Abstract
BACKGROUND: Transoesophageal echocardiography (TOE) provides accurate evaluation of mitral valve (MV) function following mitral transcatheter edge-to-edge repair (M-TEER) and may better detect complications in case of suboptimal result.
AIMS: We aimed to evaluate midterm anatomical changes and structural complications after M-TEER using TOE and investigate their association with clinical outcomes at 2 years.
METHODS: A follow-up TOE at 6 months was systematically recommended to all patients included in our institutional prospective M-TEER registry until December 2021. We assessed changes in the incidence of mitral regurgitation (MR), MV stenosis (≥5 mmHg), and partial or complete single leaflet device attachment (SLDA) between the index procedure and follow-up and evaluated MV area and annular dimensions in a subset of patients with available three-dimensional (3D) datasets. The clinical endpoint was a composite of mortality and heart failure (HF) rehospitalisation at 2 years.
RESULTS: Among the 373 patients included in the registry between February 2012 and December 2021, 128 patients (34%) underwent elective TOE at 6 months. Using TOE, severe MR was observed in 13.3% (n=17) of the patients. The number of patients with an elevated MV gradient increased from 17 (13.3%) after the procedure to 23 (18%) at 6 months, and a new partial or complete SLDA was detected in 7.8% (n=10). Based on 3D TOE measurements, significant increases in MV area, annular area, annular perimeter, and intercommissural (but not anteroposterior) diameter were observed compared to intraprocedural images. A mean MV gradient ≥5 mmHg (hazard ratio [HR] 2.30, 95% confidence interval [CI]: 1.10-4.81; p=0.023) and the presence of severe MR at 6 months (HR 3.26, 95% CI: 1.18-8.99; p=0.023) were associated with the primary endpoint, which was met in 34 (26.6%) patients at 2 years.
CONCLUSIONS: TOE follow-up allowed the detection of complications that would not be diagnosed using transthoracic echocardiography only and should therefore be used liberally in the patients presenting with a suboptimal result. A mean MV gradient ≥5 mmHg and severe MR, diagnosed at the 6-month TOE follow-up, were associated with adverse clinical outcomes.
In recent years, the landscape of mitral regurgitation (MR) management has evolved, driven by compelling findings from a series of randomised trials123. As a result, mitral transcatheter edge-to-edge repair (M-TEER) has progressively been established as a treatment option for primary (Class IIa, Level of Evidence [LoE] B or Class IIb, LoE B4) and secondary (Class IIa, LoE B) MR in patients with suitable anatomy at increased surgical risk. Real-world data have confirmed the safety, efficacy and cost-effectiveness of M-TEER567. Increasing experience and new device iterations allow for the treatment of more challenging anatomies including patients with calcified mitral annulus, extensive Barlow disease or those with previous surgical mitral annuloplasty.
Transthoracic echocardiography (TTE) is a valuable tool for the diagnosis and grading of MR severity before and after M-TEER89. Nonetheless, because of limited spatial resolution and ultrasound penetration through the barrier built by the thoracic cage and the lungs, clear visualisation of the mitral valve (MV), in particular after device implantation, may be challenging. In contrast, transoesophageal echocardiography (TOE) enables the acquisition of high-quality three-dimensional (3D) datasets used for measurements in multiplanar reconstructions. In fact, dedicated guidelines have defined TOE as the principal tool for systematic evaluation of MV anatomy as well as MR mechanism, and severity before TEER10. Moreover, TOE guidance during the procedure allows for direct device visualisation and assessment of MV functional changes post-implantation, thereby enabling prompt detection of acute complications. While most adverse events, including residual MR, single leaflet device attachment (SLDA), leaflet damage or iatrogenic mitral stenosis, can usually be detected during the procedure, some may develop during follow-up and be underdiagnosed using TTE only11. The accurate determination of the cause of recurrent MR is crucial, since it often influences the decision-making process for corrective procedures. The frequency of such late adverse events including partial leaflet device detachment is largely unknown.
The aim of the present study was to systematically analyse changes in MV anatomy and function using elective TOE follow-up at 6 months and to investigate their impact on clinical outcomes at 2 years.
Methods
PATIENT POPULATION
In this single-centre, retrospective, observational study, the data of patients undergoing M-TEER between February 2012 and December 2021 who underwent elective TOE at 6-month follow-up were extracted from our Transcatheter Mitral Valve Interventions registry (BERN TMVI registry). The protocol was approved by the local Ethics Committee (Kantonale Ethikkommission für die Forschung Bern; project ID: 2017-01104), and all patients signed an informed consent form.
FOLLOW-UP RECOMMENDATION
The postprocedural surveillance included a clinical assessment and TTE at 30 days, TOE at 6 months and TTE at 12 months after M-TEER, followed by annual evaluations by the referring cardiologist.
DATA ANALYSIS
Demographic and outcome data were extracted from our registry and patient records. We evaluated TOE performed before, during, and after the procedure, as well as TTE at discharge and 12 months, reviewing both images and echocardiographic reports. We compared paired TOE acquisitions taken at the end of the procedure and at 6-month follow-up to assess changes in MV area (MVA), MV annular dimensions, MR, MV gradient (≥5 mmHg), SLDA, and tricuspid regurgitation (TR). The results were compared to 125 patients who did not undergo TOE follow-up matched by MR aetiology and device generation.
ECHOCARDIOGRAPHIC ANALYSIS
MR and TR severity were assessed using 3 quantitative grades as described by the European and American Echocardiography Guidelines1213. The aetiology of secondary MR (SMR) was determined based on anatomical criteria. In atrial SMR, annular dilatation was the primary cause of MR, without predominant tenting and normal or mildly reduced ejection fraction. In case of prominent tenting with impaired left ventricular function, MR was classified as ventricular SMR. MR severity before and after TEER was assessed using a multiparametric approach. Significant mitral stenosis was defined as a postprocedural diastolic transvalvular gradient ≥5 mmHg measured by continuous wave Doppler. MVA was retrospectively assessed by 3D planimetry, separately for each orifice after device implantation by 1 cardiac imaging specialist (M. Kassar). The diagnosis of SLDA involved confirming detachment of the device from one of the MV leaflets, often accomplished through multiplanar reconstruction of 3D echocardiographic datasets. Classification of SLDA included assessment of mobility (partial or complete detachment), as well as timing of the diagnosis (acute: periprocedural until discharge, subacute: between discharge and 6-month follow-up, and late: diagnosed at or after 6-month follow-up). The patients were stratified according to MR severity or presence of a high (≥5 mmHg) MV gradient at 6 months.
CLINICAL ENDPOINTS
We correlated the echocardiographic parameters obtained at 6 months with clinical outcomes. The primary clinical endpoint was a composite of mortality and heart failure (HF) rehospitalisation at 2 years. Endpoints were defined according to Mitral Valve Academic Research Consortium (MVARC) criteria.
INTEROBSERVER REPRODUCIBILITY
The interobserver reproducibility of the 3D measurements with both methods was evaluated in 20 randomly selected patients by 2 blinded cardiac imaging specialists (M. Kassar and N. Brugger).
STATISTICAL ANALYSIS
We used the Shapiro-Wilk test to test for normality. Continuous variables are presented as mean±standard deviation or median and interquartile range according to the distribution. Categorical variables are presented as absolute numbers or as percentages. Differences in continuous variables between unpaired data were compared with the unpaired t-test or Mann-Whitney U test depending on normality. Unpaired nominal data were compared using Pearson’s chi-squared test.
McNemar’s test was used to compare paired dichotomised nominal data before and after intervention or between discharge and follow-up. Univariate analyses were conducted to assess the impact of MR severity grades and high (≥5 mmHg) MV gradients at 6 months on the primary clinical endpoint (death or HF hospitalisation at 2 years). The univariate analysis included parameters significantly differing between patients with or without an adverse event (death or HF rehospitalisation) (Supplementary Table 1) in addition to age, ejection fraction, MR and TR severity, and the mean gradient at 6-month follow-up. Variables were considered for multivariate analysis when they were related to the composite endpoint in univariate analysis with a p-value <0.20. The selected variables were included in the multivariate Cox regression to identify independent correlates of the outcomes of interest. Kaplan-Meier survival estimates were used to compare time to clinical endpoints between patients with and without increased MV gradients, as well as between those with different MR severity grades. Differences were tested with log-rank tests. A receiver operating characteristic (ROC) analysis was used to identify the mean gradient cutoff best predicting the occurrence of the primary endpoint at 2 years. Interobserver variability was evaluated with interclass correlation coefficient (2-way mixed, single measure) and Bland-Altman bias and limit of agreement (LoA). A propensity score-matched analysis based on device generation and MR aetiology was performed with patients who did not undergo elective TOE follow-up. The data from discharge, 1-month and 12-month TTE, as well as clinical endpoints were then compared between both groups. Results are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). A 2-sided p-value of <0.05 was considered to indicate statistical significance. All analyses were conducted with SPSS Statistics, version 18 (IBM).
Results
BASELINE
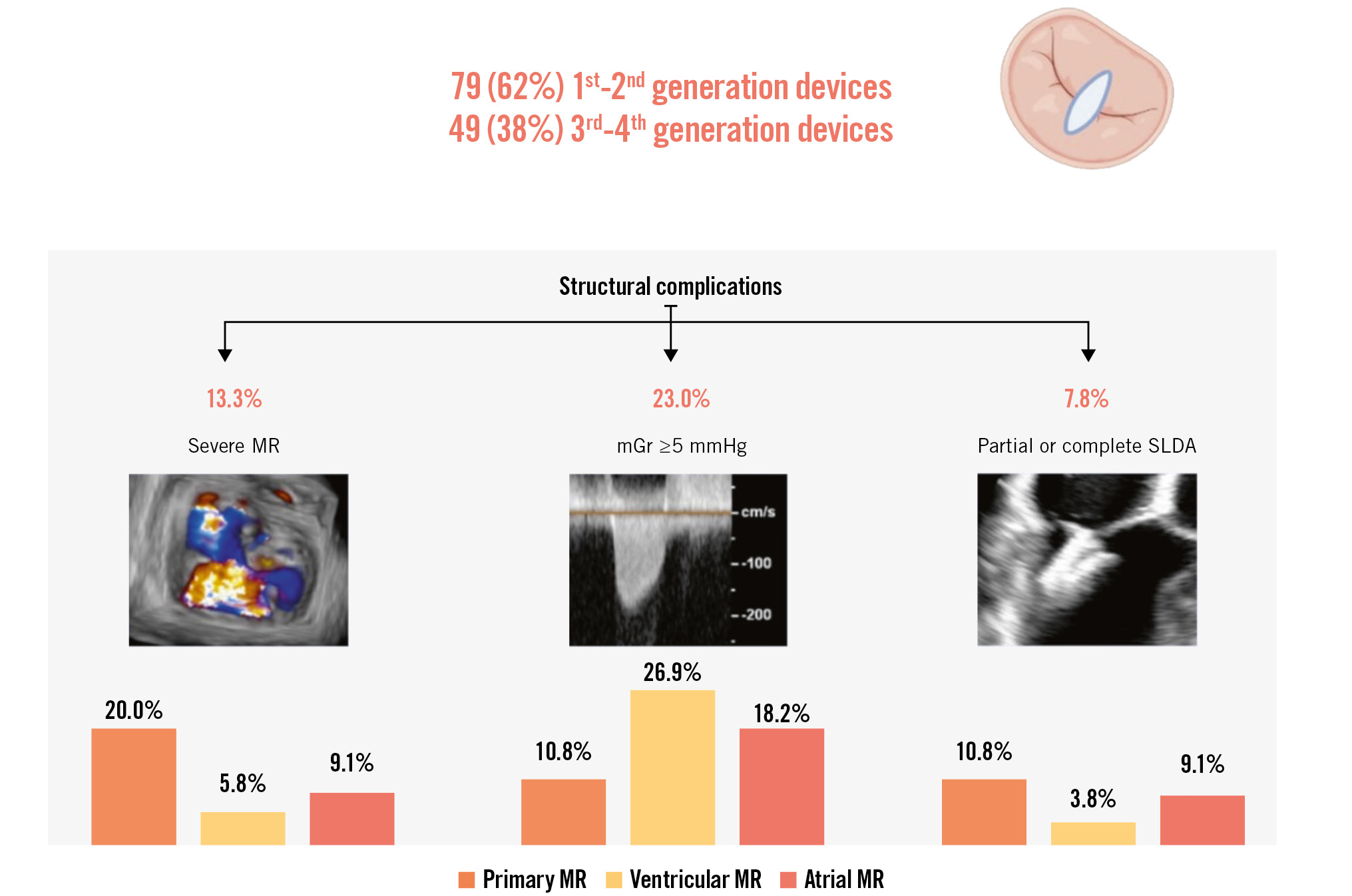
Among the 373 patients included in the registry between February 2012 and December 2021 at our institution, 128 patients had an elective TOE (34.3%) at 6 months and were included in this analysis. The median patient age was 77 (interquartile range [IQR]: 73-83) years, 64.1% (n=82) were male, and 71.9% (n=92) reported dyspnoea of New York Heart Association (NYHA) Class III or IV. Seventy-nine (61.8%) patients were treated with first- and second-generation devices and 49 (38.3%) patients with third- and fourth-generation devices (Central illustration). Hypertension was present in 102 (79.7%), atrial fibrillation or flutter in 89 (69.5%), and coronary artery disease in 74 (57.8%) patients. Primary MR (PMR) was diagnosed in 65 patients (50.8%), ventricular SMR in 52 patients (40.6%) and atrial SMR in 11 patients (8.6%). Severe MR was present in 100 patients (78.1%), and moderate MR was present in 28 (21.9%). Mild or no TR was present in 71 (55.4%), moderate TR in 40 (31.3%) and severe TR in 17 (13.3%) patients at baseline. The baseline clinical characteristics of the study population are presented in Table 1.
Central illustration. TOE follow-up at 6 months after M-TEER. mGr: mean gradient; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; SLDA: single leaflet device attachment; TOE: transoesophageal echocardiography
Table 1. Baseline characteristics at the time of mitral transcatheter edge-to-edge repair (n=128).
| Baseline characteristics | n=128 |
|---|---|
| Age, years | 77.0 [73.0-83.0] |
| Male | 82 (64.1) |
| NYHA Class | |
| II | 36 (28.1) |
| III | 83 (64.8) |
| IV | 9 (7) |
| Comorbidities | |
| Atrial fibrillation/flutter | 89 (69.5) |
| Arterial hypertension | 102 (79.7) |
| Coronary artery disease | 74 (57.8) |
| Chronic obstructive pulmonary disease | 21 (16.4) |
| Diabetes mellitus | 96 (75) |
| Dyslipidaemia | 81 (63.3) |
| Dialysis | 5 (3.9) |
| Cerebrovascular disease | 17 (13.3) |
| Severe renal dysfunction (GFR <30 ml/min/m2) | 7 (5.5) |
| Laboratory results | |
| Creatinine, µmol/l | 127 [87-145] |
| GFR, ml/min/m2 | 73.7±34.8 |
| NT pro-BNP, pg/ml | 3,880 [846-3,990] |
| Haemoglobin, g/l | 121.8±18.7 |
| Echocardiographic parameters | |
| Ejection fraction, % | 47.4±15.6 |
| MR aetiology | |
| Primary | 65 (50.8) |
| Secondary (ventricular) | 52 (40.6) |
| Secondary (atrial) | 11 (8.6) |
| MR VC, mm | 6.8±2.2 |
| MR EROA, cm2 | 0.33±0.16 |
| MR volume, ml | 49.8±24.1 |
| MR severity grades | |
| Moderate | 28 (21.9) |
| Severe | 100 (78.1) |
| TR severity grades | |
| Mild | 71 (55.5) |
| Moderate | 40 (31.3) |
| Severe | 17 (13.3) |
| Continuous data are shown as median [interquartile range] or mean±standard deviation; nominal data are shown as n (%). EROA: effective regurgitant orifice area; GFR: glomerular filtration rate; MR: mitral regurgitation; NT pro-BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; TR: tricuspid regurgitation; VC: vena contracta | |
PROCEDURE
The PASCAL device (Edwards Lifesciences) was implanted in 17 patients (13.3%) and the MitraClip (Abbott) device in 111 (86.7%). More than one device was placed in 52 (40.6%) patients. At the end of the procedure, 2 (1.6%), 37 (28.9%) and 89 (69.5%) patients had severe, moderate (grade 2+ or 3+) and mild MR, respectively. There were no significant differences between MR grades at the end of the procedure (TOE) and at discharge (TTE) (p=1.000). There were no in-hospital deaths or MV reinterventions.
SIX-MONTH TOE FOLLOW-UP
Follow-up TOE took place at a median follow-up time of 182 (IQR: 128-199) days after M-TEER. Changes in MR severity over time are shown in Figure 1. Paired analysis showed an increase of MR severity by at least 1 grade in 53 (41.4%) patients between discharge (TTE) and 6 months (TOE). Thirty-seven patients progressed to moderate MR, while 16 patients progressed to severe MR. Severe MR at 6 months was documented in 20.0% (n=13) of the 65 patients with PMR, in 5.8% (n=3) of the 52 patients with ventricular SMR, and in 9.1% (n=1) of the 11 patients with atrial SMR (Central illustration). A detailed description of all patients with severe MR is provided in Supplementary Table 2. All these patients fulfilled at least 2 criteria indicating complex MV anatomy for M-TEER.
The predominant cause of recurrent severe MR identified by TOE was partial or complete SLDA (n=6), followed by recurrent prolapse or flail (n=5). In 5 patients, the cause of recurrent MR could not be conclusively determined. Nine patients underwent a mitral reintervention (2 had repeated M-TEER, 7 underwent surgical MV replacement), and 8 patients were treated conservatively.
Either partial or complete SLDA was diagnosed in 10 patients – in 11% (n=7) of the 65 PMR patients, 4% (n=2) of the 52 ventricular SMR and 9% (n=1) of the 11 atrial SMR patients using TOE (Central illustration). All patients with combined SLDA and severe MR had PMR. The majority of the SLDA cases (70%, n=7) involved detachment from the posterior MV leaflet. Partial detachment was observed in 4 (40%) and complete detachment in 6 (60%) cases. Only two cases of SLDA were diagnosed during the hospital stay. Two other cases developed subacutely between discharge and 6 months, and 6 cases of SLDA were discovered incidentally during elective TOE. Six (60%) cases resulted in subsequent procedures: 4 patients underwent surgical MV replacement, and 2 underwent repeated M-TEER with good echocardiographic and clinical results. A detailed description of all patients with SLDA is provided in Supplementary Table 3.
MV gradients measured at 6-month TOE follow-up were lower than those at discharge (3.45±1.78 mmHg vs 3.87±1.66 mmHg; p<0.001) and higher than gradients measured at the end of the procedure (3.45±1.78 mmHg vs 3.04±1.36 mmHg; p<0.001) (Supplementary Figure 1). The number of patients with an elevated (≥5 mmHg) MV gradient increased from 17 (13.3%) at the end of the procedure to 23 (18%) at 6-month follow up – in 11% (n=7) of the 65 PMR patients, 27% (n=14) of the 52 ventricular SMR patients and 18% (n=2) of the 11 patients with atrial SMR (Central illustration). Three of them underwent surgical MV replacement (also triggered by associated relevant residual MR), one underwent percutaneous MV implantation following electrosurgical anterior leaflet laceration with clip detachment, and the remaining patients were treated conservatively.
Fifty-five patients had high-quality 3D datasets available at the end of the procedure and at 6-month follow-up. The analysis demonstrated a 15±16% increase in the total MVA (mean increase by 0.3±0.8 cm2; p<0001). Moreover, there were significant increases in both annular area and perimeter, and annular dimensions (all p-values <0.001) (Figure 2). Specifically, the anteroposterior diameter increased by 3±1 mm, while the mean increase in the lateromedial dimension was 2±1 mm after 6 months. Table 2 presents all the parameters derived from the 3D datasets and their changes over time. Patients who met the composite endpoint at 2 years exhibited a more pronounced change in MVA, annular perimeter, annular area, and lateromedial annular dimensions, as compared to those who did not meet the composite outcome (Table 3). There were no significant differences in MVA or annular changes when stratified according to MR aetiology (Supplementary Table 4), reduced ejection fraction at baseline, atrial fibrillation, left ventricular ejection fraction worsening after M-TEER, or residual MR.
There was no significant change in the severity of TR between baseline and discharge TTE (p=0.405), nor between discharge TTE and 6-month follow-up TOE (p=0.330).
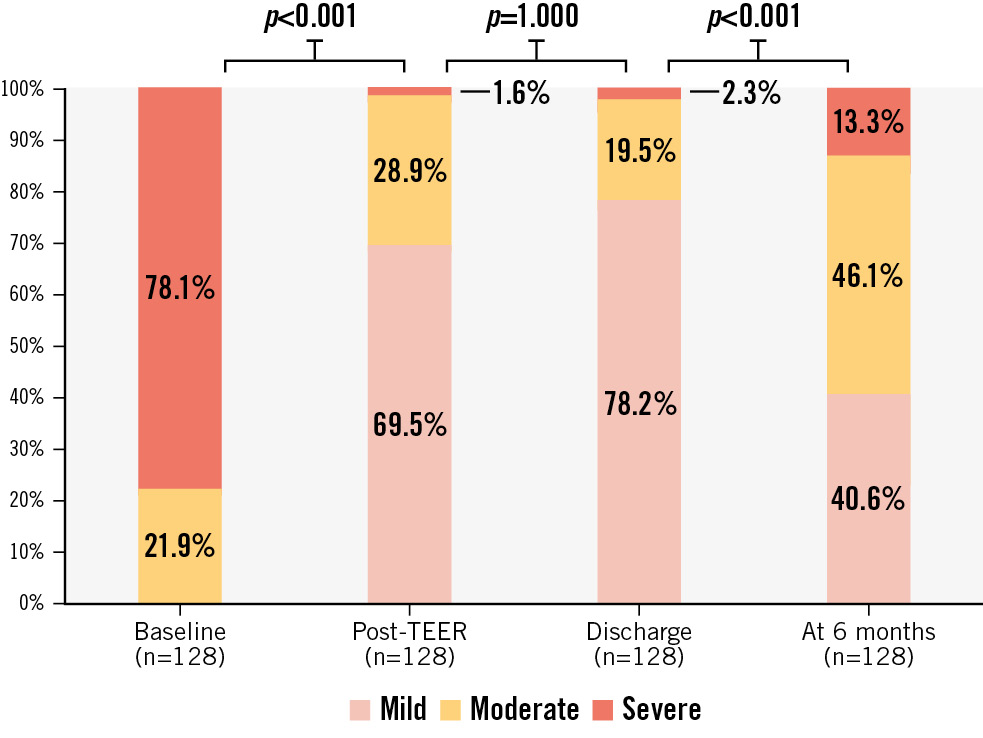
Figure 1. Changes in mitral regurgitation (MR) severity. MR was assessed at baseline (TTE or TOE), at the end of the TEER procedure (TOE), at discharge (TTE) and at six months (TOE). P-values represent the results of McNemar analysis comparing binary variables of severe versus non-severe MR. TEER: transcatheter edge-to-edge repair; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography

Central illustration. TOE follow-up at 6 months after M-TEER. mGr: mean gradient; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; SLDA: single leaflet device attachment; TOE: transoesophageal echocardiography
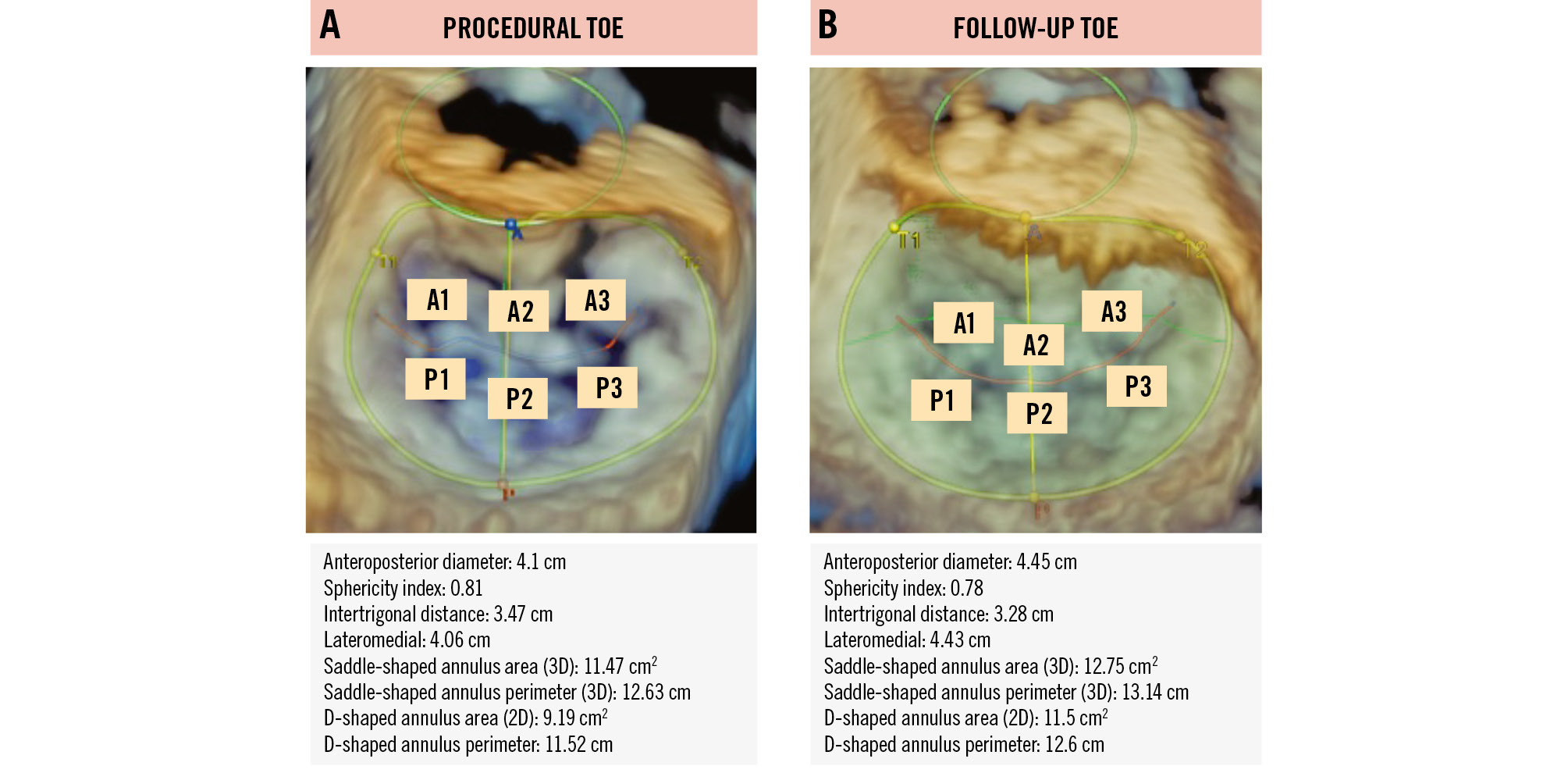
Figure 2. Procedural and follow-up TOE of the mitral valve in the same patient. Three-dimensional (3D) echocardiograms of the mitral valve were obtained from the same patient at 2 timepoints: (A) at the end of the medical procedure (procedural TOE) and (B) at 6-month follow-up (follow-up TOE), along with corresponding measurements. The results show an increase in both anteroposterior and lateromedial diameters, leading to a consecutive increase in the annular area. 2D: two-dimensional; TOE: transoesophageal echocardiography
Table 2. Evolution of 3D dimensions of the mitral valve between the end of the procedure and 6-month follow-up.
| End of index procedure | 6-month follow-up | Absolute change | Relative change, % | p-value | |
|---|---|---|---|---|---|
| 3D MVA, cm2 | 2.2±0.8 | 2.4±0.8 | 0.3±0.1 | 15±16 | <0.001 |
| 3D annular perimeter, cm | 13.7±1.6 | 14.3±1.5 | 0.6±0.1 | 5±8 | <0.001 |
| 3D annular area, cm2 | 14.1±3.3 | 15.4±3.1 | 1.3±0.3 | 11±17 | <0.001 |
| Anteroposterior annular dimension, mm | 37±6 | 40±4 | 3±0.6 | 10±27 | <0.001 |
| Lateromedial dimension, mm | 45±5 | 47±5 | 2±0.4 | 5±7 | <0.001 |
| Continuous data are shown as mean±standard deviation. 3D: three-dimensional; MVA: mitral valve area | |||||
Table 3. Comparison of three-dimensional anatomical adaptations of mitral valve apparatus following mitral valve edge-to-edge repair.
| No adverse event (N=42) | Death or heart failure rehospitalisation at 2 years (N=13) | No adverse event (N=42) | Death or heart failure rehospitalisation at 2 years (N=13) | No adverse event (N=42) | Death or heart failure rehospitalisation at 2 years (N=13) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| End of index procedure | p-value | 6-month follow-up | p-value | Relative change | p-value | ||||||
| 3D mitral valve area, cm2 | 2.19±0.73 | 2.13±1.01 | 0.071 | 3D mitral valve area, cm2 | 2.44±0.79 | 2.39±0.87 | 0.458 | 3D mitral valve area, % | 13±12 | 21±26 | <0.001 |
| 3D annular perimeter, cm | 14.0±1.5 | 12.9±1.9 | 0.206 | 3D annular perimeter, cm | 14.4±1.6 | 13.9±0.8 | 0.008 | 3D annular perimeter, % | 3±5 | 9±13 | <0.001 |
| 3D annular area, cm2 | 14.58±3.14 | 12.54±3.52 | 0.359 | 3D annular area, cm2 | 15.63±3.35 | 14.45±1.69 | 0.005 | 3D annular area, % | 8±10 | 22±30 | <0.001 |
| 3D annular AP dimension, mm | 38±6 | 34±7 | 0.184 | 3D annular AP dimension, mm | 40±5 | 38±3 | 0.079 | 3D annular AP dimension, % | 9±24 | 14±20 | 0.249 |
| 3D annular LM dimension, mm | 46±5 | 43±5 | 0.668 | 3D annular LM dimension, mm | 47±6 | 46±4 | 0.065 | 3D annular LM dimension, % | 4±5 | 8±10 | <0.001 |
| Continuous data are shown as mean±standard deviation. Comparison between the patients who met the combined endpoint of death or heart failure rehospitalisation and those who did not. Patients who met the composite endpoint exhibited more pronounced changes in mitral valve area, annular perimeter, annular area and lateromedial annular dimensions, compared to patients who did not meet the composite outcome at 2 years. 3D: three-dimensional; AP: anteroposterior; FU: follow-up; LM: lateromedial; MR: mitral regurgitation | |||||||||||
TWO-YEAR FOLLOW-UP
Throughout the 2-year observation period, 14.8% (n=19) of patients died, 18% were rehospitalised (n=23), while 26.6% (n=34) met the composite endpoint of death or HF rehospitalisation. The presence of mean MV gradients ≥5 mmHg (HR 2.30, 95% CI: 1.10-4.81; p=0.023) or severe MR (HR 3.26, 95% CI: 1.18-8.99) at 6 months were associated with an increased risk of the composite endpoint (Figure 3). There were no gender-specific differences in the risk of experiencing composite outcomes.
The association of mean gradients and severe MR at 6 months remained significant after adjusting for age, chronic obstructive pulmonary disease and severe renal dysfunction (Table 4), while no such association was found for mean gradients measured at the end of the procedure or at discharge. The ROC analysis indicated that a mean gradient of 3.65 mmHg as assessed by TOE was the most accurate in predicting the composite clinical outcome at 2 years with 62% sensitivity and 68% specificity (p=0.033, area under the curve [AUC] 0.633) (Supplementary Figure 2).
Thirteen (10.2%) patients underwent repeated MV intervention within the first 2 years after TEER. TOE enforced the diagnosis and changed the therapeutic approach, revealing partial SLDA (n=4) or refining MR grading (n=1) in 5 of these 13 cases. The most common reason for mitral reintervention was recurrent severe MR (n=11) treated with surgical MV replacement (n=9) or redo TEER (n=2).
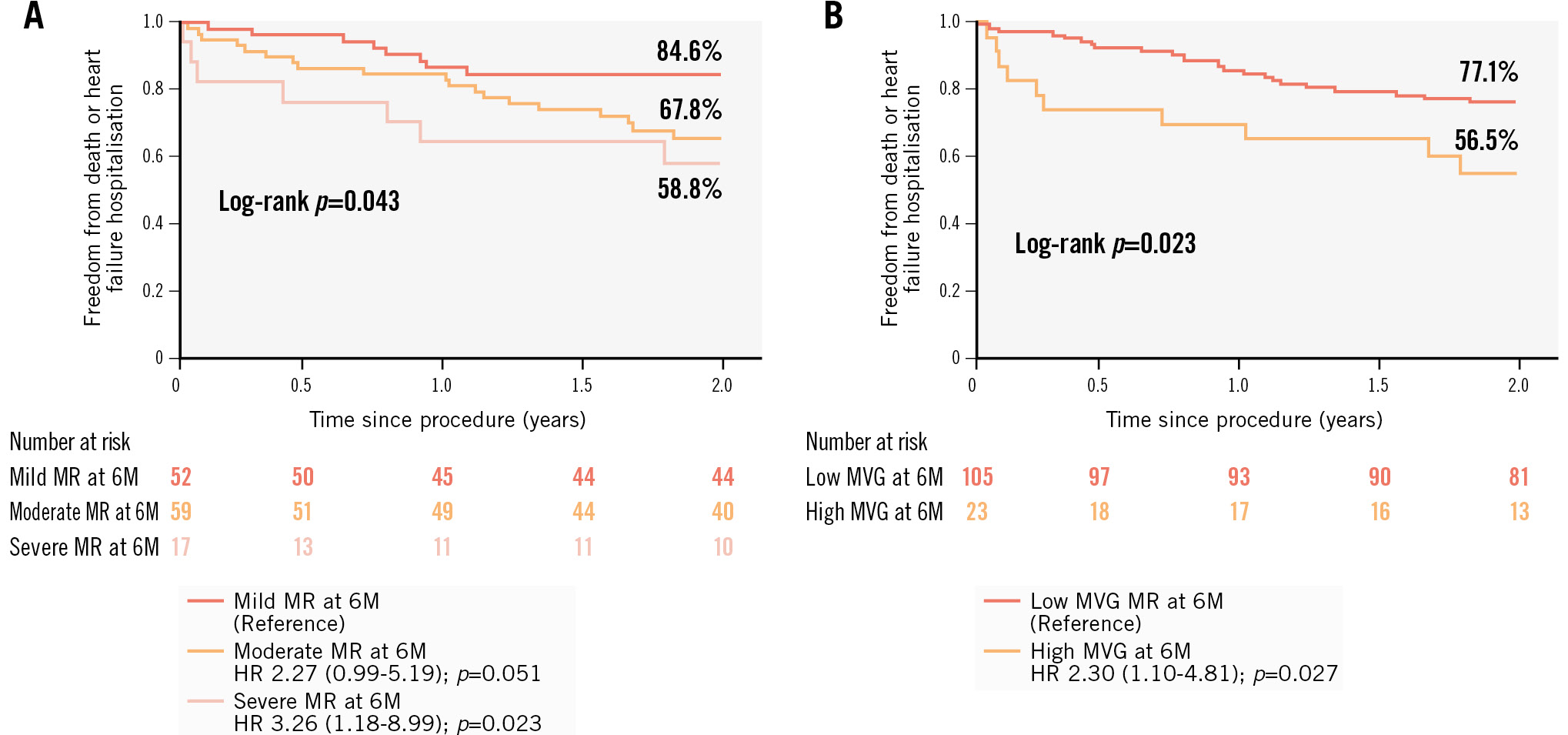
Figure 3. Kaplan-Meier analyses with log-rank test for the combined endpoint after M-TEER. A) Kaplan-Meier analysis with log-rank test for the combined endpoint (death or heart failure rehospitalisation) after M-TEER for different mitral regurgitation (MR) severity grades at 6 months (6M) with corresponding hazard ratios. B) Kaplan-Meier analysis with log-rank test for the combined endpoint (death or heart failure rehospitalisation) after M-TEER in patients with high (≥5 mmHg) and low (<5 mmHg) mean mitral valve gradients (MVG) at 6 months (6M) with the corresponding hazard ratio (HR). M-TEER: mitral transcatheter edge-to-edge repair
Table 4. Univariate and multivariate logistic regression models.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age | 0.974 | 0.938-1.012 | 0.179 | 0.981 | 0.932-1.032 | 0.458 |
| Chronic obstructive pulmonary disease | 2.390 | 1.140-5.008 | 0.021 | 2.634 | 1.094-6.339 | 0.031 |
| Severe renal dysfunction (GFR <30 ml/min/m2) |
5.218 | 1.990-13.683 | <0.001 | 6.167 | 2.204-17.256 | <0.001 |
| LVEF at 6 months | 0.986 | 0.964-1.009 | 0.224 | - | - | - |
| Mean MV gradient at 6 months | 1.265 | 1.062-1.506 | 0.008 | 1.322 | 1.082-1.615 | 0.006 |
| Severe MR at 6 months | 1.974 | 0.859-4.534 | 0.109 | 5.538 | 2.041-15.023 | <0.001 |
| Severe TR at 6 months | 1.201 | 0.423-3.409 | 0.731 | - | - | - |
| Univariate and multivariate logistic regression models evaluate the association of mitral valve mean gradients, severe mitral regurgitation and other predictors with the composite endpoint of all-cause mortality and heart failure rehospitalisation up to 2 years. CI: confidence interval; GFR: glomerular filtration rate; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MV: mitral valve; TR: tricuspid regurgitation | ||||||
MATCHED ANALYSIS
The results of the propensity-matched analysis between the patients who underwent TOE and those who did not are presented in Supplementary Table 5. There were no significant differences in the incidence of MR, SLDA, mitral reintervention, or HF hospitalisation between the TOE and no-TOE groups. MR recurrence at discharge and 2-year mortality were higher in the patients who did not undergo elective TOE follow-up.
INTEROBSERVER VARIABILITY
The interobserver variability of MVA measurement was excellent as evaluated by the interclass correlation coefficient (all values ≥0.99; p<0.001). The Bland-Altman evaluation showed a low interobserver variability (bias 0.04 cm2, LoA: 0.33-0.25).
Discussion
In this retrospective observational study reporting the results of elective TOE follow-up 6 months after M-TEER using predominantly first-generation devices, we found recurrent moderate MR (grade 2+ or 3+) in 28.9% (n=37), severe MR in 13.3% (n=17), elevated MV gradient in 18% (n=23), and a partial or complete SLDA in 7.8% (n=10) of the patients. There was a significant increase in MVA, and annular area, perimeter and dimensions at 6-month follow-up, compared to the procedural TOE under general anaesthesia. Both severe MR and elevated MV gradients at 6-month follow-up were associated with an increased risk of all-cause death or HF rehospitalisation within the first 2 years after the index procedure. In many cases, TOE allowed the detection of complications that would not be diagnosed using TTE only and revealed the exact cause of recurrent MR, such as partial SLDA or recurrent flail, impacting the decision on corrective interventions.
CLINICAL OUTCOMES AND RESIDUAL MR AT 2 YEARS
The rates of death and HF rehospitalisation are comparatively lower than those reported in other real-world registries, in which death rates at 2 years ranged from 25.0% to 31.9%141516, and the composite outcome of death and HF rehospitalisation occurred in 41.7% of patients16. The lower outcome rates in this analysis could be explained by the selection bias of patients able to undergo a TOE exam at 6 months, while higher-risk or frail patients are more likely to renounce. This seems to be confirmed by the performed propensity score analysis.
In most available registry data, TTE was scheduled at 1 and 12 months following M-TEER. Therefore, it is not possible to make direct comparisons between both imaging modalities for MR recurrence. A recent study evaluating 6-month TTE follow-up reported a lower incidence of severe MR (8.9%) compared to our study (13.3%)17. These differences could be explained by the challenges of TTE assessment because of device-related artefacts and the presence of multiple jets. Indeed, 3D colour Doppler-derived parameters assessed using TOE exhibited a stronger correlation with MR severity compared to conventional two-dimensional (2D) TTE parameters18. The predominant use of first-generation devices in our study represents another important factor, as well as the treatment of patients with challenging anatomies (Supplementary Table 2).
Patients with severe MR had a 3.3-times higher risk of death or HF rehospitalisation at 2 years. Additionally, we noted a trend towards an increased risk of adverse outcomes in patients with residual moderate MR at 6 months. Therefore, it is crucial to diagnose and treat suboptimal results early. TTE alone may not always provide a precise explanation for the cause of recurrent MR. For instance, severe MR with increased device mobility could result from partial SLDA, leaflet tear or recurrent flail. Distinguishing between these 2 entities often requires high-quality 3D multiplanar reconstruction, which is key for appropriate management. While SLDA and leaflet tear typically require surgical intervention, recurrent flail can be addressed with repeated M-TEER. Therefore, TOE should be performed liberally in patients with recurrent relevant MR to ensure accurate diagnosis and proper management.
VALVULAR REMODELLING
To our knowledge, this study is the first evaluating changes in the MV apparatus anatomy during the initial months following M-TEER. Our results demonstrate a modest but statistically significant relative increase in MVA and annular dimensions compared to the intraprocedural TOE images. Interestingly, this phenomenon was particularly evident in the patients who met the clinical composite endpoint at 2 years. Recurrent MR following M-TEER, as well as valvular (tissue weakness), atrial, and ventricular disease progression may have contributed to these findings. Despite robust and converging findings established using different measurement techniques for annular dimensions and MVA, the exact causes of the observed changes remain open, since none of our hypotheses could be validated in this small cohort using additional sensitivity analyses, and the observed changes rather appear to be a general trend, with significantly higher magnitude in patients with adverse events during follow-up.
MITRAL VALVE GRADIENTS
The reported prevalence of mitral stenosis after TEER has been found to vary widely between studies, ranging from 0% to 28%. In our analysis, 18% (n=23) of patients had an MV gradient of ≥ 5 mmHg at 6 months after the index procedure, which was associated with an increased risk of the composite endpoint of death or HF rehospitalisation at 2 years. The changes in the gradients during the follow-up period may be attributed, in part, to the use of general anaesthesia during the procedure, as opposed to no anaesthesia or brief sedation during follow-up TTE and TOE. Several studies have linked an increased gradient to adverse outcomes after M-TEER1921, particularly in patients with PMR. The cutoffs found in those analyses varied between 4.4 mmHg and 5.0 mmHg and were assessed either at the end of the procedure19 or at discharge2021. In contrast, a subanalysis of the COAPT study on SMR patients22, as well as the study by Yoon and co-authors in PMR patients21, failed to show a predictive value of increased MV gradients.
In our study, the transmitral gradient at 6 months was associated with adverse outcomes. The cutoff value that best predicted the primary endpoint was lower than previously reported, likely because it was measured under sedation during TOE. This observation is important since it shows that the MVARC definition of mitral stenosis may not apply to TOE evaluation and that a lower cutoff may already indicate clinically relevant stenosis. TOE provides a precise assessment of the underlying cause of high MV gradients (flow-related or structural), facilitating the assessment of potential treatment options.
LEAFLET DAMAGE/SLDA
The incidence of SLDA in our study was 7.8% (n=10), which is higher than in previous studies that reported 0.9-4.8% of SLDA 12 months after M-TEER232425. However, after excluding cases which were incidentally discovered during follow-up TOE, the incidence of SLDA decreased to 3.1% (4/128), which is comparable to other TTE-based reports232425 and a matched cohort without TOE follow-up. Importantly, in the present study, 60% of all SLDA cases were detected incidentally during TOE follow-up.
Limitations
The findings of the present analysis need to be interpreted in light of several limitations. First, not all M-TEER patients underwent an elective follow-up examination at 6 months. This may have led to an underestimation of clinical events. Secondly, the incidence of recurrent MR in our cohort can be explained by the considerable proportion of patients treated with first-generation devices, as well as the increasing inclusion of patients with challenging anatomies and no alternative treatment options, and is comparable with other real-world registries performed during the same period. Additionally, TTE at 6 months was not performed, thus a comparison of both modalities was not possible. Finally, the study inclusion period coincides with the early phase of our TEER programme and the learning curve linked to this complex procedure should be taken into account when interpreting our results.
Conclusions
TOE follow-up allowed the detection of complications that would not be diagnosed using TTE only and whose frequency may be underestimated. A mean MV gradient ≥5 mmHg and severe MR at 6-month TOE follow-up were associated with an increased risk of the composite outcome of death and HF hospitalisation. TOE should be performed liberally in patients with suboptimal results following M-TEER to confirm the diagnosis, determine the underlying aetiology, and evaluate the feasibility of a corrective intervention.
Impact on daily practice
Transoesophageal echocardiography (TOE) enables the detection of complications not identified by transthoracic echocardiography and accurately determines the causes of recurrent mitral regurgitation, such as partial single leaflet device attachment or recurrent flail. TOE should be used liberally in patients with suboptimal transcatheter mitral edge-to-edge repair results to identify the underlying cause and assess the feasibility of corrective interventions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
J. Bartkowiak reports a research grant from Novartis Foundation. M. Kassar reports a research grant from Swiss Heart Foundation. D. Samim reports institutional grants from Edwards Lifesciences. S. Windecker reports research, travel or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, B. Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, Cardiovalve (now Venus Medtech), Cordis Medical, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, Infraredx, Janssen-Cilag, Johnson & Johnson, MedAlliance, Medicure, Medtronic, Merck Sharp & Dohme, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Swiss, Pharming Technologies B.V., Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; and served as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V-Wave with payments to the institution but no personal payments; he is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration; he is Vice-President of the ESC and Associate Editor of JACC CV Interventions. T. Pilgrim reports research, travel, and educational grants to the institution from Boston Scientific, Biotronik, Medtronic, and Edwards Lifesciences; and speaker fees from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, and Medtronic; he is a member of clinical event adjudication committee for HighLife SAS DSMB for a study from Biosensors; and received equipment, materials, drugs, medical writing, gifts or other services from ATSens; he participates on a data safety monitoring board or advisory board for Biosensors (EMPIRE trial). F. Praz reports compensation for travel expenses from Abbott, Edwards Lifesciences, Medira, Siemens Healthineers, and InQB8 Medical Technologies; as well as a research grant to the institution from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

