Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Residual shunt (RS) after transcatheter patent foramen ovale (PFO) closure has been associated with an increased risk of recurrent stroke over long-term follow-up. However, RS prevalence, anatomical characteristics, and treatment strategies are poorly understood.
Aims: This study aimed to assess the prevalence and causes of RS, as well as to evaluate the safety and feasibility of its percutaneous treatment.
Methods: Patients with RS at transcranial Doppler after transcatheter PFO closure in three Italian high-volume centres between 2000 and 2022 were included. The prevalence and anatomical characteristics of RS, its relationship with the original occluding device, and the procedural details of percutaneous treatment were assessed.
Results: Among the 2,362 patients who underwent PFO closure, any grade and significant RS were diagnosed in 8.8% and 3.6% of patients, respectively. It was more frequently found after use of the NobleStitch system than after double-disc device implantation (20.0% vs 8.5%; p<0.00001). Among double-disc device implantations, a higher rate of shunt was found with stiffer devices (9.8% vs 7.1%; p<0.05) and with devices larger than 25 mm (13.9% vs 6.6%; p<0.00001). Intradiscal RS (type 1) was most common (43.6%), followed by extradiscal RS (type 2; 35.1%) and RS due to unusual causes (type 3; 14.9%). Percutaneous treatment was successful in 89.4% of patients using different, anatomically tailored devices.
Conclusions: RS is commonly found after transcatheter PFO closure and is significantly associated with the type and size of the occluding device implanted. It results from different mechanisms and can be safely and effectively treated by a percutaneous, patient-tailored approach in a high percentage of cases.
Transcatheter closure of patent foramen ovale (PFO) is now widely considered as the first-choice treatment for patients with cryptogenic stroke that is likely due to paradoxical embolism1234. However, residual shunt (RS) after successful percutaneous PFO closure has been reported in up to 26% of patients56789101112 and has been associated with an increased risk of recurrent cerebrovascular events and persistent PFO-related migraine101314151617. Thus, percutaneous closure of RS has recently emerged as a new therapeutic target to reduce the risk of persistent symptoms and recurrent ischaemic events567. However, large studies addressing the prevalence, causes, and treatment strategies for RS are still lacking.
The aim of this study was to assess the prevalence and causes of RS after transcatheter PFO closure in a large, multicentre registry of paediatric and adult congenital cardiology units in Italy, reporting the anatomical characteristics of RS as well as the safety and feasibility of its percutaneous treatment.
Methods
Study population
Between January 2000 and December 2022, 2,362 patients underwent percutaneous PFO closure at three Italian high-volume tertiary referral centres of paediatric cardiology and adult congenital heart disease units: Heart Hospital “G. Pasquinucci”, Tuscany Foundation “G. Monasterio”, Massa; “Ospedali dei Colli” Hospital, “L. Vanvitelli” University of Naples, Naples; and “Careggi” University Hospital, Florence. Previous stroke and transient ischaemic attack (TIA) presumably due to paradoxical embolism were the most frequent indications for PFO closure (78.3%), followed by drug-resistant migraine (14.5%), decompression disease (3.9%) and platypnoea-orthodeoxia syndrome (1.9%). Based on metallic content and mechanical properties, the originally used occluding devices were arbitrarily classified as stiff (Amplatzer device [Abbott], Occlutech device [Occlutech GmbH] and other Amplatzer-like devices) or soft devices (GORE CARDIOFORM Septal Occluder device [W. L. Gore & Associates] and Cardia Ultrasept device [Cardialogic]). The index procedure was always performed under deep sedation or general anaesthesia guided by transoesophageal (TOE) or intravascular echocardiography. PFO sizing using a static or dynamic balloon technique was performed based on the centre’s and operator’s choice. An intraoperative bubble test was always performed after device deployment, and additional sources of paradoxical shunt were consequently addressed in the same procedure.
After PFO closure, routine clinical assessment, electrocardiography and transthoracic echocardiography were performed at 1 and 12 months. Control contrast-enhanced transcranial Doppler (c-TCD) with agitated saline injection at rest and during the Valsalva manoeuvre was performed at least 12 months after device implantation. At c-TCD, a semiquantitative estimation of RS was defined according to the number of microbubbles: grade 0=none, grade 1=mild (1-10 bubbles), grade 2=moderate (>10 bubbles without a curtain pattern), and grade 3=severe (curtain or shower-like pattern)89. RS was defined as any grade of microbubble passage at rest or during the Valsalva manoeuvre, while significant RS was defined as any shunt higher than grade 1. Medical therapy after PFO closure included dual antiplatelet therapy (aspirin+clopidogrel) for 1 month, followed by aspirin monotherapy until the 1-year follow-up when patients were assessed for RS with c-TCD. If c-TCD confirmed complete closure of the PFO (no RS at c-TCD), aspirin therapy was stopped. On the other hand, in patients with confirmed RS, aspirin therapy was continued.
The study population included 207 patients with confirmed RS at c-TCD. The exclusion criterion was the presence of an extracardiac cause of paradoxical shunt. The patients’ clinical and demographic characteristics, the presence of an atrial septum aneurysm at baseline transthoracic echocardiography, and the type and size of the PFO closure device were assessed. Follow-up data regarding recurrent paradoxical embolic events, such as stroke, TIA or resistant migraine after PFO closure, were also recorded for patients. Recurrent stroke and TIA were defined by the treating neurologist. Recurrent stroke was defined as a new clinically evident and permanent neurological deficit associated with new evidence of cerebrovascular embolism at imaging (computed tomography [CT] or magnetic resonance imaging [MRI]), while TIA was defined as any associated transient ischaemic event, with or without evidence of cerebrovascular embolism on imaging. This study was approved by the ethics committee of each involved institution, which waived informed consent for this retrospective, non-invasive study.
Percutaneous RS closure
Percutaneous RS closure was considered in all patients with RS based on the following criteria: presence of recurrent symptoms after PFO closure (stroke, TIA, persistent treatment-resistant migraine); the degree of RS (moderate-to-severe vs mild RS); and patient preference about a second percutaneous procedure versus long-term aspirin therapy. The interventional procedure was performed under general anaesthesia with fluoroscopic and TOE guidance. A comprehensive TOE assessment was performed to assess the mechanism of RS and any other complication caused by the previously implanted device. All patients gave informed consent for the interventional procedure. The site of paradoxical shunt was angiographically imaged in the left anterior oblique view and confirmed by local angiography and a bubble test. Based on the anatomical characteristics, RS was classified into 3 types:
Type 1 shunt was defined as a tunnel-like intradevice shunt, located between the discs of the previously implanted device (Figure 1A, Moving image 1).
Type 2 shunt was defined as any extradevice shunt due to an accessory atrial septal defect far from the device, incomplete coverage of the PFO by an undersized device, or device malposition (Figure 2A, Moving image 2).
Type 3 shunt was defined as any other RS with characteristics not included in the two previous types. This category included unusual causes of shunt such as atrial septal fistulas (Figure 3A, Moving image 3), atrial septum tears, or incomplete PFO sealing late after a NobleStitch approach (NobleStitch EL [Heartstitch]) (Figure 4A, Moving image 4).
The site of RS was probed from femoral vein access in all but two patients, in whom the right internal jugular vein was instead used because of an unusual shunt location. Catheters and guidewires of different shapes, sizes, and characteristics were used to cross the shunt site. The closure device was selected based on the type, size, and anatomical characteristics of the shunt. In the case of a type 1 shunt, the Amplatzer Vascular Plug II or 4 devices, or the Amplatzer Duct Occluder II or Piccolo Occluder devices (all Abbott) were chosen. In the case of a type 2 shunt, a double-disc occluding device was selected after balloon sizing to better detail the defect morphology and distance from the previously implanted device. In the case of a type 3 shunt, controlled-release vascular coils or vascular plug devices were chosen in the case of atrial fistulas, or double-disc devices in the case of incomplete closure after the NobleStitch approach.
After the procedure, the device position, any RS and any potential device-related complications were assessed by right atrial contrast angiography and contrast-enhanced TOE. Procedural success was defined as device implantation without any residual shunt as assessed with contrast-enhanced TOE and without any procedural complications. All patients were discharged 24 h after the procedure and followed up by electrocardiography and transthoracic echocardiography at 1 and 12 months, as well as by c-TCD 12 months after the procedure. After RS closure, medical therapy included dual antiplatelet therapy for 1 month and, thereafter, aspirin therapy for up to 12 months. If the c-TCD at 12 months showed no RS, aspirin therapy was stopped; otherwise, aspirin therapy was continued indefinitely.
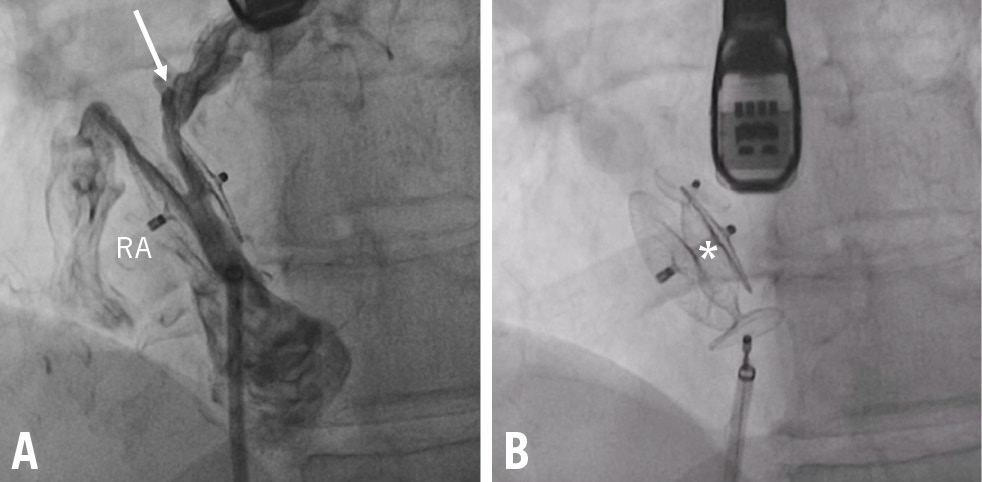
Figure 1. Transcatheter closure of a large interdiscal shunt. A) RS through a 35 mm Amplatzer PFO Occluder (Abbott; arrow) was closed by implantation of a 10 mm Amplatzer Vascular Plug II (Abbott; asterisk; B) PFO: patent foramen ovale; RA: right atrium; RS: residual shunt
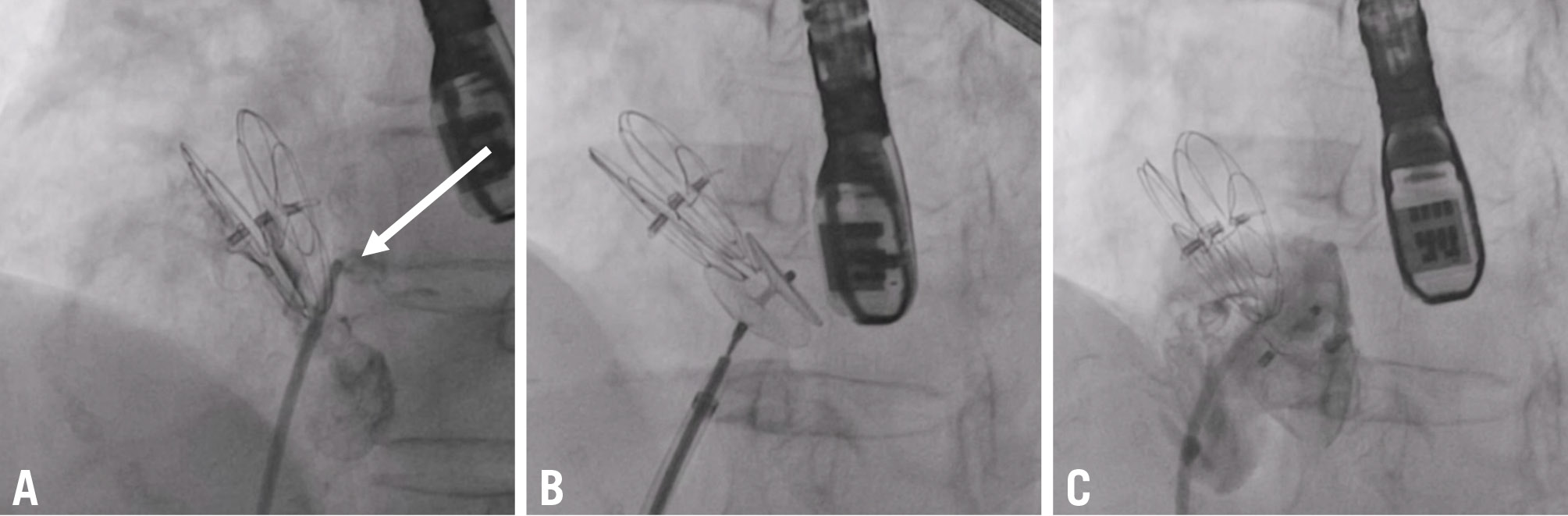
Figure 2. Transcatheter closure of an extradiscal shunt. A) The shunt was located in the lower part of a 30 mm GORE CARDIOFORM Septal Occluder device (arrow) and was occluded by implantation of a 25 mm Amplatzer PFO Occluder (B,C). PFO: patent foramen ovale
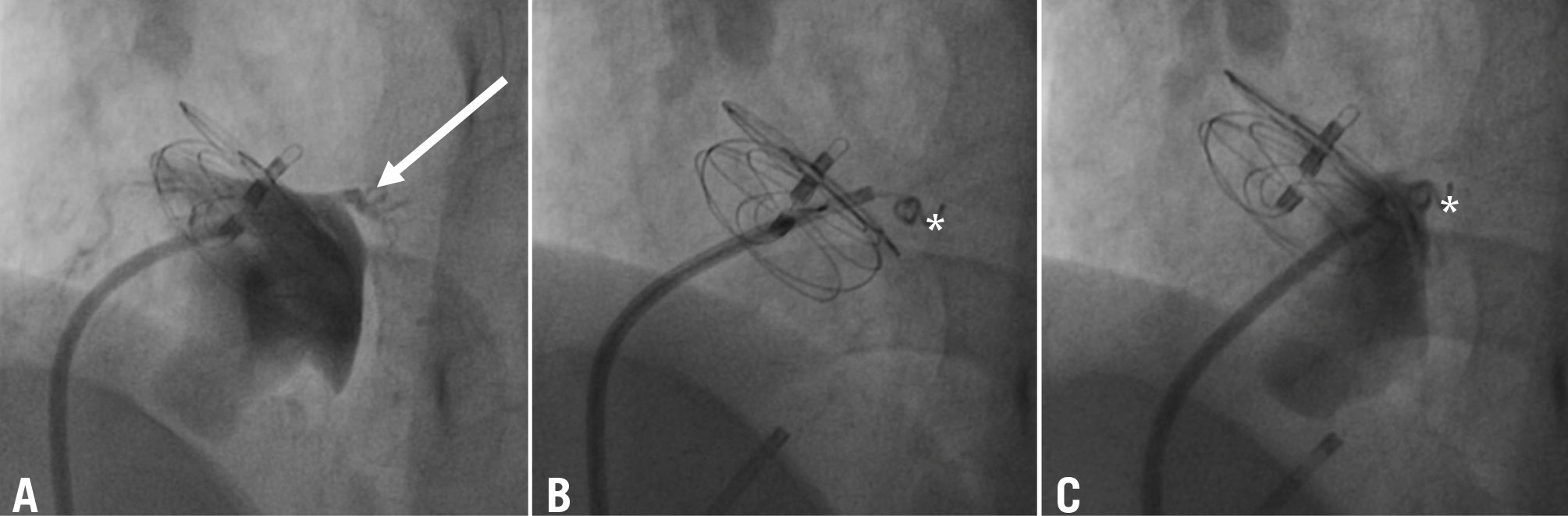
Figure 3. Coil embolisation of a serpiginous, fistulous atrial communication distant from a previously implanted 25 mm GORE CARDIOFORM Septal Occluder. The right-to-left shunt was imaged using a multipurpose catheter (A) and occluded by deployment of a 5PDA-5 controlled-release Cook coil (Cook Medical; asterisk) (B,C). The serpiginous, fistulous atrial communication is indicated with an arrow. PDA: patent ductus arteriosus
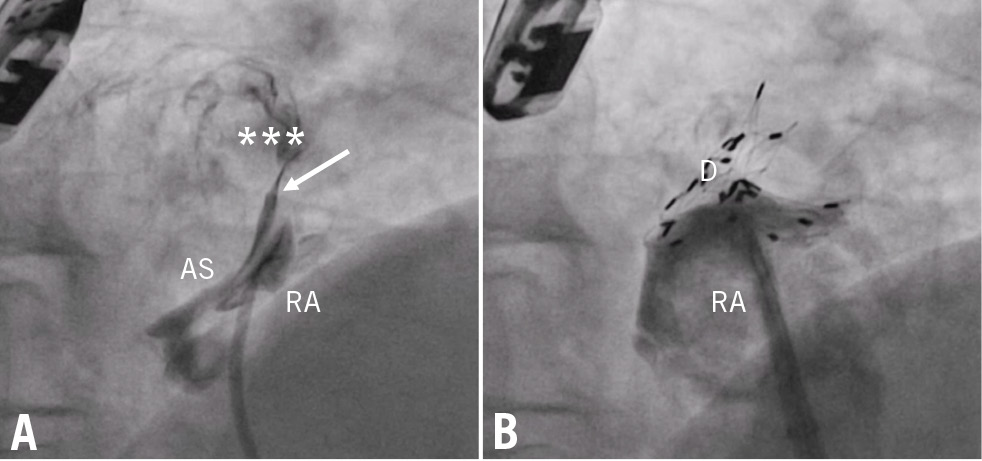
Figure 4. Transcatheter closure of a complex PFO in a patient with situs visceroatrial inversus. After the NobleStitch approach (arrow marks the occluding knot), whose aim was to straighten a severely aneurysmal septum, a tiny RS was imaged (asterisks; A) and occluded by deployment of a 30 mm Cardia Ultrasept device (B). AS: aneurysmal septum; D: Cardia Ultrasept device; PFO: patent foramen ovale; RA: right atrium; RS: residual shunt
Statistical analysis
Statistical analysis was performed using SPSS Statistics, version 29.0 (IBM). Continuous variables are expressed as mean±standard deviation for normally distributed variables and as median and percentiles for non-normally distributed variables. Categorical data are expressed in percentages. The independent samples t-test for was used to assess differences between means for normally distributed variables, while the Mann-Whitney U test was used for non-normally distributed variables. Normal distribution was tested using the Kolmogorov-Smirnov test. Categorical variables were analysed using the χ2 test, and Fisher’s exact test was used when appropriate.
Results
Patient characteristics
Among the 2,362 patients submitted to percutaneous PFO closure, any grade and significant RS were found in 207 (8.8%) and 84 (3.6%) patients, respectively. One hundred and twenty of these patients (58%) had an interatrial septal aneurysm at the time of the first procedure. RS severity was mild (grade 1) in 59.4%, moderate (grade 2) in 24.2%, and severe (grade 3) in 16.4% of patients. During a median follow-up of 3.6 (25th-75th percentile: 2.1-11.8) years after PFO closure, 2 (1%) of the 207 patients with RS had recurrent stroke, 15 (7.2%) experienced recurrent TIA and 14 (6.8%) reported residual disabling migraine (Table 1). The severity of the RS was significantly associated with recurrent symptoms (odds ratio 2.467; p<0.001; moderate-severe RS: 72.4% in symptomatic vs 35.4% in asymptomatic patients; p<0.001).
RS was observed with all types of devices but was significantly higher after the NobleStitch approach as compared to use of double-disc devices (20.0% vs 8.5%; p<0.0001). Among the patients who underwent double-disc device implantation, a higher rate of RS was found in the case of stiff prostheses compared to soft prostheses (9.8% vs 7.1%; p<0.05) and with larger protheses (>25 mm) compared to smaller ones (13.9% vs 6.6%; p<0.0001). This latter comparison was significant only in the case of stiff devices (18.3% vs 6.7%; p<0.00001), while no significant difference was found between large- and small-size soft devices (Table 2). Finally, after implantation of large devices, RS was significantly more frequent with stiff prostheses as compared to soft ones (18.3% vs 7.5%; p<0.0001).
In all, 101 patients with RS agreed to a second interventional procedure and were included in the analysis, while 106 patients (51.2%) were maintained on medical therapy. No significant differences in age, sex, baseline clinical and anatomical characteristics, or the size of the occluding device was found between the 2 groups. However, patients submitted to percutaneous closure of RS showed a higher symptom burden (any symptom: 21.8% vs 8.4%; p<0.01; recurrent stroke: 2% vs 0%; p>0.05; recurrent TIA 9.9% vs 4.7%; p<0.05; treatment-resistant migraine 9.9% vs 3.8%; p<0.05) and a higher degree of paradoxical shunt (moderate RS: 41.6% vs 7.5%; p<0.001; severe RS: 29.7% vs 3.8%; p<0.001) as compared to those remaining under pharmacological therapy.
Table 1. Clinical characteristics of the study population.
| Residual shunt (N=207) | No residual shunt closure N=106 (51.2%) | Residual shunt closure N=101 (48.8%) | p-value | |
|---|---|---|---|---|
| Age, years | 49.3±12.6 | 50±12.9 | 48.6±12.4 | 0.42 |
| Male | 86 (41.5) | 43 (40.6) | 43 (42.6) | 0.77 |
| Hypertension | 70 (33.8) | 37 (34.9) | 33 (32.7) | 0.73 |
| Diabetes | 14 (6.8) | 7 (6.7) | 7 (6.9) | 0.92 |
| Obesity | 15 (7.2) | 5 (4.7) | 10 (9.9) | 0.06 |
| Smoker | 85 (41.1) | 45 (42.5) | 40 (39.6) | 0.81 |
| IAS aneurysm | 120 (58.0) | 68 (64.2) | 52 (51.5) | 0.065 |
| Prosthesis diameter, mm | 30 (25-35) | 28 (25-35) | 30 (25-30) | 0.68 |
| Indication for PFO closure: stroke/TIA | 162 (78.3) | 81 (78.6) | 81 (80.2) | 0.51 |
| Indication for PFO closure: treatment-resistant migraine | 30 (14.5) | 13 (12.3) | 17 (16.8) | 0.35 |
| Recurrent stroke after PFO closure | 2 (1) | 0 (0) | 2 (2) | N/A |
| Recurrent TIA after PFO closure (imaging-negative) | 15 (7.2) | 5 (4.7) | 10 (9.9) | 0.15 |
| Recurrent stroke or TIA after PFO closure | 17 (8.2) | 5 (4.7) | 12 (11.9) | 0.06 |
| Resistant migraine after PFO closure | 14 (6.8) | 4 (3.8) | 10 (9.9) | 0.12 |
| Any symptom after PFO closure | 31 (14.9) | 9 (8.4) | 22 (21.8) | 0.01* |
| Shunt severity: mild | 123 (59.4) | 94 (88.7) | 29 (28.7) | <0.001* |
| Shunt severity: moderate | 50 (24.2) | 8 (7.5) | 42 (41.6) | <0.001* |
| Shunt severity: severe | 34 (16.4) | 4 (3.8) | 30 (29.7) | <0.001* |
| Significant shunt: moderate/severe RS | 84 (40.6) | 12 (11.3) | 72 (71.3) | <0.001* |
| Data are n (%), mean±standard deviation, or median (interquartile range). *p<0.05. TIA is included with or without evidence on imaging. IAS: interatrial septal; N/A: not applicable; PFO: patent foramen ovale; RS: residual shunt; TIA transient ischaemic attack | ||||
Table 2. Residual shunts according to PFO closure device.
| PFO device | Patients who underwent PFO closure 2000-2022 | Residual shunt§ | Residual shunt ø ≤25 mm, % |
Residual shunt ø ≤25 mm, % | p-value |
|---|---|---|---|---|---|
| All devices | 2,362 | 207 (8.8) | 6.6 | 13.9 | p<0.0001* |
| Amplatzer PFO Occludera | 1,437 | 136 (9.5) | 6.3 | 16.7 | p<0.0001* |
| Occlutech PFO Occludera | 66 | 11 (16.7) | 5.9 | 60.0 | p<0.0001* |
| GORE CARDIOFORM Septal Occluderc | 389 | 33 (8.5) | 6.1 | 11.3 | p=NS |
| Cardia Ultraseptd | 204 | 10 (4.9) | 8.5 | 2.5 | p=NS |
| Stiff devices (Amplatzer+Occlutech) | 1,503 | 148 (9.8)& | 6.7 | 18.3@ | p<0.00001* |
| Soft devices (GORE+Cardia) | 593 | 42 (7.1)& | 6.7 | 7.5@ | p=NS |
| Other double-disc devices | 206 | 5 (2.4) | $ | $ | |
| NobleStitch ELe | 60 | 12 (20.0)# | N/A | N/A | N/A |
| All double-disc devices | 2,302 | 195 (8.5)# | $ | $ | |
| Data are n or n (%), unless otherwise indicated. Other devices: CeraFlexf, STARflexg, Helexc, CardioSEALg, FlatStenth, Solysafei. *Shows statistical significance. #, @ and & indicate the p-values for the chi-square test between two variables as follows: #NobleStitch RS 20% vs all double-disc devices RS 8.5%; &stiff devices RS 9.8% vs soft devices RS 7.1% (all sizes); @stiff devices 18.3% vs soft devices 7.5% (ø >25 mm). &p-value for comparison of RS rates between all devices in the column.#p<0.0001; &p<0.001; @p<0.0001; &p<0.05; $analysis not performed. aBy Abbott; bby Occlutech GmbH; cby W. L. Gore & Associates; dby Cardialogic; eby Heartstitch; fby LifeTech Scientific Corporation; gby NMT Medical; hby Coherex Medical; iby Swissimplant AG. N/A: not applicable; NS: non-significant; PFO: patent foramen ovale; RS: residual shunt | |||||
RS characteristics and percutaneous treatment
At cardiac catheterisation, paradoxical intracardiac shunt was confirmed in 94 patients (93.1%) (Central illustration). One patient (1%) exhibited an extracardiac shunt due to a small pulmonary arteriovenous fistula, while in the remaining 6 patients (5.9%), no shunt site was found despite multiple angiographies and intraprocedural bubble test injections. Type 1 (intradevice) shunt was found in 41 patients (43.6%). Type 2 (extradevice) shunt was observed in 33 (35.1%) patients. It was caused by device dislocation in 1 patient, incomplete sealing of the PFO by an undersized device in 2 patients, and an accessory interatrial septal defect in the remaining 30 patients. Fourteen patients (14.9%) showed type 3 residual shunt, caused by an interatrial septum fistula in 2 cases and an incomplete PFO closure after the NobleStitch approach in 12 patients. In 2 patients, a potential late-onset atrial septum tear caused by the NobleStitch EL device was suspected at intraprocedural transoesophageal evaluation, while in the remaining 10 patients, loosening of the occluding knot was considered as a potential cause of the RS since it had not been evident at the end of the first procedure. Finally, 6 (6.4%) patients had multiple mechanisms of shunt due to combinations of the 3 types of shunts.
Transcatheter closure of RS was successful in 84 patients (89.4% success rate). The most used devices were vascular plugs, which were implanted in 51.1% of cases. Double-disc devices were used in 31.9% of patients and controlled-release coils in 4.3% of patients. A combination of different devices was used in 5.3% of patients (Table 3).
The choice of the closure device was influenced by the type and mechanism of RS. Type 1 shunts were treated with a vascular plug device in 80.4% of cases, while coils or double-disc devices were used in 4.8% and 2.4% of cases, respectively. Type 2 shunts were treated with a second non-self-centring device in 51.5% of cases and with a vascular plug in 36.4% of cases. Type 3 shunts were treated with a second non-self-centring device in 78.6% of patients and with a vascular plug or controlled-release coils in the remaining patients (Central illustration). Mixed-type shunts were treated with a combination of devices tailored to the specific type of shunt (Figure 5), as shown in Table 4. The success rate was slightly, though not significantly, lower in type 1 (82.9%) as compared to type 2 (90.9%) and type 3 shunts (100%).
Among the 10 patients (10.6%) in whom the closure procedure failed, 7 had type 1 shunts and 3 had type 2 shunts. In 7 patients, the RS could not be closed because of a failure to pass the guidewire through the previously implanted device, while in 3 patients, the new implanted device did not completely seal the shunt site. No major or device-related complications were reported, and only 1 minor complication (a femoral haematoma requiring local prolonged compression and immobilisation) occurred. Of the 94 patients submitted to RS closure, 79 (84%) completed the recommended 1-year follow-up and underwent c-TCD assessment. In these patients, any grade RS was found in 12 (15.2%) patients (9 with type 1 and 3 with type 2 RS), while significant RS was still present in 7 (8.9%) patients (5 with type 1 and 2 with type 2 RS). No recurrent ischaemic events were observed during the 1-year follow-up, and only 3 patients showed persistent treatment-resistant migraine. Finally, no erosions, pericardial effusions, or other mechanical complications, nor any episodes of atrial fibrillation were reported during this follow-up period.
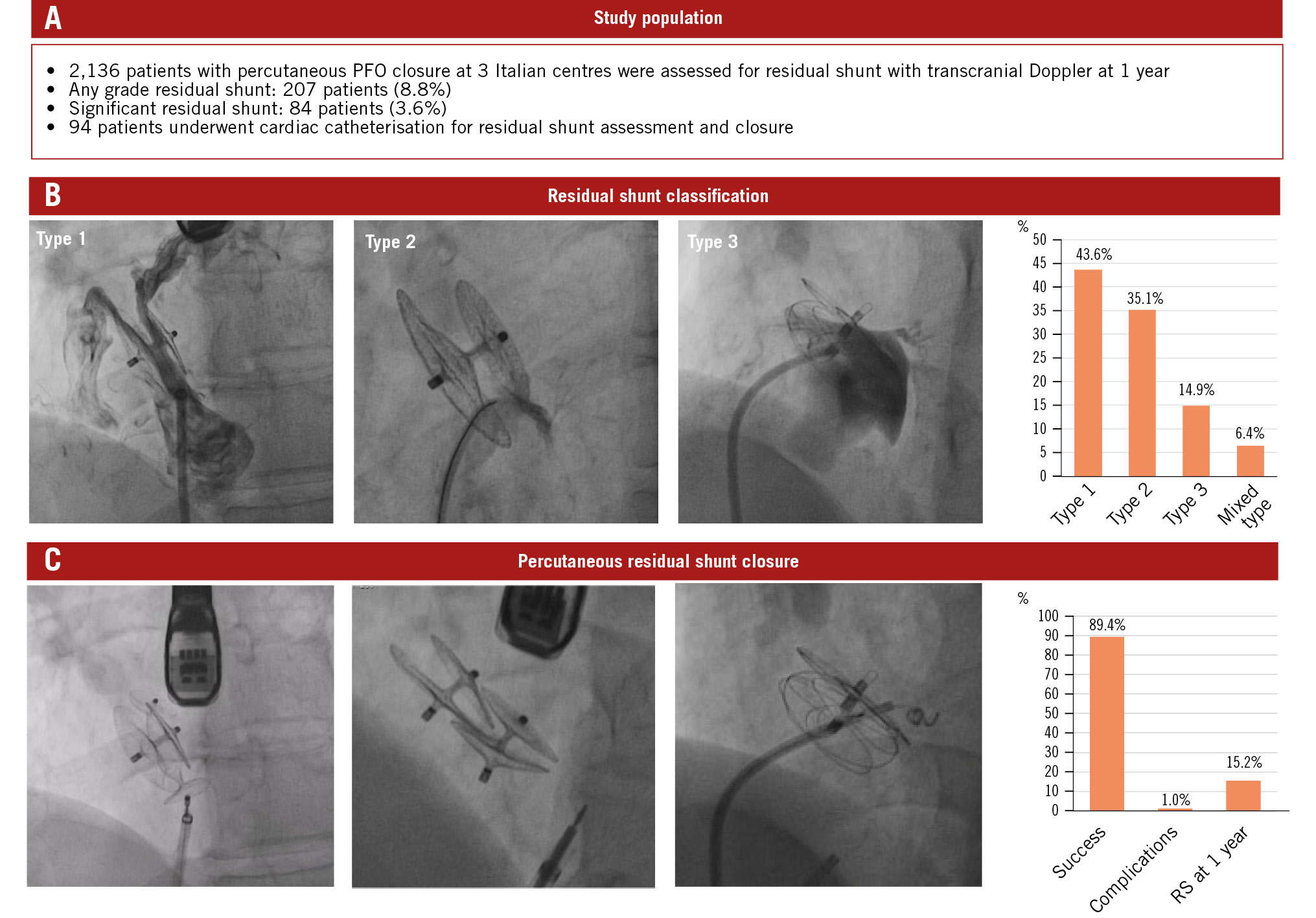
Central illustration. Residual shunt after PFO closure. A) Study population details. B) Classification system and prevalence of RS. Type 1 shunt: a tunnel-like intradevice shunt located between the discs of the previously implanted device. Type 2 shunt: any extradevice shunt due to incomplete coverage of the PFO, device dislocation, or an accessory atrial septal defect. Type 3 shunt: any other residual shunt with characteristics not included in the two previous types. C) RS closure techniques and results. PFO: patent foramen ovale; RS: residual shunt
Table 3. Residual shunt classification and devices used for closure.
| Intracardiac confirmed shunt (n=94) | |
|---|---|
| Type 1 shunt (intradiscal) | 41 (43.6) |
| Type 2 shunt (extradiscal/accessory defect) | 33 (35.1) |
| Type 3 shunt (other) | 14 (14.9) |
| Mixed shunt | 6 (6.4) |
| New double-disc device implantation | 30 (31.9) |
| Vascular device implantation | 48 (51.1) |
| Coil implantation | 4 (4.3) |
| Multiple device implantation | 5 (5.3) |
| No device implantation | 7 (7.4) |
| Treatment success – no RS at end-procedure TOE bubble test | 84 (89.4) |
| Procedural major complications | 0 (0) |
| Procedural minor complications | 1 (1) |
| Device-related complications | 0 (0) |
| Atrial fibrillation at follow-up* | 0 (0) |
| Stroke or TIA at follow-up* | 0 (0) |
| Persistent RS at 1-year follow-up: any grade shunt* | 12 (15.2) |
| Persistent RS at 1 year follow-up: significant shunt only* | 7 (8.9) |
| Device erosion at follow-up* | 0 (0) |
| Pericardial effusion at follow-up* | 0 (0) |
| Any mechanical complication at follow-up* | 0 (0) |
| Data are n (%). *1-year follow-up was available for only 79 (81.4%) patients. RS: residual shunt; TIA: transient ischaemic attack; TOE: transoesophageal echocardiography | |
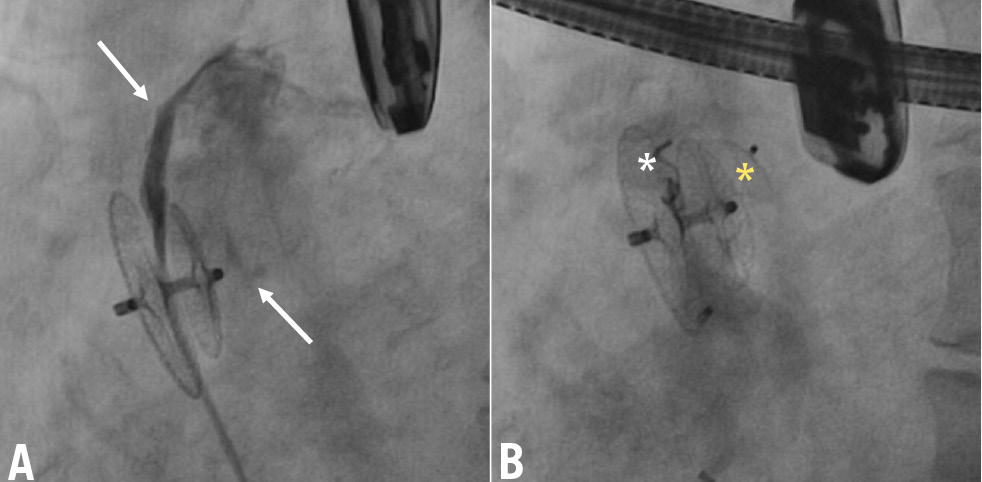
Figure 5. Mixed-type RS caused by multiple mechanisms successfully treated with multiple devices. Multiple RS sites (arrows) after closure of a large PFO by implantation of a 35 mm Amplatzer PFO device (A). The tunnel-like intradevice shunt was treated by implantation of a controlled-release Cook coil (white asterisk) and the extradevice shunt by implantation of an ADO II (yellow asterisk) device (B). ADO: Amplatzer Duct Occluder; PFO: patent foramen ovale; RS: residual shunt
Table 4. Treatment of RS according to the shunt type.
| Type 1 (n=41) | Type 2 (n=33) | Type 3 (n=14) | Mixed shunt (n=6) | |
|---|---|---|---|---|
| Device diameter, mm* | 30 (25-30) | 25 (25-35) | 30 (25-35) | 30 (25-30) |
| PFO device | 1 (2.4) | 17 (51.5) | 11 (78.6) | 1 (16.6) |
| Vascular plug | 33 (80.4) | 12 (36.4) | 1 (7.1) | 2 (33.3) |
| Coil | 2 (4.8) | 0 (0) | 2 (14.2) | 0 (0) |
| Multiple device types | 1 (2.4) | 1 (3.0) | 0 (0) | 3 (50.0) |
| Procedural success* | 34 (82.9) | 30 (90.9) | 14 (100) | 6 (100) |
| Persistent RS at 1-year follow-up& | 9 (21.9) | 3 (9.1) | 0 (0) | 0 (0) |
| Data are n (%) or median (interquartile range). *Procedural success was not statistically significant between the 3 types of RS (p=ns). &Assessment of RS with transcranial Doppler 1 year after the procedure was available only for 79 (81.4%) patients. NS: non-significant; PFO: patent foramen ovale; RS: residual shunt | ||||
Discussion
Percutaneous PFO closure is currently recommended as the first-line treatment of ischaemic stroke caused by PFO-related paradoxical embolism234. However, persistence of significant shunt after a seemingly successful transcatheter closure has been reported with different prevalence rates in previous studies, mainly due to differences in definitions, diagnostic techniques and grading methods1011121314. Overall, the rate of any grade RS ranged from 23% to 26%1314, while the rate of significant RS has been reported between 5%11 and 16%101214. In our study, the rates of any grade and significant RS detected by c-TCD were 8.8% and 3.6%, respectively. These figures are lower than previously reported in literature, possibly due to the larger population size and the more detailed approach to PFO sizing and prosthesis selection in our series.
Although the clinical and prognostic impact of RS is still debated, mild RS does not appear to predict significant adverse outcomes15, whereas higher grades of shunt may be associated with an increased risk of recurrent stroke, peripheral embolism, and residual migraine burden16171819. In accordance with the current literature, in our experience, patients with significant RS showed a higher prevalence of symptoms and more frequently agreed to a second interventional approach. Over time, transcatheter treatment of high-grade RS has emerged as a novel, cost-effective therapeutic option compared to chronic pharmacological therapy567. However, although previous studies have shown transcatheter closure of residual shunt to be safe and feasible, little is known as to whether further intervention is necessary compared to medical therapy alone. Furthermore, there is no consensus on the optimal device for the treatment of this condition. Susuri et al and Diaz et al used a second double-disc device, while Butera et al selected the type of device based on the anatomical characteristics of the shunt5611. In accordance with the latter study, we identified multiple mechanisms of RS, prompting a detailed anatomical assessment in each patient to choose the most appropriate closure approach.
Paradoxical RS was significantly more common following the NobleStitch technique than with standard double-disc devices. In this latter group, the rate of RS was significantly dependent on the mechanical properties and the size of the occluding device. Indeed, it was more frequently found after implantation of stiff and large prostheses. This finding might presumably be due to their lower capacity to adhere to the entire surface of the fossa ovalis compared to softer and smaller devices. A potential role may be played by the original anatomical characteristics of the septum at the time of the first procedure, since a floppy, highly mobile, aneurysmal atrial septum with severe paradoxical shunt often prompted the use of larger stabilising devices, which less frequently sealed the entire fossa ovalis, as confirmed by the current literature10. In our opinion, a key role in causing this sequela might be played by the different thickness between the septum secundum and the thinner and hollower septum primum, which precludes 360-degree adherence of the device to the bottom of the fossa ovalis. In fact, this anatomical arrangement causes the posterior-inferior part of the device not to adhere to the septum, and this phenomenon is mainly evident in the case of stiffer devices and results in a higher risk of intradevice RS. Indeed, in our experience, RS was less common after implantation of softer devices, likely due to their higher anatomical compliance and adherence to the atrial septum in the case of aneurysmal and mobile septa1014. However, there was no significant difference in RS rates between stiffer and softer devices in the case of small-size prostheses, while the difference was statistically significant with larger ones. Thus, it could be cost-effective to prefer softer devices in the case of aneurysmal, floppy, mobile atrial septa with large PFOs, while no significant difference exists among the different commercially available brands in the case of small PFOs that require small-sized occluding devices.
Several mechanisms may underlie paradoxical RS after PFO closure, and an understanding of these mechanisms is crucial to selecting the best therapeutic approach. In our study, nearly half of cases were due to incomplete sealing of the device discs, resulting in interdiscal, tunnel-like communication. The prevalence of this mechanism was significantly higher with large occluding devices, and it was treated by implanting single or multiple vascular plugs inside the previously deployed device (Figure 1B, Moving image 1). The second most frequent cause of RS was extradevice interatrial communication due to incomplete coverage of the PFO by an undersized or dislocated device or an accessory atrial septal defect outside the fossa ovalis and far from the previously implanted device. This type of RS was mainly found in patients with an aneurysmal septum, in whom detecting accessory sites of shunt at the time of the first procedure may have been challenging because of the high mobility of the septum. Additionally, significant device traction on the atrial septum, particularly in cases with a stiff PFO tunnel, may lead to type 2 RS caused by a septum primum tear near the posterior edge of the device. In all these cases, the use of a second non-self-centring or self-centring device should be suggested as the best choice (Figure 2B, Figure 2C, Moving image 2). Less common causes of RS included unusual right-to-left communications, such as a serpiginous fistula into the atrial septum or right atrium-to-pulmonary vein fistulas. In these cases, an individualised treatment using detachable coils (Figure 3B, Figure 3C, Moving image 3) or vascular plugs could be the best approach. A novel type of RS identified in our study was the recurrence of paradoxical shunt following the NobleStitch approach2021. It was presumably due to loosening of the occlusion knot or atrial septal tears and appeared over a short-term follow-up after a seemingly successful procedure. The higher rate of RS associated with the NobleStitch approach raises questions about whether this technique should continue to be used in clinical practice. In such cases, implanting a small double-disc non-self-centring device might be the preferred option for RS treatment (Figure 4B, Moving image 4). Finally, RS was caused by a combination of different mechanisms of paradoxical shunt in a small percentage of patients. These cases were successfully treated with a combination of devices targeted to the specific anatomical type of shunt (Figure 5B, Moving image 5).
In our approach, the closure strategy and device selection were strongly dependent on the anatomical characteristics of each patient. Thus, a detailed assessment of the atrial septum, along with the availability of different devices, was crucial for effectively performing this complex procedure. Based on these considerations, the treatment of RS was successful in a high percentage of cases with a low risk of complications, in line with the current literature561122, and without any significant difference between the 3 types of RS. Failure to cross small intradisc shunt sites or incomplete shunt sealing due to tortuous anatomy were the most frequent causes of procedure failure. Persistence of significant RS after the second procedure was still found in nearly 9% of cases. However, it was deemed not prognostically relevant, since it was presumably due to intraplug foaming and therefore not worthy of further interventional treatment since the occluding device acted as a mechanical barrier to large clots. Thus, these patients remained on chronic antiplatelet therapy.
Limitations
This retrospective study has several limitations, primarily due to its multicentre nature, which precluded a standardised approach to both the original anatomical characteristics and the technique in the previous PFO closure procedure and the RS closure procedure. Specifically, the anatomical description of the septum and the choice of the type and size of the occluding device were not based on the same criteria across the three centres. Consequently, it was not possible to identify any predictive risk factors for RS. However, the aim of this study was to report the prevalence and the anatomical classification of RS in a real-world experience and to describe an anatomically tailored approach for transcatheter treatment of this condition. Another limitation is that RS was identified by a positive c-TCD rather than TOE, which may have overestimated the true prevalence of RS. Nonetheless, c-TCD is routinely used as an alternative to TOE for RS screening, with a high sensitivity and specificity61013. Additionally, the 1-year c-TCD follow-up was not available for all patients who underwent percutaneous treatment of RS, so the efficacy of the procedure could only be assessed based on the end-procedure TOE bubble test. Finally, we were unable to perform a comparative analysis between medically and interventional-treated patients with RS, resulting in a significant gap in the evidence regarding whether RS closure is indicated. Further prospective studies are necessary to assess the true benefit of this approach.
Conclusions
Residual paradoxical shunt is frequently found after transcatheter PFO closure and is significantly associated with the type and size of the occluding device. The causes of RS are multiple, and a comprehensive assessment of their mechanisms, as well as detailed imaging of atrial septal anatomy, is crucial in defining a patient-tailored approach to shunt closure. Percutaneous treatment of RS with different dedicated or off-label devices is safe and effective in a high percentage of cases.
Impact on daily practice
Residual paradoxical shunt is frequently found after patent foramen ovale closure and is associated with the type and size of the occluding device. The mechanisms of residual shunt (RS) are multiple and can be classified into 3 categories: type 1: intradevice; type 2: extradevice; type 3: any other unusual cause of RS. Transcatheter closure of RS can be safely and effectively performed in a high percentage of patients, regardless of its mechanism, by using different dedicated or off-label devices.
Conflict of interest statement
G. Gaio is a proctor for W. L. Gore & Associates and Heartstitch. F. Meucci is a proctor for Boston Scientific and Innova HTS. G. Santoro is a proctor for Abbott, W. L. Gore & Associates, and Occlutech. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Transcatheter closure of an intradiscal RS by implantation of an Amplatzer Vascular Plug type II.
Moving image 2. Transcatheter closure of an extradevice RS by implantation of an Amplatzer PFO Occluder.
Moving image 3. Transcatheter closure of multiple atrial septum fistulas by deployment of controlled-release coils.
Moving image 4. Closure of RS after the NobleStitch technique in a complex PFO in situs visceroatrial inversus by implantation of a 30 mm Cardia Ultrasept.
Moving image 5. Transcatheter closure of multiple shunts of different types by implantation of an Amplatzer Duct Occluder II and a controlled-release Cook coil.

