Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Mitral annular calcification (MAC) presents challenges for transcatheter edge-to-edge repair (TEER). Limited data exist on how the anatomical features of MAC, assessed by computed tomography (CT), may be associated with TEER outcomes.
Aims: We sought to examine the association between CT features of MAC and clinical outcomes after TEER at 3 years.
Methods: This retrospective observational study included patients who underwent TEER and preprocedural CT. Patients were classified into no/mild MAC and moderate/severe MAC groups. Classification was determined by scoring calcium thickness, distribution, trigone involvement, and leaflet calcification. The primary outcome was all-cause mortality 3 years after TEER.
Results: Among 391 patients who underwent pre-TEER cardiac CT, 318 (81.3%) had no/mild MAC, and 73 (18.7%) had moderate/severe MAC. At 3 years, all-cause mortality was comparable between the groups (17.6% vs 24.7 %; p=0.17), whereas patients with no/mild MAC had a significantly better New York Heart Association Class than those with moderate/severe MAC (p=0.029). Calcium thickness >5 mm and leaflet involvement were significant predictors of all-cause mortality at 3 years (odds ratio [OR] 2.38, 95% confidence interval [CI]: 1.08-5.25; p=0.032; OR 6.71, 95% CI: 3.28-13.7; p<0.001); patients exhibiting both of these indicators had a significantly higher incidence of all-cause mortality compared to those with calcium thickness ≤5 mm and no leaflet calcification.
Conclusions: Overall, all-cause mortality did not significantly differ between patients with varied MAC severity. However, greater calcium thickness and leaflet involvement were associated with worse clinical outcomes in patients undergoing TEER. Detailed preoperative CT evaluation can facilitate the prediction and management of TEER outcomes.
Transcatheter edge-to-edge repair (TEER) has been established as an effective and safe alternative to surgical intervention for primary mitral regurgitation (MR) in patients considered high risk for surgery12. Additionally, TEER is the standard of care for secondary MR in patients with low left ventricular ejection fraction, regardless of surgical risk34. Although TEER has demonstrated substantial benefits with respect to symptom relief and quality of life improvement, its effectiveness can be significantly influenced by anatomical and pathological features of the mitral valve, especially mitral annular calcification (MAC). MAC is characterised by dense, fibrous calcium deposits in the mitral valve annulus and is more prevalent in older individuals and those with chronic kidney disease, among other cohorts56. The presence of MAC is associated with poor outcomes of surgical mitral valve repair or replacement78. The presence of MAC poses unique technical challenges during TEER and thus affects the feasibility of the procedure; however, the relationship between MAC and TEER outcomes remains unclear.
Echocardiography is the standard imaging modality for the functional assessment of MR and the structural evaluation of the mitral valve; however, its ability to visualise calcification details and quantitatively assess the extent of calcification is limited. Computed tomography (CT) offers superior spatial resolution, which allows detailed visualisation of calcified patterns and their spatial relation to the mitral leaflets9. Several studies have reported that CT imaging is a useful modality for predicting clinical outcomes in patients undergoing TEER1011. However, data regarding CT characterisation of MAC and its association with TEER outcomes are lacking.
In the present study, we sought to examine CT phenotypes of MAC and their association with clinical outcomes in patients undergoing TEER. A comprehensive understanding of negative prognostic markers identified on CT will inform improved patient selection and clinical outcomes in this challenging population.
Methods
Study design and patient population
This retrospective observational study was conducted at the Cedars-Sinai Medical Center in Los Angeles, California, USA. Patients aged ≥18 years who underwent TEER for MR between April 2015 and March 2021 were evaluated. Among them, only patients who received preprocedural CT scans before TEER were included in the study. We excluded patients who underwent concomitant heart valve interventions with TEER to mitigate the confounding effects of other procedures. This study was conducted in accordance with the Declaration of Helsinki (1975) and approved by the Institutional Review Board of the Cedars-Sinai Medical Center. Informed consent was obtained from all patients. Patient information was retrospectively obtained from an established interventional cardiology laboratory database at our institution, during outpatient visits, and through telephone interviews.
CT acquisition and analysis of the mitral valve
Electrocardiogram-gated multidetector CT scans were performed using a 128-row dual-source CT system (Siemens SOMATOM Definition Flash [Siemens Healthineers]) with a collimation of 128×0.625 mm. The maximum tube current was automatically adjusted for each patient using CareDose (Siemens Healthineers), and the tube potential was fixed at 100-120 kV. A standardised dose of 100 mL Omnipaque (GE HealthCare) was administered at 6 mL/s for contrast enhancement. The scan range extended from the aortic arch to the diaphragm in a craniocaudal direction. Images were reconstructed at a slice thickness of 0.6 mm with a 0.3 mm overlap using iterative reconstruction; subsequently, they were evaluated at 10% intervals from 0% to 90% of the RR interval using 3mensio Valves software, version 9.0 (Pie Medical Imaging). These images were reviewed in a specialised CT core laboratory.
Images from the late diastolic phase with minimal motion artefacts were selected for mitral valve analysis. The mitral annulus was segmented by placing 16 seeding points along the insertion of the posterior leaflet and the contour of the fibrous continuity, using stepwise rotation in the long-axis view. The D-shaped annulus was defined by truncating the saddle shape at the trigone-to-trigone distance. The 3mensio Valves software automatically calculated the mitral annulus area, perimeter, and anterior-posterior diameter; moreover, the medial-lateral diameter was measured perpendicular to the anterior-posterior line at its longest length (Supplementary Figure 1).
MAC severity was evaluated using 3mensio Valves software based on established criteria. The scoring system included four main parameters based on previously established criteria12, as follows: (1) average annulus calcium thickness in millimetres; (2) the extent of calcium distribution around the annulus circumference in degrees; (3) presence of calcification in either or both of the fibrous trigones; and (4) calcification extending ≥5 mm from the leaflet insertion point at the annulus in either or both mitral leaflets. Each criterion is divided into subcategories, with points assigned based on severity, as illustrated in Figure 1. The total MAC score is the sum of the points from each category, with a maximum possible score of 10. MAC severity was classified as mild (≤3 points), moderate (4-6 points), or severe (≥7 points).
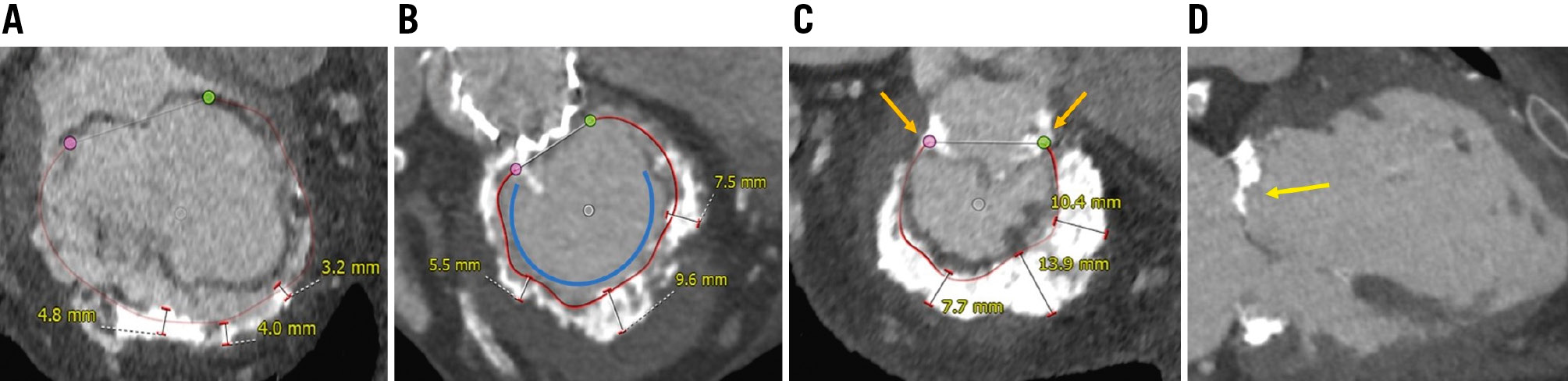
Figure 1. Cardiac computed tomography-based measurements of the calcified mitral annulus using 3mensio Structural Heart Mitral Workflow. The scoring system for MAC includes four main criteria: (A) average annulus calcium thickness, scored as follows: <5 mm (1 point), 5-9.9 mm (2 points), or ≥10 mm (3 points); (B) calcium distribution around the annulus circumference, scored as follows: <180° (1 point), 180-270° (2 points), or >270° (3 points), shown by the blue curve; (C) presence of calcification in the fibrous trigones, scored as follows: none (0 points), anterolateral (1 point), or posteromedial (1 point), indicated by the orange arrows; and (D) calcification extending ≥5 mm from the leaflet insertion point at the annulus, scored as follows: none (0 points), anterior (1 point), or posterior (1 point), illustrated by the yellow arrow. MAC severity was categorised based on the total points: mild MAC (≤3 points), moderate MAC (4-6 points), and severe MAC (≥7 points). MAC: mitral annular calcification
Devices and procedures
The indication for TEER was moderate-to-severe MR accompanied by symptomatic heart failure (HF) according to the New York Heart Association (NYHA) Functional Classification in patients at a high surgical risk. A multidisciplinary Heart Team at Cedars-Sinai Medical Center discussed the indication for TEER. Procedure details, including proper device selection and access site, were determined based on preoperative echocardiography. TEER was performed using the MitraClip device (Abbott) according to standard clinical techniques and published guidelines under fluoroscopic and echocardiographic guidance13. Procedures were performed under general anaesthesia through femoral vein access.
Echocardiographic assessment
All patients underwent follow-up transthoracic echocardiography at 30 days and 1 year after TEER as part of a routine post-TEER echocardiographic assessment, which included an evaluation of valvular function. MR severity was assessed using preprocedural transthoracic or transoesophageal echocardiography and was classified as 0 (none or trivial), 1+ (mild), 2+ (moderate), 3+ (moderate-severe), or 4+ (severe). Echocardiograms were performed and interpreted by experienced sonographers and level 3-trained echocardiologists, following the relevant American Society of Echocardiography guidelines14.
Outcomes
The primary outcome was all-cause mortality at 3 years following TEER. Secondary outcomes included HF hospitalisation and mitral valve reintervention. All clinical outcomes were assessed according to the Mitral Valve Academic Research Consortium criteria15. Moreover, the NYHA Functional Class was evaluated at 30 days and 1 year after the procedure. Procedural success was defined as residual MR grade ≤2+ with a transmitral mean pressure gradient (TMPG) <5 mmHg at 30 days after TEER.
Statistical analysis
Continuous data are presented as the mean±standard deviation or median (interquartile range), and categorical variables are expressed as numbers and percentages. The Student’s t-test or the Mann-Whitney U test was used for between-group comparisons of continuous variables. One-way analysis of variance and the Kruskal-Wallis test were applied to normally and non-normally distributed data, respectively. Categorical variables were assessed using the chi-squared test. The cumulative rates of adverse events were analysed using the Kaplan-Meier method. Univariate logistic regression models were used to assess the relationships between clinical variables and residual MR ≥moderate at 30 days as well as all-cause mortality at 3 years post-TEER. Subsequently, multivariable logistic regression analyses were conducted to calculate adjusted odds ratios (ORs) for all clinical variables with a p-value<0.10 in the univariate analyses, along with their corresponding 95% confidence intervals (CIs). Multicollinearity among independent variables was assessed by measuring the variance inflation factor (VIF) and tolerance (1/VIF). A VIF >5 and tolerance value <0.2 were indicative of significant multicollinearity. Covariates included in the multivariable regression analyses are listed in Supplementary Table 1. Statistical analyses were performed using SPSS, version 26 (IBM), and statistical significance was defined as p<0.05.
Results
Among 996 consecutive patients undergoing TEER, 412 patients received preprocedural CT scans. The baseline characteristics of patients who underwent TEER with and without preprocedural CT are shown in Supplementary Table 2. After applying the exclusion criteria, a total of 391 patients were retrospectively analysed. These patients were divided into groups based on the severity of MAC assessed by CT. Among them, 318 (81.3%) had no/mild MAC, while 73 (18.7%) had moderate/severe MAC (Figure 2). Table 1 summarises the baseline characteristics and echocardiographic findings. The mean age was 73.8±13.1 years, and 229 (58.6%) patients were male. Patients with moderate or severe MAC had a higher TMPG than did those with no or mild MAC (3.49±1.67 mmHg vs 2.85±1.75 mmHg; p=0.004). Table 2 presents preprocedural CT measurements for both groups at baseline. Compared with patients with no/mild MAC, those with moderate/severe MAC had a greater average calcium thickness, wider calcium distribution, and more frequent involvement of trigones and leaflets (all p<0.001). There were no significant differences in the mitral annular area, perimeter, or diameter between the groups.
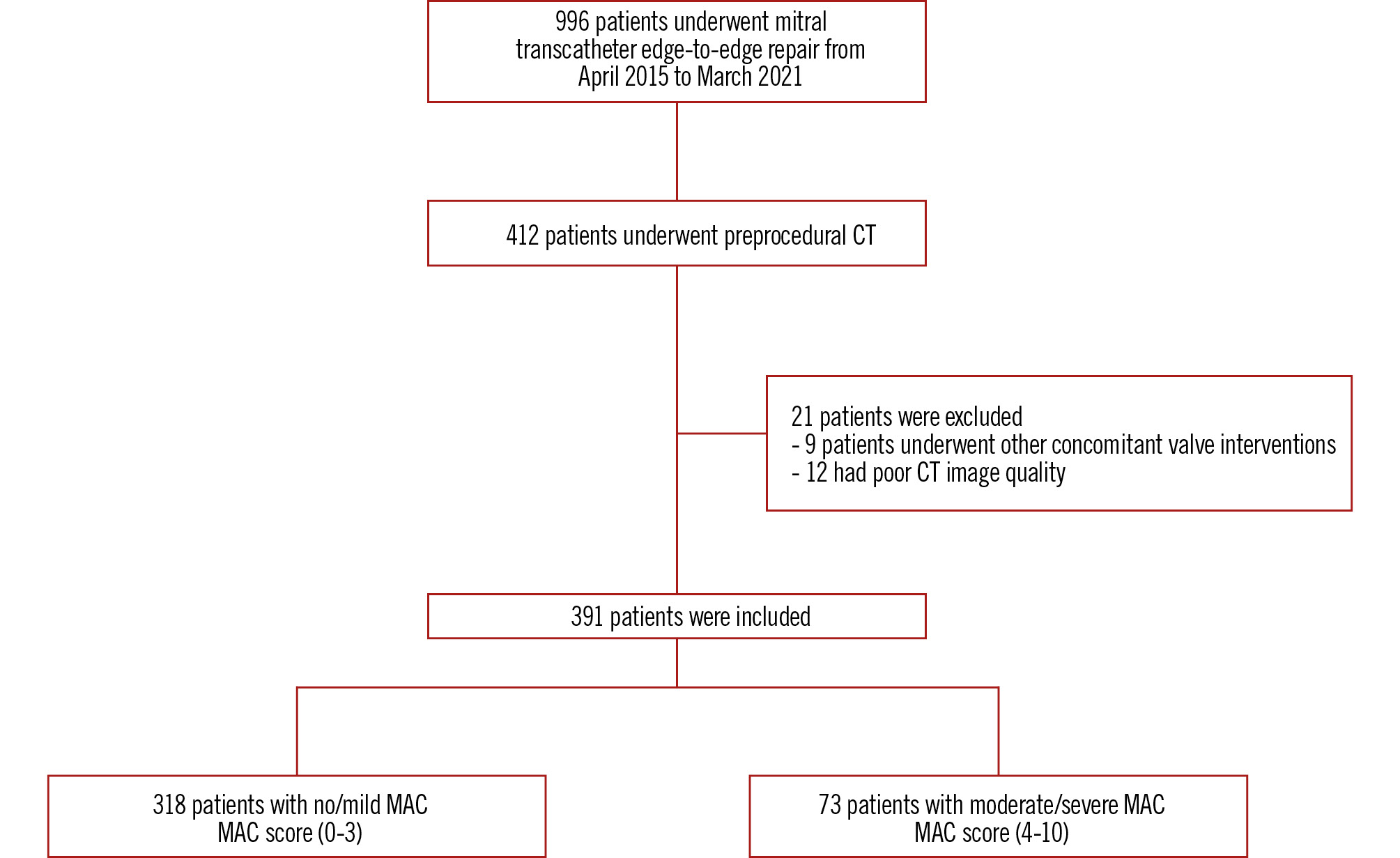
Figure 2. Study flowchart. Number of enrolled patients in the study and the inclusion and exclusion criteria. CT: computed tomography; MAC: mitral annular calcification
Table 1. Baseline patient characteristics.
| Baseline characteristics | Overall n=391 |
No or mild MAC n=318 |
Moderate or severe MAC n=73 |
p-value |
|---|---|---|---|---|
|
Age, years |
73.8±13.1 |
71.9±12.5 |
81.9±12.3 |
<0.001 |
|
Male |
229 (58.6) |
191 (60.1) |
38 (52.1) |
0.21 |
|
Body mass index, kg/m2 |
25.9±6.2 |
26.1±6.2 |
25.0±6.2 |
0.16 |
|
STS risk score for mitral valve repair, % |
7.5±8.1 |
7.2±8.4 |
8.8±6.8 |
0.085 |
|
Hypertension |
325 (83.1) |
264 (83.0) |
61 (83.6) |
0.91 |
|
Previous stroke or TIA |
37 (9.5) |
33 (10.4) |
4 (5.5) |
0.2 |
|
Porcelain aorta |
22 (5.6) |
18 (5.7) |
4 (5.5) |
0.85 |
|
Smoker |
16 (4.1) |
15 (4.7) |
1 (1.4) |
0.19 |
|
Diabetes |
122 (31.2) |
98 (30.8) |
24 (32.9) |
0.73 |
|
Current dialysis |
39 (10.0) |
30 (9.4) |
9 (12.3) |
0.46 |
|
Chronic lung disease |
57 (14.6) |
45 (14.2) |
12 (16.4) |
0.62 |
|
Prior heart failure hospitalisation |
212 (54.2) |
177 (55.7) |
35 (47.9) |
0.23 |
|
Peripheral vascular disease |
50 (12.8) |
37 (11.6) |
13 (17.8) |
0.15 |
|
Previous permanent pacemaker implantation |
50 (12.8) |
38 (11.9) |
12 (16.4) |
0.3 |
|
Previous cardiac resynchronisation therapy |
71 (18.2) |
63 (19.8) |
8 (11.0) |
0.077 |
|
Atrial fibrillation or flutter |
213 (54.5) |
174 (55.4) |
39 (50.6) |
0.84 |
|
Previous myocardial infarction |
99 (25.3) |
84 (26.4) |
15 (20.5) |
0.3 |
|
Prior CABG |
74 (18.9) |
58 (18.2) |
16 (21.9) |
0.47 |
|
Prior aortic valve replacement |
70 (17.9) |
45 (14.2) |
25 (34.2) |
<0.001 |
|
Prior percutaneous coronary intervention |
117 (29.9) |
91 (28.6) |
26 (35.6) |
0.24 |
|
NYHA Class |
0.98 |
|||
|
II |
6 (1.5) |
6 (1.9) |
0 (0) |
|
|
III |
100 (25.6) |
79 (24.8) |
21 (28.8) |
|
|
IV |
285(72.9) |
233 (73.2) |
52 (71.2) |
|
|
BNP, pg/mL |
1,166±1,213.9 |
1,216.7±1,234.1 |
950.9±1,105.7 |
0.11 |
|
Creatinine, mg/dL |
1.7±2.1 |
1.7±1.4 |
1.6±1.3 |
0.8 |
| Medication | ||||
|
Aspirin |
176 (45.0) |
140 (44.0) |
36 (49.3) |
0.41 |
|
Beta blocker |
298 (76.2) |
242 (81.2) |
56 (76.7) |
0.91 |
|
ACEi or ARB or ARNI |
223 (57.0) |
176 (55.3) |
47 (64.4) |
0.16 |
|
Mineralocorticoid receptor antagonists |
70 (17.9) |
59 (18.6) |
11 (15.1) |
0.48 |
|
Loop diuretic |
294 (75.2) |
236 (74.2) |
58 (79.5) |
0.35 |
|
Thiazides |
20 (5.1) |
14 (4.4) |
6 (8.2) |
0.18 |
|
Anticoagulant |
156 (39.9) |
129 (40.6) |
27 (37.0) |
0.57 |
| Echocardiographic characteristics | ||||
|
Severe mitral regurgitation |
321 (82.1) |
261 (82.1) |
60 (82.2) |
0.98 |
|
Degenerative mitral regurgitation |
138 (35.3) |
110 (34.6) |
28 (38.4) |
0.54 |
|
Functional mitral regurgitation |
257 (65.7) |
211 (66.4) |
46 (63.0) |
0.59 |
|
Mitral valve area by planimetry, cm2 |
5.01±2.20 |
5.0±2.17 |
5.21±2.53 |
0.8 |
|
Transmitral mean pressure gradient, mmHg |
2.97±1.75 |
2.85 ±1.75 |
3.49±1.67 |
0.004 |
|
Mitral regurgitant volume by PISA, mL |
48.8±15.1 |
48.5±15.2 |
50.1±14.5 |
0.87 |
|
Left ventricular ejection fraction, % |
43.1±19.6 |
42.8±19.6 |
44.3±19.6 |
0.55 |
|
Left ventricular end-systolic diameter, cm |
4.3±1.4 |
4.50±1.42 |
3.44±0.91 |
<0.001 |
|
Left ventricular end-diastolic diameter, cm |
5.5±1.2 |
5.67±1.17 |
4.83±0.82 |
<0.001 |
|
Left atrium volume, mL |
104.5±49.9 |
103.0±49.2 |
116.4±50.5 |
0.15 |
|
Right atrial pressure, mmHg |
11.7±5.7 |
11.69±5.89 |
11.71±5.04 |
0.98 |
|
Pulmonary arterial systolic pressure, mmHg |
46.9±15.9 |
47.41±16.38 |
45.28±14.24 |
0.44 |
|
TAPSE, mm |
17.5±5.1 |
17.5±5.0 |
17.8±5.5 |
0.7 |
|
AR ≥moderate |
35 (9.0) |
31 (9.7) |
4 (5.5) |
0.25 |
|
TR ≥moderate |
228 (58.1) |
182 (57.2) |
46 (63.0) |
0.37 |
|
Moderate TR |
142 (36.3) |
115 (36.1) |
27 (37.0) |
|
|
Moderate to severe TR |
42 (10.7) |
34 (10.7) |
8 (11.0) |
|
|
Severe TR |
24 (6.1) |
18 (5.7) |
6 (8.2) |
|
|
Very severe/massive TR |
18 (4.6) |
14 (4.4) |
4 (5.4) |
|
|
Torrential TR |
2 (0.5) |
1 (0.3) |
1 (1.3) |
|
|
Data are reported as mean±SD or n (%). ACEi: angiotensin-converting enzyme inhibitors; AR: aortic regurgitation; ARB: angiotensin II receptor blocker; ARNI: angiotensin receptor-neprilysin inhibitor; BNP: B-type natriuretic peptide; CABG: coronary artery bypass graft; MAC: mitral annular calcification; NYHA: New York Heart Association; PISA: proximal isovelocity surface area; SD: standard deviation; STS: Society of Thoracic Surgeons; TAPSE: tricuspid annular plane systolic excursion; TIA: transient ischaemic attack; TR: tricuspid regurgitation |
||||
Table 2. Preprocedural computed tomography measurements.
| Overall n=391 |
No or mild MAC n=318 |
Moderate or severe MAC n=73 | p-value | |
|---|---|---|---|---|
|
Mitral annular area, cm2 |
12.4±3.9 |
12.6±3.8 |
12.0±4.0 |
0.42 |
|
Mitral annular perimeter, mm |
104.8±16.9 |
105.6±17.3 |
102.5±16.0 |
0.33 |
|
Anteroposterior diameter, mm |
35.2±6.2 |
35.6±6.2 |
34.2±5.9 |
0.26 |
|
Mediolateral diameter, mm |
41.9±6.7 |
42.1±6.5 |
41.5±7.3 |
0.68 |
| MAC | ||||
|
Average calcium thickness, short axis |
<0.001 |
|||
|
≤5 mm (1 point) |
337 (86.2) |
313 (98.5) |
24 (32.9) |
|
|
>5 mm to <10 mm (2 points) |
33 (8.4) |
5 (1.6) |
28 (38.4) |
|
|
≥10 mm (3 points) |
21 (5.4) |
0 (0) |
21 (28.8) |
|
|
Calcium distribution |
<0.001 |
|||
|
<180° (1 point) |
339 (86.7) |
311 (97.8) |
28 (38.4) |
|
|
180° to 270° (2 points) |
32 (8.2) |
7 (2.2) |
25 (34.2) |
|
|
>270° (3 points) |
20 (5.1) |
0 (0) |
20 (27.4) |
|
|
Trigone involvement |
<0.001 |
|||
|
None (0 point) |
293 (74.9) |
288 (90.6) |
5 (6.8) |
|
|
One (1 point) |
57 (14.6) |
25 (7.9) |
32 (43.8) |
|
|
Both (2 points) |
41 (10.5) |
5 (1.6) |
36 (49.3) |
|
|
Leaflet involvement |
<0.001 |
|||
|
None (0 point) |
332 (84.9) |
298 (93.7) |
34 (46.6) |
|
|
One leaflet (1 point) |
50 (12.8) |
20 (6.3) |
30 (41.1) |
|
|
Both leaflets (2 points) |
9 (2.3) |
0 (0) |
9 (12.3) |
|
|
MAC grade |
||||
|
No-mild (0-3 points) |
318 (81.3) |
318 (81.3) |
0 (0) |
<0.001 |
|
Moderate (4-6 points) |
48 (12.3) |
0 (0) |
48 (12.3) |
|
|
Severe (7-10 points) |
25 (6.4) |
0 (0) |
25 (6.4) |
|
|
Data are reported as the mean±SD or n (%). MAC: mitral annular calcification; SD: standard deviation |
||||
Procedural data
The procedural success rate was 81.3% overall, with no significant difference between the no/mild MAC group and the moderate/severe MAC group (81.3% vs 78.1%; p=0.43) (Supplementary Table 3). The post-procedure TMPG was significantly higher in the moderate/severe MAC group (p=0.006) than in the no/mild MAC group, while other procedural metrics and in-hospital complications did not significantly differ between the groups.
Clinical outcomes
There were no significant between-group differences in the 3-year clinical outcomes after TEER, including all-cause mortality, cardiovascular death, HF hospitalisation, valve reintervention, and stroke (Table 3, Supplementary Figure 2A-Supplementary Figure 2B-Supplementary Figure 2C). After adjusting for age, sex, previous cardiac resynchronisation therapy defibrillator use, and prior aortic valve replacement, there were no significant differences in the outcomes between the two groups. There was no significant difference in the NYHA Class at baseline and 30 days after TEER (Supplementary Figure 3); however, at 1 year and 3 years after TEER, patients with no/mild MAC had a significantly better NYHA Class than those with moderate/severe MAC (p=0.021 and 0.029, respectively). Echo assessment showed no significant difference in residual MR between groups at 30 days after TEER (p=0.098). However, at 1 year, patients with moderate/severe MAC had significantly greater residual MR than those with no/mild MAC (p=0.036) (Supplementary Figure 4A). The TMPG was significantly higher in the moderate/severe MAC group compared with that in the no/mild MAC group at baseline, discharge, 30 days, and 1 year post-procedure (all p<0.01) (Supplementary Figure 4B).
Table 3. Clinical outcomes at 3 years following TEER.
| Overall
N=391 |
No/mild MAC N=318 | Moderate/severe MAC N=73 | Unadjusted OR (95% CI) | p-value | Adjusted OR* (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
|
All-cause mortality |
72 |
56 |
18 |
1.53 (0.84-2.81) |
0.17 |
1.63 (0.81-3.28) |
0.17 |
|
CV death |
55 |
43 |
12 |
1.26 (0.63-2.53) |
0.52 |
1.51 (0.67-3.37) |
0.32 |
|
HF hospitalisation |
74 |
58 |
16 |
1.26 (0.68-2.35) |
0.47 |
1.41 (0.68-2.89) |
0.35 |
|
Valve reintervention |
18 |
13 |
5 |
1.73 (0.60-5.00) |
0.32 |
1.85 (0.56-6.15) |
0.31 |
|
Stroke |
19 |
17 |
2 |
0.5 (0.11-2.21) |
0.36 |
0.51 (0.12-2.89) |
0.51 |
|
Tricuspid intervention |
7 |
6 |
1 |
0.68 (0.8-5.69) |
0.72 |
0.65 (0.74-5.6) |
0.69 |
|
Data are reported as n (%). *Adjusted by age, male sex, previous CRTD, prior AVR. AVR: aortic valve replacement; CI: confidence interval; CRTD: cardiac resynchronisation therapy defibrillator; CV: cardiovascular; HF: heart failure; MAC: mitral annular calcification; OR: odds ratio; TEER: transcatheter edge-to-edge repair |
|||||||
Predictors
Supplementary Table 4 presents the predictors of residual MR ≥moderate at 30 days after TEER. Anatomical features of MAC, including average calcium thickness >5 mm and leaflet involvement, were independent predictors of residual MR ≥moderate at 30 days after TEER (OR 2.67, 95% CI: 1.26-5.65; p=0.01 and OR 3.69, 95% CI: 1.79-7.63; p<0.001, respectively). Table 4 presents the univariate and multivariable analyses of predictors for all-cause mortality at 3 years after TEER. Significant predictors in the multivariable analysis included the Society of Thoracic Surgeons risk score for mitral valve repair and left ventricular ejection fraction (OR 1.04, 95% CI: 1.01–1.07; p=0.006, and OR 0.72, 95% CI: 0.61-0.84; p<0.001, respectively). Additionally, an average calcium thickness >5 mm and leaflet involvement were significant predictors of all-cause mortality (OR 2.38, 95% CI: 1.08-5.25; p=0.032; OR 6.71, 95% CI: 3.28-13.70; p<0.001, respectively).
Table 4. Predictors of all-cause mortality at 3 years after TEER.
| Univariate | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
|
Age, years |
0.99 (0.98-1.01) |
0.57 |
||
|
Male sex |
1.05 (0.63-1.75) |
0.86 |
||
|
Body mass index, kg/m2 |
1.01 (0.97-1.05) |
0.56 |
||
|
STS risk score for mitral valve repair, per 1% increase |
1.05 (1.02-1.08) |
<0.001 |
1.04 (1.01-1.07) |
0.006 |
|
Atrial fibrillation or flutter |
1.05 (0.63-1.74) |
0.86 |
||
|
Chronic lung disease |
1.67 (0.87-3.20) |
0.13 |
||
|
Current dialysis |
2.08 (0.99-4.33) |
0.05 |
1.5 (0.66-3.44) |
0.33 |
|
Previous myocardial infarction |
1.21 (0.69-2.14) |
0.5 |
||
|
Prior CABG |
1.36 (0.74-2.51) |
0.33 |
||
|
Anticoagulation therapy |
1.36 (0.82-2.26) |
0.24 |
||
|
LVEF, per 10% increase |
0.98 (0.97-0.99) |
0.014 |
0.72 (0.61-0.84) |
<0.001 |
|
Left ventricular end-diastolic diameter, per 1 mm increase |
1.17 (0.94-1.47) |
0.16 |
||
|
LA volume, per 10 mL increase |
1.01 (0.95-1.08) |
0.72 |
||
|
AR ≥moderate |
1.08 (0.85-1.36) |
0.54 |
||
|
TR ≥moderate |
1.89 (1.10-3.27) |
0.022 |
1.72 (0.93-3.17) |
0.082 |
|
Mitral annular area, per 1 cm2 increase |
1.03 (0.92-1.17) |
0.59 |
||
|
Presence of MAC in A2 or P2 |
1.42 (0.82-2.46) |
0.21 |
||
|
Average calcium thickness >5 mm |
2.59 (1.37-4.90) |
0.01 |
2.38 (1.08-5.25) |
0.032 |
|
Calcium distribution >180° |
0.75 (0.34-1.67) |
0.49 |
||
|
Trigone involvement (one or two) |
0.95 (0.53-1.7) |
0.87 |
||
|
Leaflet involvement (one or two leaflets) |
5.3 (2.91-9.67) |
<0.001 |
6.71 (3.28-13.70) |
<0.001 |
|
AR: aortic regurgitation; CABG: coronary artery bypass graft; CI: confidence interval; LA: left atrium; LVEF: left ventricular ejection fraction; MAC: mitral annular calcification; OR: odds ratio; STS: Society of Thoracic Surgeons; TEER: transcatheter edge-to-edge repair; TR: tricuspid regurgitation |
||||
Subgroup analysis
Based on the multivariable analysis results, we categorised the patients into three groups: those with an average calcium thickness ≤5 mm and no leaflet calcification, those with either an average calcium thickness >5 mm or leaflet calcification, and those with both conditions. We compared the baseline characteristics and clinical outcomes among these groups (Supplementary Table 5). Kaplan-Meier survival analysis revealed that patients with both significant leaflet calcification and an average calcium thickness >5 mm had significantly higher incidences of all-cause mortality (Central illustration), HF hospitalisation, and reintervention than the other subgroups (Supplementary Figure 5A, Supplementary Figure 5B). Implantation success was significantly lower in patients with both an average calcium thickness >5 mm and leaflet involvement compared with the other groups (Supplementary Table 6). Additionally, in patients with both significant leaflet calcification and an average calcium thickness >5 mm, TMPG ≥5 mmHg and residual MR >moderate at 30 days after TEER were more prevalent than in the other groups (Supplementary Table 7).
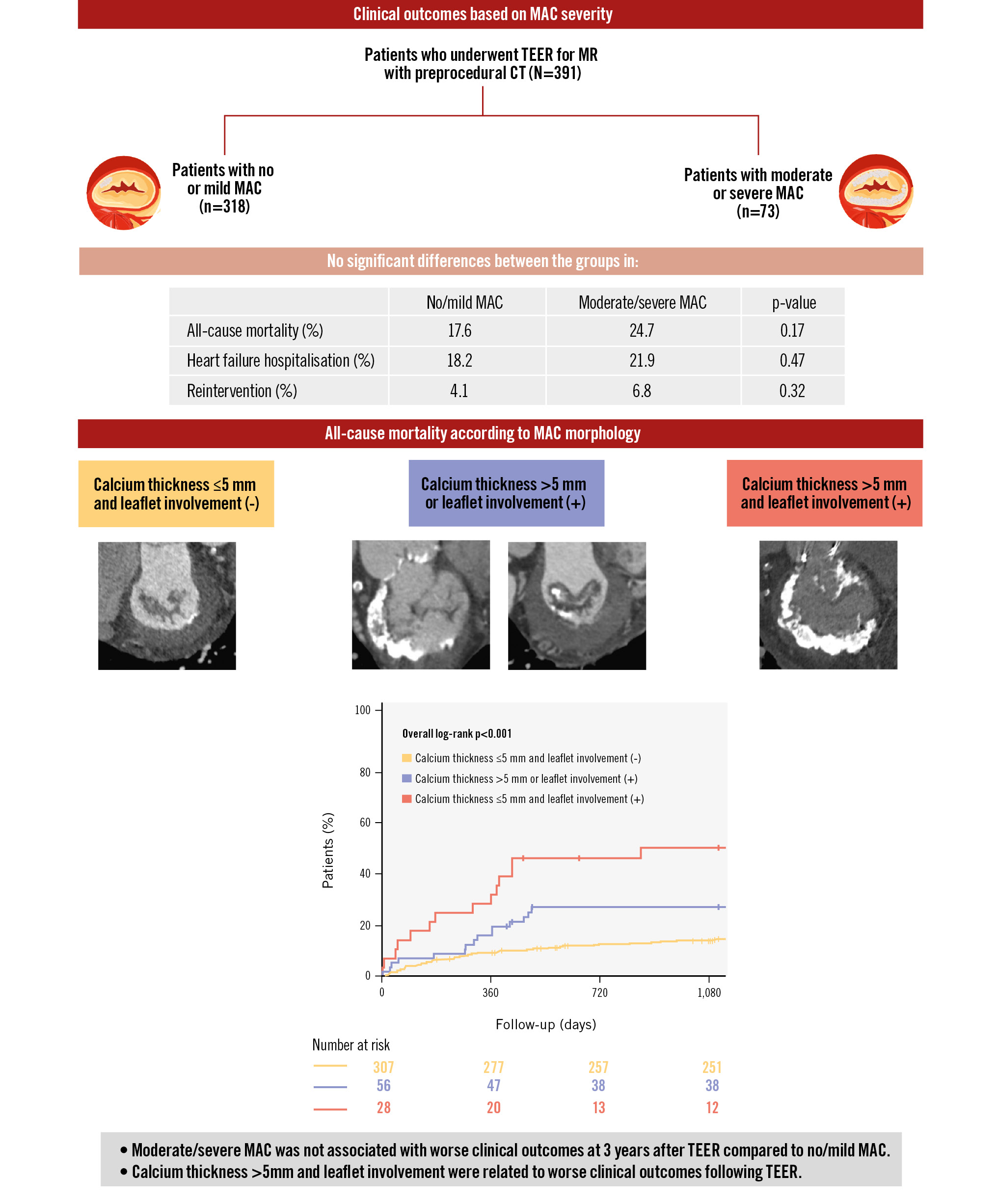
Central illustration. Impact of anatomical features of mitral annular calcification on transcatheter edge-to-edge repair outcomes. CT: computed tomography; MAC: mitral annular calcification; MR: mitral regurgitation; TEER: transcatheter edge-to-edge repair
Discussion
This study investigated the impact of CT-derived MAC scores on procedural success and prognosis. Additionally, we analysed detailed parameters, including calcium thickness, calcium distribution, trigone involvement, and leaflet involvement, to identify prognostic factors. To our knowledge, this is the first study to conduct such a comprehensive analysis.
The main findings of this study are as follows: (1) no significant differences were noted between the no/mild MAC and moderate/severe MAC groups in 3-year outcomes, including all-cause mortality and HF rehospitalisation; (2) implantation and procedural success rates were similar between the groups. There were no significant between-group differences in the NYHA Class at baseline or at 30 days post-procedure, although a significant difference emerged after 1 year; and (3) average calcium thickness >5 mm and leaflet involvement emerged as independent predictors of residual MR ≥2+ at 30 days post-TEER and all-cause mortality at 3 years. Stratification based on these parameters showed that patients with both significant leaflet calcification and an average calcium thickness >5 mm had significantly higher incidences of all-cause mortality, HF hospitalisation, and reintervention compared with the other subgroups.
The impact of MAC on prognosis following TEER remains controversial. Previous studies have demonstrated that procedural success and the durability of MR reduction in patients undergoing TEER are not associated with the presence or severity of MAC as determined by echocardiography161718. Contrastingly, several studies have shown that patients with moderate/severe MAC had high 1-year mortality and less symptom improvement following TEER19. In our study, despite patients in the moderate/severe MAC group being approximately 10 years older, survival outcomes were similar. This may be partly explained by the relatively younger age profile of our cohort (mean age 73.8±13.1 years), leading to a lower event rate. However, the moderate/severe MAC group showed a numerical trend towards worse outcomes, including higher all-cause mortality, cardiovascular death, and HF hospitalisation. A longer follow-up may further clarify these differences.
Tanaka et al found that the presence of MAC detected with CT was associated with worse procedural and clinical outcomes in patients undergoing TEER compared to those without MAC, and they observed that higher MAC scores and calcium volume of the mitral valve were related to less favourable procedural outcomes of TEER11. In our study, we categorised patients into no/mild MAC and moderate/severe MAC groups and found no significant differences in the procedural success or prognosis between the two groups. However, while there was no significant difference in residual MR between the no/mild MAC group and the moderate/severe MAC group up to 30 days, a significant difference emerged after 1 year. This raises concerns regarding the long-term durability of interventions in patients with moderate/severe MAC due to the potential for calcification progression and mitral valve degeneration. A significant difference in the NYHA Functional Class became evident after 1 year, suggesting that residual MR may influence patient symptoms. Additionally, the baseline TMPG was significantly higher in patients with moderate/severe MAC and remained elevated after the procedure. This is consistent with previous reports showing that extended MAC or a baseline TMPG ≥4 mmHg is a risk factor for elevated TMPG after TEER20. Furthermore, a postprocedural TMPG ≥5 mmHg has been suggested to be a predictor of poor prognosis21. Thus, although there were no significant differences in all-cause mortality or HF rehospitalisation at 3 years post-TEER in the current study, further follow-up is warranted to elucidate how disparities in TMPG and residual MR following TEER between patients with varied MAC severity influence clinical symptoms and prognosis over a longer follow-up period.
The TEER procedure involved minimal complications, with no in-hospital mortality in the moderate/severe MAC group and only a 1.4% rate of conversion to surgery. Although MAC may increase the risk of complications, such as leaflet tearing or device embolisation, during the TEER procedure2223, we observed no significant differences in these complications between the two groups. This indicates that the TEER procedure is safe even in patients with moderate/severe MAC. Our study did not involve the use of the first-generation MitraClip; accordingly, the use of relatively newer-generation devices may have contributed to the safety of the procedure in patients with MAC24.
Notably, our findings indicated that an average calcium thickness >5 mm and leaflet involvement were independent predictors of residual MR ≥2 at 30 days post-TEER and of all-cause mortality within 3 years. Thick calcium deposits make the mitral leaflets stiffer and less flexible, reducing their mobility25. This reduced mobility impedes the TEER device from effectively grasping and approximating the leaflets, which leads to inadequate coaptation and recurrent MR. Kaewkes et al reported that leaflet calcification is an independent prognostic factor for postprocedural residual MR26, which is consistent with our findings. The surface irregularities created by calcified leaflets lead to inadequate grasping by the TEER device, resulting in ineffective clip application and failure to properly reduce MR. Furthermore, calcified leaflet areas limit their ability to fully extend or move, thereby shortening their functional length even if the anatomical length remains unchanged. Moreover, stratification based on the combination of calcium thickness and leaflet involvement revealed that patients with both severe calcium thickness and leaflet involvement had significantly higher incidences of all-cause mortality, HF hospitalisation, and reintervention. Moreover, echocardiographic findings at 30 days post-TEER showed that patients with both significant leaflet calcification and an average calcium thickness >5 mm had significantly greater residual MR and TMPG, and lower implantation success; these factors might negatively affect clinical outcomes27. As no significant differences in outcomes were observed between the no/mild MAC and moderate/severe MAC groups, our findings suggest that the presence of MAC alone is not the primary determinant of outcomes; rather, leaflet involvement and calcium thickness are the key prognostic factors.
Although echocardiography is the gold standard for mitral valve assessment in TEER, cardiac CT offers the advantages of high spatial resolution and the ability to perform measurements in any plane. Cardiac CT enhances calcium visibility, suggesting added utility in assessing patients with MAC. Additionally, echocardiographic severity assessment is based on calcium distribution, which is one parameter of the MAC score2829. Therefore, detailed evaluation using CT allows for a more comprehensive assessment of MAC and the identification of prognostic factors. This suggests that preoperative CT evaluation should be considered in patients with MAC to better predict residual MR and prognosis following TEER.
Limitations
This study has several limitations. Given its retrospective design, this study has some potential biases. Additionally, we only included patients who underwent preoperative CT. Some patients may have been excluded from TEER and instead underwent surgical mitral valve replacement or transcatheter mitral valve replacement due to anatomical incompatibilities, including MAC. Although this study was conducted at a highly experienced centre, it is a single-centre study; accordingly, further multicentre prospective studies are warranted. Furthermore, certain parameters related to valve characteristics, including leaflet length, thickness, coaptation length, and mobility, were partially missing. The impact of MAC on these characteristics was not assessed, and specific evaluations of the leaflets grasped were not performed. Finally, echo follow-up data at 3 years are lacking.
Conclusions
In patients undergoing TEER, the presence of moderate/severe MAC, as assessed by CT, was not associated with increased all-cause mortality at 3 years compared with no/mild MAC. However, greater calcium thickness and leaflet involvement were associated with decreased procedural success and worse clinical outcomes following TEER.
Impact on daily practice
Mitral annular calcification (MAC) complicates transcatheter edge-to-edge repair (TEER) procedures and is linked to worse outcomes. Computed tomography (CT) imaging provides detailed visualisation of MAC, allowing for stratification by severity and aiding procedural planning. In this study, procedural success and 3-year all-cause mortality were similar between MAC severity groups. However, greater calcium thickness and leaflet calcification identified by CT independently predicted residual mitral regurgitation and mortality. Patients with both leaflet calcification and an average calcium thickness >5 mm had higher risks of mortality, heart failure hospitalisation, and reintervention. These findings support the use of CT imaging during preoperative assessment to improve TEER outcomes in patients with MAC.
Acknowledgements
The study was supported in part by the California Chapter of the American College of Cardiology through the Save A Heart Foundation.
Conflict of interest statement
R.R. Makkar received grant support from Edwards Lifesciences; he is a consultant for Abbott, Cordis, and Medtronic; and holds equity in Entourage Medical. T. Chakravarty is a consultant, proctor, and speaker for Edwards Lifesciences and Medtronic; he is a consultant for Abbott; and a consultant and speaker for Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

