Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The role of optical coherence tomography (OCT) guidance during percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) remains inconclusive.
Aims: This study aimed to evaluate the impact of OCT-guided PCI in ACS patients with complex lesions.
Methods: The Optical CoherenCe Tomography-gUided Coronary Intervention in Patients With Complex Lesions (OCCUPI) Trial compared PCI with OCT guidance versus angiography guidance in patients who required drug-eluting stent implantation for complex lesions. This post hoc analysis focused on participants presenting with ACS. The primary outcome was 1-year major adverse cardiac events (a composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation).
Results: Out of 1,604 randomised patients, 790 (49.3%) and 814 (50.7%) presented with ACS and chronic coronary syndrome (CCS), respectively. Among patients with ACS, the incidence of the primary outcome was 4.9% in the OCT-guided group and 9.5% in the angiography-guided group (hazard ratio [HR] 0.50, 95% confidence interval [CI]: 0.29-0.87; p=0.011). Among patients with CCS, its incidence was 4.4% and 5.4%, respectively (HR 0.80, 95% CI: 0.43-1.50; p=0.479). No significant interaction between clinical presentation and imaging guidance strategy was observed for the primary outcome (pinteraction=0.273). Among patients with ACS randomised to OCT guidance, the achievement of stent optimisation by OCT was associated with a lower incidence of the primary outcome compared with suboptimisation (2.9% vs 9.7%; HR 0.29, 95% CI: 0.12-0.72; p=0.004).
Conclusions: In ACS patients with complex lesions, OCT-guided PCI demonstrated an evident cardiovascular benefit over angiography-guided PCI, a finding endorsed by current guidelines. (ClinicalTrials.gov: NCT03625908)
Intravascular imaging guidance during percutaneous coronary intervention (PCI) is expected to improve clinical outcomes compared with angiography guidance, particularly in patients with complex lesions and those presenting with acute coronary syndrome (ACS)1234567. Timely revascularisation is the cornerstone of treatment for patients with ACS, with intravascular imaging guidance facilitating culprit lesion identification and optimising stent implantation1348. Among intravascular imaging techniques, optical coherence tomography (OCT) provides more intuitive, higher-resolution images than intravascular ultrasound (IVUS)18. Nonetheless, while intravascular imaging guidance exclusively with IVUS has been investigated in patients with ACS, evidence supporting the impact of OCT guidance in patients with ACS remains insufficient91011. Previous randomised trials have investigated the impact of PCI with OCT guidance versus angiography guidance in patients with complex lesions1213. Although ACS and chronic coronary syndrome (CCS) differ in their underlying pathophysiology, plaque vulnerability, and the risk of future cardiovascular events, the role of OCT – particularly in patients with ACS – was left unexplored in these trials23481213. Recently, the Optical CoherenCe Tomography-gUided Coronary Intervention in Patients With Complex Lesions (OCCUPI) Trial has demonstrated the superiority of OCT guidance over angiography guidance with respect to 1-year major adverse cardiac events in patients requiring drug-eluting stent (DES) implantation for complex lesions14. Therefore, the present post hoc analysis of the OCCUPI Trial aimed to evaluate the impact of OCT guidance versus angiography guidance in patients undergoing PCI for complex lesions, particularly those with ACS.
Methods
Study design and patient population
The study design and rationale of the OCCUPI Trial has been previously described in detail14. Briefly, the OCCUPI Trial was an investigator-initiated, multicentre, randomised, open-label, superiority trial that evaluated PCI with OCT guidance versus angiography guidance in 1,604 patients at 20 centres in South Korea14. Patients requiring PCI with DES implantation for complex lesions were eligible to participate in the trial14. Complex lesions were defined as acute myocardial infarction, long lesion, bifurcation lesion, small vessel disease, unprotected left main artery disease, in-stent restenosis, calcified lesion, intracoronary thrombus visible on angiography, chronic total occlusion, bypass graft lesion, or stent thrombosis14. The full inclusion and exclusion criteria are provided in Supplementary Appendix 114. The trial was approved by the institutional review board of each participating centre and followed the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to their enrolment. The present post hoc analysis evaluated the impact of OCT guidance during PCI for complex lesions according to clinical presentation, with a specific focus on patients with ACS. The diagnosis of ACS was determined by the investigators at the time of enrolment, primarily based on chest pain characteristics, cardiac biomarker levels, and electrocardiographic findings, in accordance with clinical guidelines and expert consensus1516.
Randomisation and treatment
Consenting patients were randomly assigned to undergo PCI with OCT guidance (OCT-guided group) or angiography guidance without OCT (angiography-guided group) at a 1:1 ratio14. PCI was performed following the conventional standard methods using everolimus-eluting stents (XIENCE Alpine or XIENCE Sierra [both Abbott]). Detailed descriptions regarding OCT-guided and angiography-guided PCI are provided in the previous study and in Supplementary Appendix 214. In patients assigned to the OCT-guided group, device sizing, landing, and stent optimisation were assessed under OCT guidance14. In the OCCUPI Trial, the OCT-defined stent optimisation criteria – comprising stent expansion, apposition, and edge dissection – were prespecified14. Achievement of stent optimisation by OCT was defined as meeting the following acceptability criteria for all three components (stent expansion, apposition, and edge dissection) on the final post-stent OCT evaluation14. Acceptable stent expansion was defined as meeting any of the following: minimal stent area ≥80% of the mean reference lumen area, ≥100% of the distal reference lumen area, or absolute minimal stent area >4.5 mm2 (for a non-left main artery lesion). Acceptable stent apposition was indicated by acute malapposition with a distance of <400 μm. Acceptable edge dissection was defined as the absence of major dissection, which was characterised by the presence of any of the following: dissection flap circumference ≥60°, dissection length ≥3 mm, or deeper vessel injury (intramural haematoma, penetration into media or adventitia). In the OCT-guided group, patients who satisfied the prespecified stent optimisation criteria were further classified as the stent optimisation group, whereas those that did not were classified as the stent suboptimisation group14. In patients assigned to the angiography-guided group, device size was recommended to be determined based on quantitative angiographic assessment (Supplementary Appendix 2)14.
Study outcomes and follow-up
The primary outcome was 1-year major adverse cardiac events, defined as a composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation14. The secondary outcomes included the individual components of the primary outcome, as well as all-cause death, any revascularisation, stroke, bleeding, contrast-induced nephropathy, and stent optimisation, as confirmed on the final post-stent OCT evaluation14. As in the primary report of the OCCUPI Trial, post hoc composite outcomes included the composite of cardiac death, myocardial infarction, or stent thrombosis; the composite of cardiac death, spontaneous myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation; and the composite of cardiac death, spontaneous myocardial infarction, or stent thrombosis14. Definitions of each clinical outcome are detailed in Supplementary Appendix 31617181920. All clinical outcomes were adjudicated by an independent clinical endpoint committee, which was blinded to the random assignments and primary results of the trial14. Clinical follow-up was performed at 1, 3, 6, and 12 months after PCI, and dual antiplatelet therapy (DAPT) was recommended for at least 6 months after PCI14. Guideline-directed medical therapy for the management of cardiovascular risk factors was strongly encouraged, irrespective of group assignment14.
Statistical analysis
The full statistical analysis plan of the OCCUPI Trial has been described previously14. All analyses were performed on an intention-to-treat basis. The cumulative incidence of the clinical outcomes was evaluated using a Kaplan-Meier survival analysis based on the time of enrolment to the occurrence of the first event of interest during follow-up. The event rates between the two groups were compared using log-rank tests. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using a Cox proportional hazard model. The interaction terms between clinical presentation (ACS vs CCS) and imaging guidance strategy (OCT guidance vs angiography guidance) for the primary, secondary, and post hoc composite outcomes were evaluated using formal interaction testing. Further details on statistical analyses are provided in Supplementary Appendix 4142122. A two-sided p-value of <0.05 was considered significant for all tests, with no adjustment for multiple comparisons. All statistical analyses were performed using SPSS software, version 25.0 (IBM) and R software, version 4.2.2 (R Foundation for Statistical Computing).
Results
Study population and baseline characteristics
Between January 2019 and September 2022, a total of 1,604 patients requiring PCI with DES implantation for complex lesions were enrolled in the OCCUPI Trial. Among them, 790 (49.3%) and 814 (50.7%) presented with ACS and CCS, respectively (Figure 1). Among patients with ACS, 327 (41.4%) had acute myocardial infarction. Baseline characteristics according to clinical presentation are presented in Table 1. Patients with ACS were younger, exhibited a higher body mass index, and included a higher proportion of current smokers than patients with CCS. Additionally, patients with ACS were more likely to be discharged with potent P2Y12 inhibitors and were also found to have lower proportions of hypertension, dyslipidaemia, and prior PCI, as well as a lower left ventricular ejection fraction. The baseline characteristics between the OCT-guided and angiography-guided groups were well balanced in both patients with ACS and those with CCS, except for body mass index and left ventricular ejection fraction in patients with CCS (Supplementary Table 1). 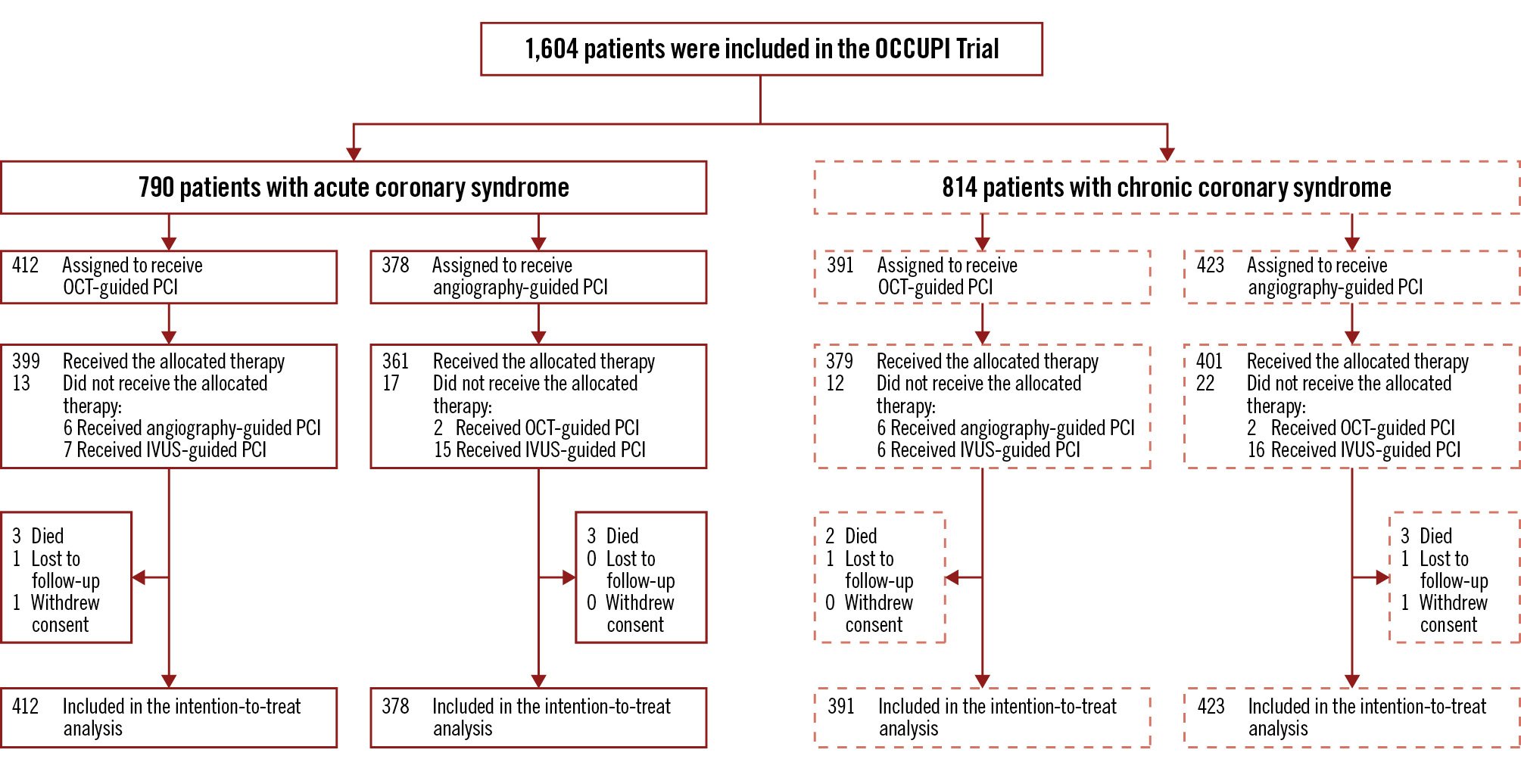
Figure 1. Study flow of participants. IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Table 1. Baseline characteristics according to clinical presentation.
| Total population (N=1,604) | ACS patients (N=790) | CCS patients (N=814) | p-value | |
|---|---|---|---|---|
| Age, years | 64 (57-70) | 63 (56-70) | 65 (59-71) | 0.001 |
| Sex | 0.075 | |||
| Female | 314 (19.6) | 140 (17.7) | 174 (21.4) | |
| Male | 1,290 (80.4) | 650 (82.3) | 640 (78.6) | |
| Body mass index, kg/m2 | 24.7 (22.9-26.6) | 25.0 (23.2-26.9) | 24.4 (22.8-26.3) | <0.001 |
| Hypertension | 917 (57.2) | 428 (54.2) | 489 (60.1) | 0.020 |
| Diabetes mellitus | 523 (32.6) | 252 (31.9) | 271 (33.3) | 0.588 |
| Chronic kidney diseasea | 110 (6.9) | 59 (7.5) | 51 (6.3) | 0.393 |
| Dyslipidaemia | 1,345 (83.9) | 647 (81.9) | 698 (85.7) | 0.043 |
| Current smoker | 307 (19.1) | 208 (26.3) | 99 (12.2) | <0.001 |
| Prior myocardial infarction | 82 (5.1) | 37 (4.7) | 45 (5.5) | 0.513 |
| Prior percutaneous coronary intervention | 330 (20.6) | 143 (18.1) | 187 (23.0) | 0.019 |
| Prior coronary bypass graft surgery | 24 (1.5) | 13 (1.6) | 11 (1.4) | 0.780 |
| Prior stroke | 84 (5.2) | 44 (5.6) | 40 (4.9) | 0.633 |
| Clinical presentation | <0.001 | |||
| CCS | 814 (50.7) | - | 814 (100) | |
| Unstable angina | 463 (28.9) | 463 (58.6) | - | |
| Non-ST-segment elevation myocardial infarction | 223 (13.9) | 223 (28.2) | - | |
| ST-segment elevation myocardial infarction | 104 (6.5) | 104 (13.2) | - | |
| Multivessel coronary artery disease | 416 (25.9) | 211 (26.7) | 205 (25.2) | 0.522 |
| Left ventricular ejection fraction, % | 59.0 (57.3-65.3) | 59.0 (54.0-65.0) | 59.0 (58.0-66.0) | <0.001 |
| Antiplatelet therapy at discharge | ||||
| Aspirin | 1,571 (97.9) | 774 (98.0) | 797 (97.9) | 1.000 |
| P2Y12 inhibitor | ||||
| Any | 1,582 (98.6) | 779 (98.6) | 803 (98.6) | 1.000 |
| Clopidogrel | 1,232 (76.8) | 481 (60.9) | 751 (92.3) | <0.001 |
| Potent P2Y12 inhibitorb | 350 (21.8) | 298 (37.7) | 52 (6.4) | <0.001 |
| Data are median (interquartile range), or number (%). aChronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2 of body surface area. bPotent P2Y12 inhibitor indicates ticagrelor or prasugrel. ACS: acute coronary syndrome; CCS: chronic coronary syndrome | ||||
Lesion and procedural characteristics
Lesion and procedural characteristics according to clinical presentation are presented in Table 2. Patients with ACS showed a higher proportion of target lesions with intracoronary thrombus and lower proportions of long lesions and bifurcation lesions than those with CCS. During PCI, patients with ACS had a lower number of stents implanted, less frequent use of adjunct post-dilation, a smaller amount of contrast volume, and a shorter procedural time. Quantitative coronary angiographic analyses revealed that patients with ACS had a larger postprocedural minimal lumen diameter and smaller diameter stenosis, although the preprocedural minimal lumen diameter and diameter stenosis did not differ between patients with ACS and those with CCS. The lesion characteristics between the OCT-guided and angiography-guided groups were well balanced in both patients with ACS and those with CCS (Supplementary Table 2). During PCI, the OCT-guided group had a larger stent diameter, more frequent use of high-pressure post-dilation, and a higher maximal inflation pressure than the angiography-guided group, irrespective of clinical presentation. However, the amount of contrast volume used and the procedural time were greater in the OCT-guided group, in both patients with ACS and those with CCS. While the preprocedural minimal lumen diameter and diameter stenosis did not differ between the OCT-guided and angiography-guided groups, the OCT-guided group exhibited a larger postprocedural minimal lumen diameter and smaller diameter stenosis than the angiography-guided group, regardless of clinical presentation.
Table 2. Lesion and procedural characteristics according to clinical presentation.
| Total population (N=1,604) | ACS patients (N=790) | CCS patients (N=814) | p-value | |
|---|---|---|---|---|
| Complex lesion characteristicsa | ||||
| Acute myocardial infarction | 327 (20.4) | 327 (41.4) | - | - |
| Long lesionb | 1,152 (71.8) | 519 (65.7) | 633 (77.8) | <0.001 |
| Bifurcation lesion | 381 (23.8) | 165 (20.9) | 216 (26.5) | 0.009 |
| Small vessel diseasec | 267 (16.6) | 138 (17.5) | 129 (15.8) | 0.421 |
| Unprotected left main artery disease | 229 (14.3) | 101 (12.8) | 128 (15.7) | 0.107 |
| In-stent restenosis | 171 (10.7) | 78 (9.9) | 93 (11.4) | 0.355 |
| Calcified lesiond | 149 (9.3) | 66 (8.4) | 83 (10.2) | 0.236 |
| Intracoronary thrombus visible on angiography | 130 (8.1) | 113 (14.3) | 17 (2.1) | <0.001 |
| Chronic total occlusion | 115 (7.2) | 61 (7.7) | 54 (6.6) | 0.455 |
| Bypass graft lesion | 3 (0.2) | 1 (0.1) | 2 (0.2) | 1.000 |
| Stent thrombosis | 1 (0.1) | 1 (0.1) | 0 (0) | 0.493 |
| Procedural characteristicse | ||||
| Total number of treated complex lesions | 1,894 | 944 | 950 | - |
| Number of stents implanted per patient | 1 (1-2) | 1 (1-2) | 1 (1-2) | 0.049 |
| Stent diameter, mm | 3.1 (3.0-3.5) | 3.0 (3.0-3.5) | 3.1 (3.0-3.5) | 0.895 |
| Total stent length, mm | 37.0 (28.9-53.2) | 36.1 (28.8-54.2) | 37.5 (29.2-51.1) | 0.959 |
| Adjunct post-dilation | 1,808 (95.5) | 883 (93.5) | 925 (97.4) | <0.001 |
| High-pressure post-dilation | 1,048 (55.3) | 508 (53.8) | 540 (56.8) | 0.201 |
| Maximal inflation pressure, atmosphere | 16.0 (14.0-18.0) | 16.0 (14.0-18.0) | 16.0 (14.0-18.0) | 0.755 |
| Contrast volume used per patient, mL | 250 (200-330) | 220 (200-310) | 250 (200-350) | <0.001 |
| Procedural time per patient, min | 49.0 (34.0-65.0) | 47.0 (34.0-61.0) | 50.0 (33.5-69.0) | 0.024 |
| Quantitative coronary angiographic analysese | ||||
| Preprocedural reference vessel diameter, mm | 2.81 (2.52-3.12) | 2.80 (2.53-3.12) | 2.83 (2.51-3.11) | 0.954 |
| Preprocedural minimal lumen diameter, mm | 0.70 (0.46-0.94) | 0.70 (0.45-0.95) | 0.70 (0.48-0.92) | 0.897 |
| Preprocedural diameter stenosis, % | 75.0 (67.2-83.3) | 75.1 (66.4-84.0) | 74.9 (68.0-82.8) | 0.799 |
| Lesion length, mm | 32.1 (24.3-48.4) | 31.1 (24.1-50.1) | 32.6 (24.7-47.0) | 0.978 |
| Postprocedural minimal lumen diameter, mm | 2.67 (2.42-2.98) | 2.69 (2.44-3.02) | 2.65 (2.39-2.94) | 0.007 |
| Postprocedural diameter stenosis, % | 13.0 (8.6-18.0) | 12.5 (7.9-18.0) | 14.0 (9.0-18.0) | 0.002 |
| Data are median (interquartile range), n, or n (%). aPatients may have had more than one qualifying characteristic for complex lesions. bA long lesion was defined as a lesion requiring a stent length ≥28 mm. cSmall vessel disease was defined as a lesion w with a ith reference vessel diameter <2.5 mm. dA calcified lesion was defined as a lesion with severe calcification, showing radiopacities without cardiac motion before contrast injection, usually affecting both sides of the arterial lumen. eProcedural characteristics and quantitative coronary angiographic analyses data are per-lesion analyses, unless stated otherwise. ACS: acute coronary syndrome; CCS: chronic coronary syndrome | ||||
Clinical outcomes
Patients were followed up for a median of 365 days (interquartile range [IQR] 365-365 days). The primary outcome occurred in 56 patients (7.1%) among those with ACS and in 40 patients (4.9%) among those with CCS (HR 1.46, 95% CI: 0.97-2.18; p=0.067). During follow-up, DAPT was maintained for a median of 6 months (IQR 6-12 months), with details on its duration according to clinical presentation and complex lesion characteristics presented in Supplementary Table 3. The primary outcome according to clinical presentation and randomised imaging guidance strategy is presented in Table 3. Among patients with ACS, the incidence of the primary outcome was 4.9% in the OCT-guided group and 9.5% in the angiography-guided group (HR 0.50, 95% CI: 0.29-0.87; p=0.011) (Figure 2). Among patients with CCS, the incidence of the primary outcome was 4.4% in the OCT-guided group and 5.4% in the angiography-guided group (HR 0.80, 95% CI: 0.43-1.50; p=0.479) (Supplementary Figure 1). No significant interaction between clinical presentation and imaging guidance strategy was observed for the primary outcome (pinteraction=0.273). The secondary and post hoc composite outcomes are presented in Table 3. With respect to the individual components of the primary outcome, the incidence of myocardial infarction was lower in the OCT-guided group than in the angiography-guided group among patients with ACS. The incidence of cardiac death and ischaemia-driven target vessel revascularisation showed a trend towards being lower in the OCT-guided group. The incidence of stent thrombosis did not differ between the two groups. As for the other secondary and post hoc composite outcomes, compared with the angiography-guided group, the OCT-guided group showed a lower incidence of any revascularisation; composite of cardiac death, myocardial infarction, or stent thrombosis; and composite of cardiac death, spontaneous myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation. No difference in the incidence of contrast-induced nephropathy was noted between the two groups. No significant interactions between clinical presentation and imaging guidance strategy were observed for the secondary and post hoc composite outcomes, except for periprocedural myocardial infarction. The subgroup analysis according to the ACS type is presented in Supplementary Table 4. No significant interactions between the ACS type and imaging guidance strategy were observed for the study outcomes. Among patients with ACS, sensitivity analyses for the primary outcome showed consistent results in those meeting multiple complex lesion criteria defined in the trial (496 [62.8%]) or alternative criteria (392 [49.6%]) (Supplementary Figure 2).
Table 3. Clinical outcomes according to clinical presentation and randomised imaging guidance strategy.
| ACS patients (N=790) | CCS patients(N=814) | pinteractiona | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OCT-guided (N=412) | Angiography-guided (N=378) | HR(95% CI) | p-value | OCT-guided(N=391) | Angiography-guided(N=423) | HR(95% CI) | p-value | ||
| Primary outcome | |||||||||
| Major adverse cardiac events (composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation) | 20 (4.9) | 36 (9.5) | 0.50(0.29-0.87) | 0.011 | 17 (4.4) | 23 (5.4) | 0.80(0.43-1.50) | 0.479 | 0.273 |
| Secondary outcomes | |||||||||
| All-cause death | 3 (0.7) | 3 (0.8) | 0.92(0.19-4.54) | 0.915 | 2 (0.5) | 3 (0.7) | 0.72(0.12-4.31) | 0.718 | 0.844 |
| Cardiac death | 0 (0) | 3 (0.8) | - | 0.071 | 1 (0.3) | 2 (0.5) | 0.54(0.05-5.96) | 0.610 | - |
| Myocardial infarction | 15 (3.6) | 26 (6.9) | 0.53(0.28-0.99) | 0.041 | 14 (3.6) | 14 (3.3) | 1.09(0.52-2.28) | 0.828 | 0.144 |
Spontaneous myocardial infarction |
4 (1.0) | 8 (2.1) | 0.46(0.14-1.51) | 0.188 | 3 (0.8) | 11 (2.6) | 0.29(0.08-1.04) | 0.058 | 0.616 |
| Target vessel related | 3 | 6 | 2 | 11 | |||||
Non-target vessel related |
1 | 2 | 1 | 0 | |||||
Periprocedural myocardial infarction |
11 (2.7) | 18 (4.8) | 0.56(0.26-1.18) | 0.119 | 11 (2.8) | 4 (0.9) | 2.98(0.95-9.36) | 0.061 | 0.016 |
| Stent thrombosis | 3 (0.7) | 4 (1.1) | 0.69(0.15-3.06) | 0.619 | 1 (0.3) | 6 (1.4) | 0.18(0.02-1.49) | 0.072 | 0.310 |
| Definite | 1 | 3 | 1 | 1 | |||||
| Probable | 2 | 1 | 0 | 5 | |||||
| Ischaemia-driven target vessel revascularisation | 8 (1.9) | 15 (4.0) | 0.49(0.21-1.14) | 0.091 | 4 (1.0) | 18 (4.3) | 0.24(0.08-0.70) | 0.004 | 0.303 |
Target lesion revascularisation |
6 | 12 | 3 | 16 | |||||
Non-target lesion revascularisation |
2 | 3 | 1 | 2 | |||||
| Any revascularisation | 9 (2.2) | 21 (5.6) | 0.39(0.18-0.84) | 0.013 | 10 (2.6) | 25 (5.6) | 0.42(0.20-0.88) | 0.017 | 0.874 |
| Stroke | 1 (0.2) | 2 (0.5) | 0.46(0.04-5.06) | 0.514 | 0 (0) | 3 (0.7) | - | 0.095 | - |
| Bleeding (BARC Type 3 or 5) | 3 (0.7) | 2 (0.5) | 1.38(0.23-2.26) | 0.723 | 1 (0.3) | 3 (0.7) | 0.36(0.04-3.45) | 0.354 | 0.361 |
| Contrast-induced nephropathy | 5 (1.2) | 4 (1.1) | 1.15(0.31-4.27) | 0.838 | 5 (1.3) | 3 (0.7) | 1.80(0.43-7.55) | 0.412 | 0.648 |
| Achievement of stent optimisation by OCTb | 279/393 (71.0) | 266/374 (71.1) | |||||||
| Post hoc composite outcomesc | |||||||||
| Cardiac death, myocardial infarction, or stent thrombosis | 15 (3.6) | 27 (7.1) | 0.51(0.27-0.95) | 0.029 | 15 (3.8) | 15 (3.5) | 1.09(0.53-2.22) | 0.822 | 0.115 |
| Cardiac death, spontaneous myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation | 9 (2.2) | 18 (4.8) | 0.46(0.20-1.00) | 0.047 | 6 (1.5) | 20 (4.7) | 0.32(0.13-0.79) | 0.009 | 0.559 |
| Cardiac death, spontaneous myocardial infarction, or stent thrombosis | 4 (1.0) | 8 (2.1) | 0.46(0.14-1.51) | 0.188 | 4 (1.0) | 12 (2.8) | 0.36(0.12-1.10) | 0.061 | 0.769 |
| Data are number (% of the cumulative rates at 1 year according to Kaplan-Meier event rates), unless stated otherwise. aP-value for the interaction between clinical presentation and imaging guidance strategy. bData are the number of patients/total number of patients (%). Stent optimisation was defined as acceptable stent expansion, apposition, and edge dissection, and was assessed in the OCT-guided group with available final post-stent OCT evaluation, which included 393 patients with ACS and 374 patients with CCS. cAnalysed as post hoc endpoints in the OCCUPI Trial. ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CI: confidence interval; HR: hazard ratio; OCCUPI: Optical CoherenCe Tomography-gUided Coronary Intervention in Patients With Complex Lesions; OCT: optical coherence tomography | |||||||||
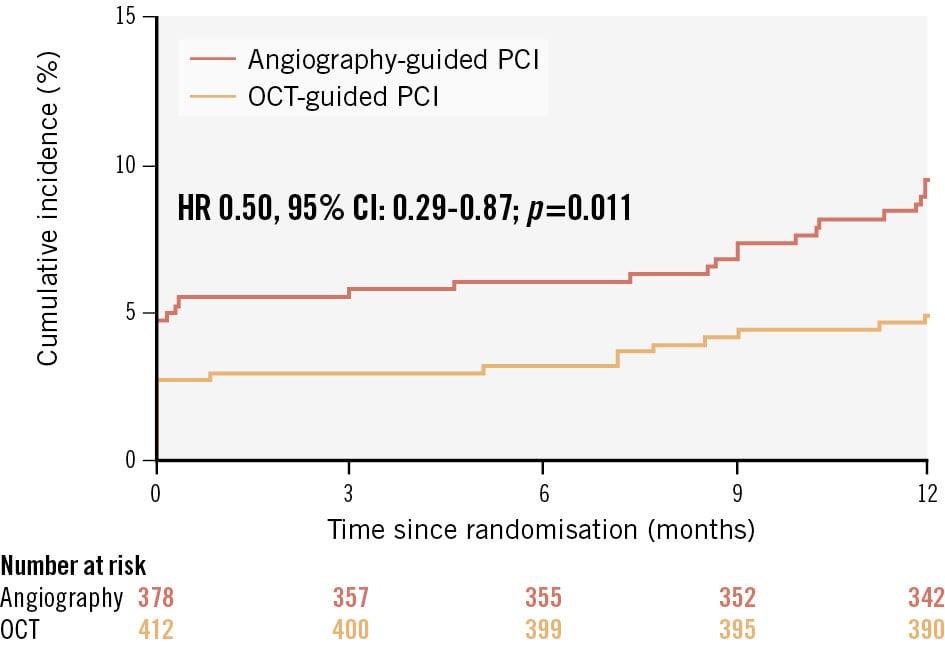
Figure 2. Time-to-event curves for the primary outcome according to randomised imaging guidance strategy among patients with ACS. Kaplan-Meier survival curves for the primary outcome (major adverse cardiac events, defined as a composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation) according to randomised imaging guidance strategy among patients with ACS. ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Stent optimisation by OCT
Out of 803 patients assigned to the OCT-guided group in the trial, 767 (393 with ACS and 374 with CCS) had final post-stent OCT available for stent optimisation evaluation. Among patients with ACS, 279 (71.0%) satisfied the prespecified stent optimisation criteria by OCT. ACS patients with stent optimisation showed a lower incidence of the primary outcome than those with stent suboptimisation (2.9% vs 9.7%; HR 0.29, 95% CI: 0.12-0.72; p=0.004) (Figure 3, Supplementary Table 5). CCS patients with stent optimisation showed a trend towards a lower incidence of the primary outcome compared with those with stent suboptimisation (Supplementary Figure 3, Supplementary Table 5). Among patients with ACS, a smaller stent diameter and lower maximal inflation pressure were identified as independent determinants of stent suboptimisation following OCT-guided PCI, whereas among patients with CCS, a long lesion, smaller stent diameter, and smaller reference vessel diameter were identified as independent determinants (Supplementary Table 6). 
Figure 3. Time-to-event curves for the primary outcome. Kaplan-Meier survival curves for the primary outcome according to stent optimisation among patients with ACS assigned to OCT guidance. Patients with stent optimisation were defined as those with acceptable stent expansion, apposition, and edge dissection on the final post-stent OCT evaluation. ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; OCT: optical coherence tomography
Discussion
The main findings of this post hoc analysis of the OCCUPI Trial, which investigated the impact of OCT guidance versus angiography guidance with a particular focus on ACS patients, were as follows (Central illustration): (1) among patients with ACS requiring DES implantation for complex lesions, the risk of 1-year major adverse cardiac events – defined as a composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation – was significantly lower in the OCT-guided group than in the angiography-guided group; (2) among ACS patients with OCT guidance, the achievement of the prespecified stent optimisation criteria by OCT was associated with a significantly lower risk of major adverse cardiac events; and (3) the advantage of OCT guidance and achieving stent optimisation in reducing the risk of major adverse cardiac events was especially evident in patients with ACS, although no significant interaction between clinical presentation and imaging guidance strategy was observed for major adverse cardiac events. Overall, our findings support the use of OCT to guide PCI in ACS patients with complex lesions. The latest guidelines strongly recommend intravascular imaging guidance for patients undergoing PCI for complex lesions23. Moreover, patients with ACS are expected to derive substantial benefits from intravascular imaging during PCI167. In the previous multicentre registry study and meta-analysis, the benefit of IVUS guidance in reducing major adverse cardiac events was particularly evident in patients with ACS67. Additionally, the recent Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS) trial, which exclusively enrolled patients with ACS, confirmed the benefit of IVUS-guided PCI in reducing target vessel failure, defined as a 1-year composite of cardiac death, target vessel myocardial infarction, or clinically driven target vessel revascularisation9. However, whether OCT-guided PCI could reduce the risk of cardiovascular events in patients with ACS has not been sufficiently evaluated. The Optical Coherence Tomography Guided Percutaneous Coronary Intervention With Nobori Stent Implantation in Patients With Non ST Segment Elevation Myocardial Infarction (OCTACS) and Does Optical Coherence Tomography Optimise Results of Stenting (DOCTORS) trials explored the role of OCT guidance in patients with non-ST-segment elevation myocardial infarction; however, both trials enrolled only a small number of patients (100 and 240 patients, respectively) and defined the procedural endpoints (strut coverage and post-PCI fractional flow reserve, respectively) as the primary outcome1011. In contrast, the current study focused on the ACS subgroup in the OCCUPI Trial; it included 790 patients with all ACS types (i.e., unstable angina, non-ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction) and conducted clinical follow-up for up to 1 year. Among enrolled patients with ACS, the primary outcome of 1-year major adverse cardiac events occurred less frequently in the OCT-guided group than in the angiography-guided group. Moreover, while no significant interaction between clinical presentation and imaging guidance strategy was observed, the advantage of OCT guidance in reducing the risk of major adverse cardiac events was especially evident in patients with ACS. These observations align with previous evidence regarding the use of intravascular imaging in patients with ACS and may support current recommendations for OCT guidance in patients with ACS undergoing PCI for complex lesions13678. Presumably, the exceptional advantages of high-resolution OCT in identifying the culprit lesions, detecting thrombus, and delineating luminal discontinuity and plaque composition in patients with ACS may have contributed to these substantial benefits134823. As observed in this study, it may be hypothesised that the higher incidence of myocardial infarction in the angiography-guided group, compared with the OCT-guided group, reflects missed culprit lesions. Nevertheless, further studies are required before any causative effect can be established or rebutted. In this study, OCT guidance was associated with a significant 50% relative reduction in the risk of 1-year major adverse cardiac events, compared with angiography guidance, in patients with ACS undergoing PCI for complex lesions; this was mainly driven by the reduction in myocardial infarction and target vessel revascularisation. Our findings are consistent with the primary results of the OCCUPI Trial and similar to those of the IVUS-ACS trial, which evaluated the role of IVUS guidance in patients with ACS914. Regarding contrast-induced nephropathy after PCI, despite the greater contrast volume and longer procedural time in the OCT-guided group, as noted in previous trials, the incidence was similar between the OCT-guided and angiography-guided groups, regardless of clinical presentation1213. Meanwhile, a significant interaction between clinical presentation and OCT guidance strategy was observed for periprocedural myocardial infarction. Given that the proportion of patients who achieved stent optimisation by OCT was similar between those with ACS and those with CCS (71%), the higher prevalence of long and bifurcation lesions, along with the longer procedural time of OCT-guided PCI in patients with CCS than in those with ACS, may have contributed to our findings regarding periprocedural myocardial infarction. These factors are known to increase the risk, likely owing to the more aggressive procedures required to meet the prespecified stent optimisation criteria2425. Additional studies considering clinical presentation and OCT guidance are needed to investigate the impact of potential periprocedural myocardial infarction during the process of stent optimisation on long-term cardiovascular events. Although previous trials have consistently reported improved clinical outcomes with stent optimisation guided by intravascular imaging in complex lesions, the impact of stent optimisation by OCT in patients with ACS has not been thoroughly evaluated5926. In the subgroup analysis of the Randomized Controlled Trial of Intravascular Imaging Guidance Versus Angiography-Guidance on Clinical Outcomes After Complex Percutaneous Coronary Intervention (RENOVATE-COMPLEX-PCI), achieving stent optimisation resulted in a reduced risk of target vessel failure, particularly in patients with ACS27. However, in their study, IVUS guidance was mainly evaluated, whereas OCT was used in only 25% of patients527. In the OCCUPI Trial, OCT was exclusively used as the intravascular imaging modality, and achieving stent optimisation according to the prespecified, protocol-defined OCT criteria was associated with a lower risk of major adverse cardiac events compared with suboptimisation14. Moreover, the current subgroup analysis showed that the advantage of achieving stent optimisation by OCT in reducing the risk of major adverse cardiac events was especially evident in patients with ACS. The three key parameters of OCT-defined stent optimisation – stent expansion, apposition, and edge dissection – are important OCT predictors of cardiovascular events after PCI. These parameters may have a greater influence on patients with ACS than on those with CCS because of their vulnerable features, such as increased thrombogenicity8272829. Furthermore, given that additional post-stent procedures required for stent optimisation are particularly challenging in the ACS setting because of concerns regarding potential complications such as no-reflow or distal embolisation, the pre- and post-stent OCT assessments may have facilitated a more effective and safer application of adjunctive high-pressure post-dilation or additional stenting1830. However, achieving stent optimisation was demanding, and meticulous OCT-based decision-making regarding procedural strategy, including stent diameter and inflation pressure, may improve the likelihood of successful optimisation in patients with ACS. Furthermore, to gain a deeper understanding of stent optimisation, additional studies are warranted to explore the impact of more extensive optimisation on clinical outcomes. 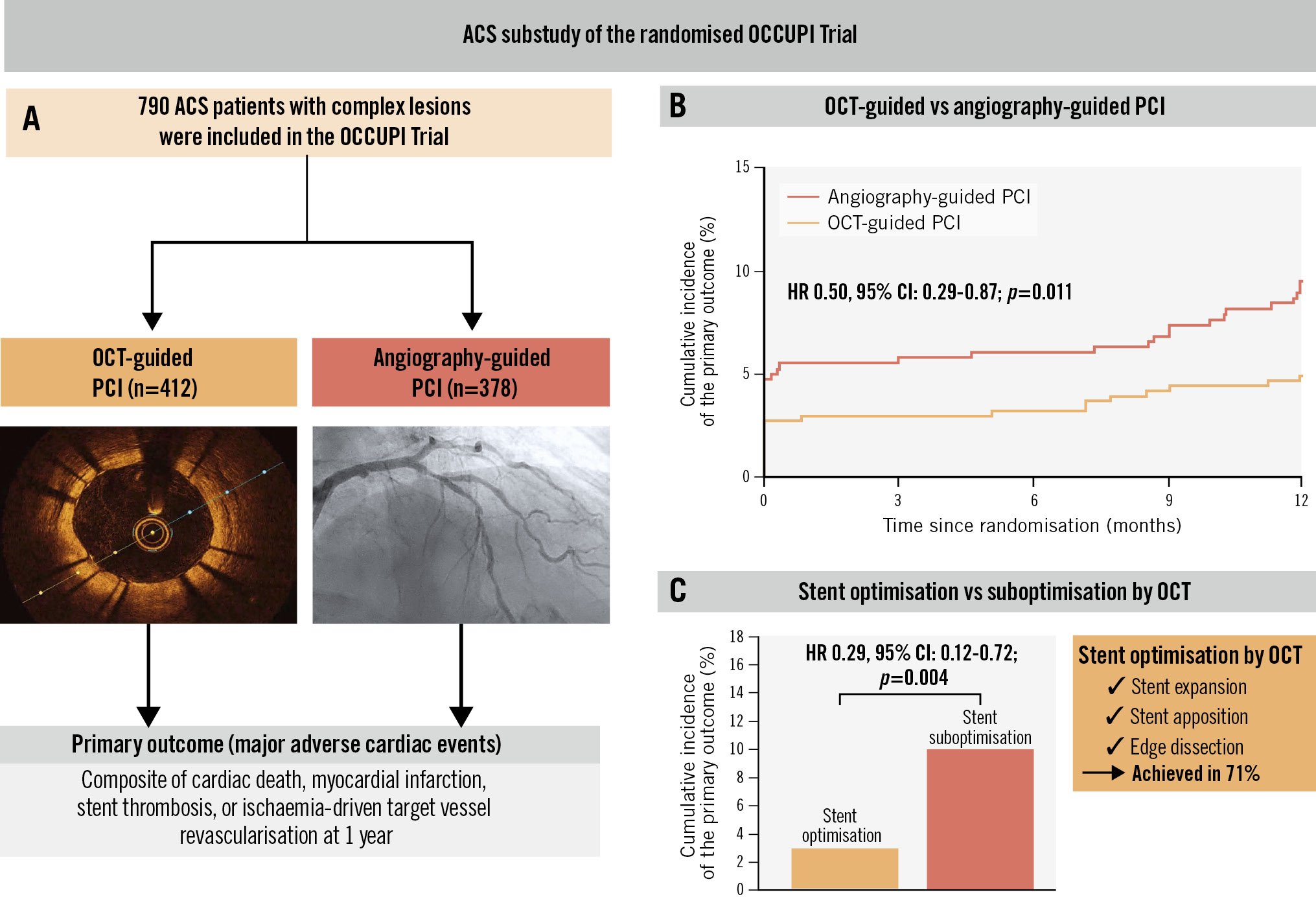
Central illustration. Optical coherence tomography guidance during percutaneous coronary intervention in acute coronary syndrome patients with complex lesions. The impact of OCT guidance versus angiography guidance during PCI for complex lesions in patients presenting with ACS in the OCCUPI Trial. Achievement of stent optimisation by OCT was prespecified based on stent expansion, apposition, and edge dissection. A) Study details; (B) incidence of the primary outcome according to imaging guidance strategy (OCT vs angiography); (C) incidence of the primary outcome according to whether patients achieved stent optimisation or not. ACS: acute coronary syndrome; CI: confidence interval; HR: hazard ratio; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Limitations
This study has some limitations. First, as this study was a post hoc subgroup analysis of a randomised clinical trial based on clinical presentation, potential selection bias should be considered. In addition, the number of patients in each clinical presentation may have limited the statistical power to derive definite conclusions regarding the impact of OCT-guided PCI. Second, comparing each component of the primary outcome might be difficult because of the small number of events; therefore, the results should be interpreted with caution. Third, the OCCUPI Trial was an open-label trial in which physicians were not blinded to treatment allocation. However, an independent clinical endpoint committee that was blinded to treatment assignments adjudicated all clinical outcomes in the trial. Fourth, the trial’s criteria for defining complex lesions are arbitrary and may limit the generalisability of the findings. Fifth, specific guidance on optimisation criteria – especially concerning clinical presentation – was not established in the trial. Sixth, this study lacked detailed qualitative assessment of OCT images, including the evaluation of calcified plaque. Seventh, the follow-up period in this study was relatively short, lasting just 1 year. Our findings should therefore be considered only as hypothesis-generating, which underscores the need for future clinical trials with a larger number of patients and a longer follow-up period.
Conclusions
This post hoc analysis focusing on ACS patients in the OCCUPI Trial demonstrated the evident cardiovascular benefit of OCT guidance over angiography guidance in reducing the risk of a 1-year composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target vessel revascularisation. Such benefit was particularly observed in patients who achieved stent optimisation by OCT. Our findings support the use of OCT as an effective intravascular imaging technique to guide PCI in ACS patients with complex lesions, in line with current guidelines. Given that this was a post hoc analysis of a randomised trial, the findings should be interpreted as hypothesis-generating and warrant confirmation in future dedicated trials conducted for each clinical presentation.
Impact on daily practice
The current post hoc analysis of the randomised OCCUPI Trial demonstrated that, in patients with acute coronary syndrome (ACS) requiring drug-eluting stent implantation for complex lesions, optical coherence tomography (OCT) guidance was associated with a significantly lower risk of 1-year major adverse cardiac events compared with angiography guidance, particularly in those who achieved stent optimisation by OCT. However, as these findings are based on a post hoc analysis, they should be considered hypothesis-generating. Dedicated randomised clinical trials with larger patient populations and longer follow-up are warranted to confirm the role of OCT in guiding percutaneous coronary intervention for ACS patients with complex lesions.
Funding
This study was funded by Abbott Vascular (Santa Clara, CA, USA) and supported by the Cardiovascular Research Center (Seoul, Republic of Korea) and ENCORE Seoul (Seoul, Republic of Korea). The funder/sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest statement
B.-K. Kim reports institutional research grants and speaker or consultant fees from Abbott, Yuhan Corporation, Samjin Pharmaceutical, Chong Kun Dang, Medtronic, Boston Scientific, Biotronik, Genoss, CGBIO, MicroPort, Daewoong Pharmaceutical, Amgen, Bayer Korea, DIO Medical, Hanmi, AstraZeneca, and Novartis. M.-K. Hong reports institutional research grants from Samjin Pharmaceutical and Chong Kun Dang; as well as honoraria for lectures from Medtronic and Edwards Lifesciences. Y. Jang reports an institutional research grant from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

