical reintervention was 60.2% for mechanical valves versus 76.3% for bioprostheses (log-rank p=0.004); 60.9% for mitral PVL involvement versus 76.4% for aortic PVL (log-rank p=0.007); 56.0% for patients with haemolytic anaemia versus 75.4% without (log-rank p=0.001); 45.1% for a transapical approach versus 69.0% for a non-transapical approach (log-rank p=0.002); and 32.5% for patients without clinical success at 1 month versus 76.6% with clinical success (log-rank p<0.001).
Multivariate analysis
Multivariate analysis identified the following parameters as independent risk factors for death or surgical reintervention (Table 2): mitral PVL involvement (hazard ratio [HR] 1.92, 95% CI: 1.03-3.70; p=0.041); mechanical valve (HR 1.88, 95% CI: 1.12-3.22; p=0.017); haemolytic anaemia (HR 1.96, 95% CI: 1.17-3.29; p=0.010). Use of a transapical approach tended to be associated with worse outcomes but was not significant (HR 1.91, 95% CI: 0.98-3.75; p=0.059). The statistical concordance index (C-index) for this multivariate model was 78%, indicating good predictive performance. When the absence of clinical success at 1 month was added to a second, separate multivariate model alongside the other variables, it emerged as the strongest risk factor (HR 5.00, 95% CI: 2.70-9.09; p=0.001).
Haemolytic anaemia
Of the 118 procedures (49.8%) in patients who presented with haemolytic anaemia, 17 cases (7.2%) presented with haemolytic anaemia alone. After these procedures, death or surgical reintervention was reported in 9 cases (52.9%).
Transapical approach
A transapical approach was performed in 6.8% of procedures, exclusively for mitral PVL cases. No significant differences were observed in terms of baseline characteristics or procedural success at 1 month when comparing the procedure with transapical versus transfemoral access for mitral PVLs (Supplementary Table 1). After the transapical approach, surgical reintervention was required in 3 cases (18.8%) versus 41/221 in the remaining cohort (18.6%). After transapical procedures, the rate of death was high: 10 patients died (62.5%) including one after a surgical reintervention, while 35 deaths were recorded in total after other mitral or aortic PVLc (15.8%). Kaplan-Meier curves confirmed a lower survival without reintervention after the transapical approach versus transfemoral mitral PVLc (45.1% [95% CI: 25.4-80.4] vs 63.6% [95% CI: 55.4-73.0] at 2 years; log-rank p=0.034) (Supplementary Figure 1).
Discussion
To our knowledge, this prospective multicentre study represents one of the most comprehensive evaluations of medium-term outcomes following transcatheter PVLc. While our findings confirm that this high-risk population remains exposed to significant rates of mortality and surgical reintervention, they also provide compelling evidence of the durable benefits of transcatheter PVLc when early clinical success is achieved. Importantly, clinical success at 1 month emerged as the strongest independent predictor of favourable medium-term outcomes, demonstrating a substantial reduction in the risk of death or surgical reintervention. This highlights the crucial role of early symptomatic improvement in securing the medium-term efficacy of transcatheter PVLc. These results support the notion that, when performed successfully, this minimally invasive procedure offers a meaningful and sustained therapeutic benefit, even in patients deemed at high or prohibitive surgical risk. Our study reaffirms previously known risk factors, such as mitral PVL involvement and haemolytic anaemia, but also introduces new prognostic insights, notably the negative impact of mechanical valves and transapical approach on medium-term outcomes, as shown in the Central illustration. The identification of these factors can significantly enhance patient selection and procedural planning, ensuring that the benefits of PVLc are maximised in appropriately selected individuals.
The inclusion criteria of this study were closely aligned with current European and American guidelines910, focusing on symptomatic patients at high or prohibitive surgical risk as reflected by a median EuroSCORE II of 6 (IQR 4-10). This alignment reinforces the clinical relevance and generalisability to real-world practice, particularly for long-term outcome assessment in this challenging patient population. While significant progress has been made in transcatheter PVLc techniques over recent years, our study highlights that morbidity and mortality remain considerable, with approximately 34% of patients experiencing either death or surgical reintervention within 2 years. These figures underscore the persistent complexity of managing paravalvular leaks in high-risk patients, despite less invasive therapeutic options. These results are consistent with previous mid- and long-term cohort studies161718192021, thereby contributing robust, prospective multicentre data that confirm the ongoing need for optimised patient selection and procedural strategies to further improve outcomes in this setting.
Our findings confirm that mitral PVLs are associated with worse long-term outcomes following transcatheter closure, a result consistent with several, though not all, previous studies1718. The higher pressure gradients associated with mitral PVLs likely contribute to more severe clinical presentations, often combining heart failure and haemolytic anaemia, as reflected by lower clinical success rates, increased heart failure readmissions, and greater transfusion needs2223. Despite the inherent complexity of mitral PVLs, our data suggest that patients achieving early clinical improvement still derive substantial long-term benefit.
Haemolytic anaemia, a debated prognostic factor, was associated with worse long-term outcomes in our cohort. While some studies align with this finding16202425, others do not171826. Persistent haemolysis despite PVL reduction may account for cases of clinical failure requiring surgical reintervention. However, when haemolysis is effectively resolved, transcatheter PVLc offers a clear advantage by reducing the need for repeat surgery in this high-risk population.
This study introduces two additional prognostic parameters: (1) mechanical valves as a predictor of poorer outcomes compared to bioprostheses and (2) the transapical approach as a risk factor for adverse medium-term prognosis. The presence of mechanical valves may increase morbidity due to high-velocity jets through PVLs, promoting complications such as haemolysis and posing technical challenges related to device positioning and occluder sizing5. While the transapical approach has shown promising preliminary results in experienced centres2127, our study suggests an association with worse mid- to long-term outcomes in Kaplan-Meier curve analysis and a trend towards worse outcomes in multivariate analysis (p=0.059).
Interestingly, heart failure symptoms prior to the procedure were associated with better outcomes in univariate analysis, suggesting that patients treated primarily for heart failure may benefit more from PVLc than those treated for haemolysis – although this effect did not persist in multivariate analysis. Finally, our univariate analysis demonstrated that the use of multiple devices for PVL closure was not associated with poorer outcomes, supporting the feasibility and safety of this approach in selected cases.
Overall, this study highlights that transcatheter PVLc is not only a safe and effective alternative to surgery, but when successful, it offers sustained reductions in morbidity and mortality. These findings advocate for early and optimised intervention strategies, aiming for clinical success as a pivotal goal to secure long-term benefits. Further research is essential to refine patient selection criteria and explore novel techniques, such as covered stents or dedicated devices, to further expand the therapeutic potential of PVLc28.
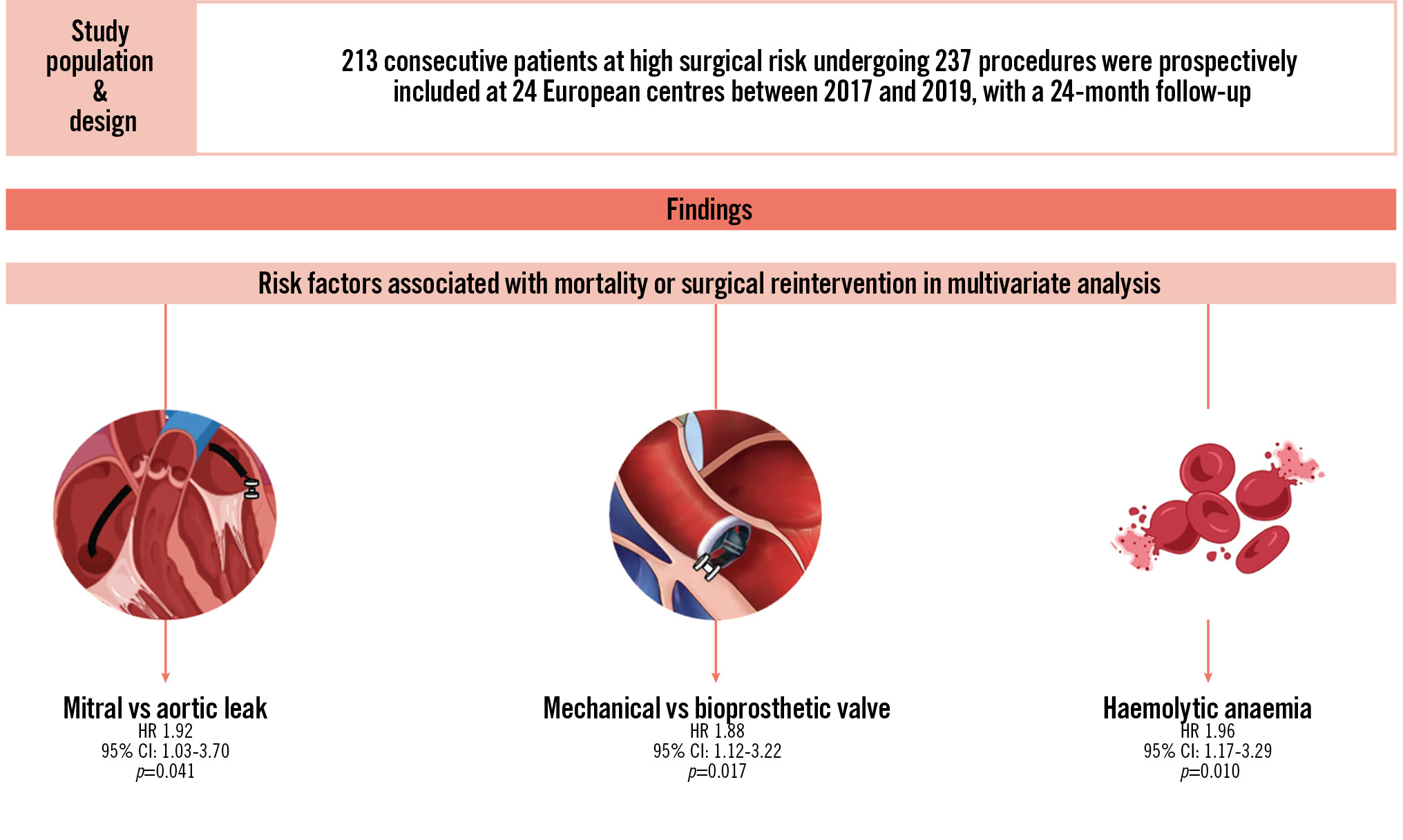
Central illustration. Medium-term outcomes and prognostic factors after transcatheter paravalvular leak closure: an international prospective multicentre registry. CI: confidence interval; HR: hazard ratio
Limitations
This study provides valuable insights into the medium-term outcomes of transcatheter paravalvular leak closure; however, several limitations should be acknowledged. Firstly, the absence of a control group (e.g., patients managed conservatively or by repeat surgery) limits direct comparisons with alternative treatment strategies. Secondly, the multicentre design introduces variability in operator experience and procedural techniques, which may have influenced outcomes. Thirdly, the inclusion of diverse procedural approaches – such as transapical access and the use of multiple devices – adds heterogeneity that could affect generalisability. Finally, although the 2-year follow-up provides robust midterm data, it remains insufficient to fully assess long-term device durability and PVL progression. However, these limitations are inherent to real-world cohort studies, which reflect routine clinical practice and, therefore, the true performance of transcatheter PVL closure in diverse and complex patient populations. This pragmatic design enhances the external validity of our findings and ensures their applicability to everyday clinical settings. Despite these considerations, this study remains one of the most comprehensive evaluations to date, identifying key prognostic factors and offering valuable guidance for optimising patient selection, procedural strategy, and future research directions.
Conclusions
Transcatheter PVL closure is a durable therapeutic option in high-risk patients, provided early clinical success is achieved, while mitral valve involvement, a mechanical prosthesis, and haemolytic anaemia remain key predictors of adverse outcomes.
Impact on daily practice
This large, prospective, multicentre study confirms that transcatheter paravalvular leak closure is a viable long-term therapeutic option for high-risk, symptomatic patients. Early clinical success is crucial as it is a strong predictor of long-term outcomes and can inform early patient management. Identifying key prognostic factors, such as mitral position, mechanical prosthesis, haemolytic anaemia, and transapical access, can support the tailoring of procedural planning and postprocedural surveillance.
Acknowledgements
We thank Stephane Morisset for assisting with the statistical analysis, Vincent Roth for developing the electronic case report form, and Florence Lecerf for managing the project.
Funding
The study was coordinated by the clinical research unit of Marie-Lannelongue Hospital (Le Plessis-Robinson, France).
Conflict of interest statement
S. Hascoët, E. Brochet, and G. Bonnet report proctoring for Abbott. G. Smolka reports proctoring for and fees or honoraria for lectures, presentations, and educational events from Abbott and Occlutech. F. Bouisset received consulting fees from B. Braun; and honoraria for lectures from Boston Scientific and Abbott. G. Leurent is a speaker for and received proctoring fees from Abbott and Edwards Lifesciences. N. Dumonteil received consulting fees and fees or honoraria for lectures and presentations from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Ancora Heart; and reports participation in a data safety monitoring board or advisory board for Ancora Heart. M. Nejjari is a proctor for Abbott, Boston Scientific, and Medtronic; and received consulting fees from Boston Scientific, Abbott, Medtronic, and Robocath. N. Hammoudi received consulting fees from Abbott, Philips, Boehringer Ingelheim, Bayer, and Novartis; fees or honoraria for lectures from Abbott, Philips, Novartis, and GE HealthCare; support for attending meetings and/or travel from Boehringer Ingelheim, Bayer, and Novartis; and participation in a data safety monitoring board or advisory board for Novartis. D. Champagnac received proctoring fees from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

