Cory:
Unlock Your AI Assistant Now!
The pullback pressure gradient (PPG) is a novel metric that quantifies coronary artery disease (CAD) patterns as focal or diffuse on a scale from 0 (diffuse) to 1 (focal)1. PPG predicts blood flow improvement after percutaneous coronary intervention (PCI): a high PPG (focal disease) is associated with greater flow improvement and angina relief, while a low PPG (diffuse disease) is linked to higher periprocedural complications23. Traditionally, PPG has been derived from fractional flow reserve (FFR) pullbacks. However, assessment using non-hyperaemic pressure ratios (NHPR) can shorten procedure time and eliminate the need for a hyperaemic agent. This study aimed to assess the agreement between resting and hyperaemic PPG.
This study was a prespecified subanalysis of the PPG Global Registry, a prospective, investigator-initiated, multicentre, international study (ClinicalTrials.gov: NCT04789317)2. Eligible patients had at least one haemodynamically significant lesion (FFR ≤0.80) scheduled for PCI, with those undergoing both resting and hyperaemic pressure pullbacks included (Supplementary Figure 1). The pressure wire (PressureWire X [Abbott]) was placed in the distal coronary artery to measure resting distal coronary pressure/aortic pressure, resting full-cycle ratio (RFR), and FFR. Manual pullbacks were performed over 20-30 seconds. PPG was automatically calculated online using CoroFlow v3.5.1 software (Coroventis Research AB) (Supplementary Figure 2). Resting PPG was calculated by the core lab (CoreAalst BV) using resting pressure pullbacks with the same algorithm. We classified functional patterns using the median hyperaemic PPG value of 0.62, derived from the entire PPG Global cohort, and applied the same threshold for resting PPG. Continuous variables were compared using Mann-Whitney U tests and categorical variables using chi-square tests. Pearson’s correlation assessed associations, and Bland-Altman analysis, Passing-Bablok regression, and Cohen’s Kappa evaluated agreement. The diagnostic performance of resting PPG was evaluated based on sensitivity, specificity, positive predictive value, and negative predictive value. Receiver operating characteristic analysis assessed the predictive capacity for optimal PCI outcomes (post-PCI FFR ≥0.88), with the area under the curve (AUC) comparisons performed using the DeLong method.
Between December 2020 and September 2023, 1,004 patients (1,057 vessels) were enrolled, of whom 88 patients (90 vessels) underwent both resting and hyperaemic pressure pullbacks. Patient and procedural characteristics are shown in Supplementary Table 1 and Supplementary Table 2. The mean resting and hyperaemic PPG were 0.67±0.14 and 0.63±0.16 (Figure 1A), respectively, with a mean difference of 0.04 (95% limits of agreement: −0.23 to 0.15) and a strong correlation (Figure 1B, Figure 1C). Passing-Bablok regression showed systematic and proportional differences, with coefficient A at 0.13 (95% confidence interval [CI]: 0.05 to 0.22) and coefficient B at 0.86 (95% CI: 0.73 to 0.98). Correlation between resting and hyperaemic PPG among vessels with FFR in the grey zone (0.75
This study demonstrated a strong correlation between resting and hyperaemic PPG and substantial agreement in CAD pattern classification, suggesting that resting PPG may serve as a practical alternative to hyperaemic PPG for assessing CAD patterns. Vessels with a higher resting PPG achieved greater flow improvement after PCI, with predictive accuracy comparable to hyperaemic PPG. The agreement between resting and hyperaemic PPG was approximately 80%, similar to the concordance observed between NHPR and FFR. Given its procedural simplicity, shorter duration, and comparable diagnostic performance, resting PPG presents a practical alternative to hyperaemic PPG for quantifying CAD patterns.
This study has several limitations. Potential selection bias may be present, and the predominance of left anterior descending artery lesions (83%) may limit generalisability. Additionally, the sample size was relatively small, and the study only included vessels with FFR ≤0.80. The optimal PPG cutoff remains undefined, and the study is underpowered to detect significant differences in predictive capacity. Therefore, these findings should be interpreted as hypothesis-generating, warranting further validation in larger and more diverse cohorts.
In conclusion, resting PPG demonstrated a high level of agreement with hyperaemic PPG and was associated with improved PCI outcomes, with vessels exhibiting a high resting PPG achieving greater post-PCI FFR and RFR than those with a low resting PPG.
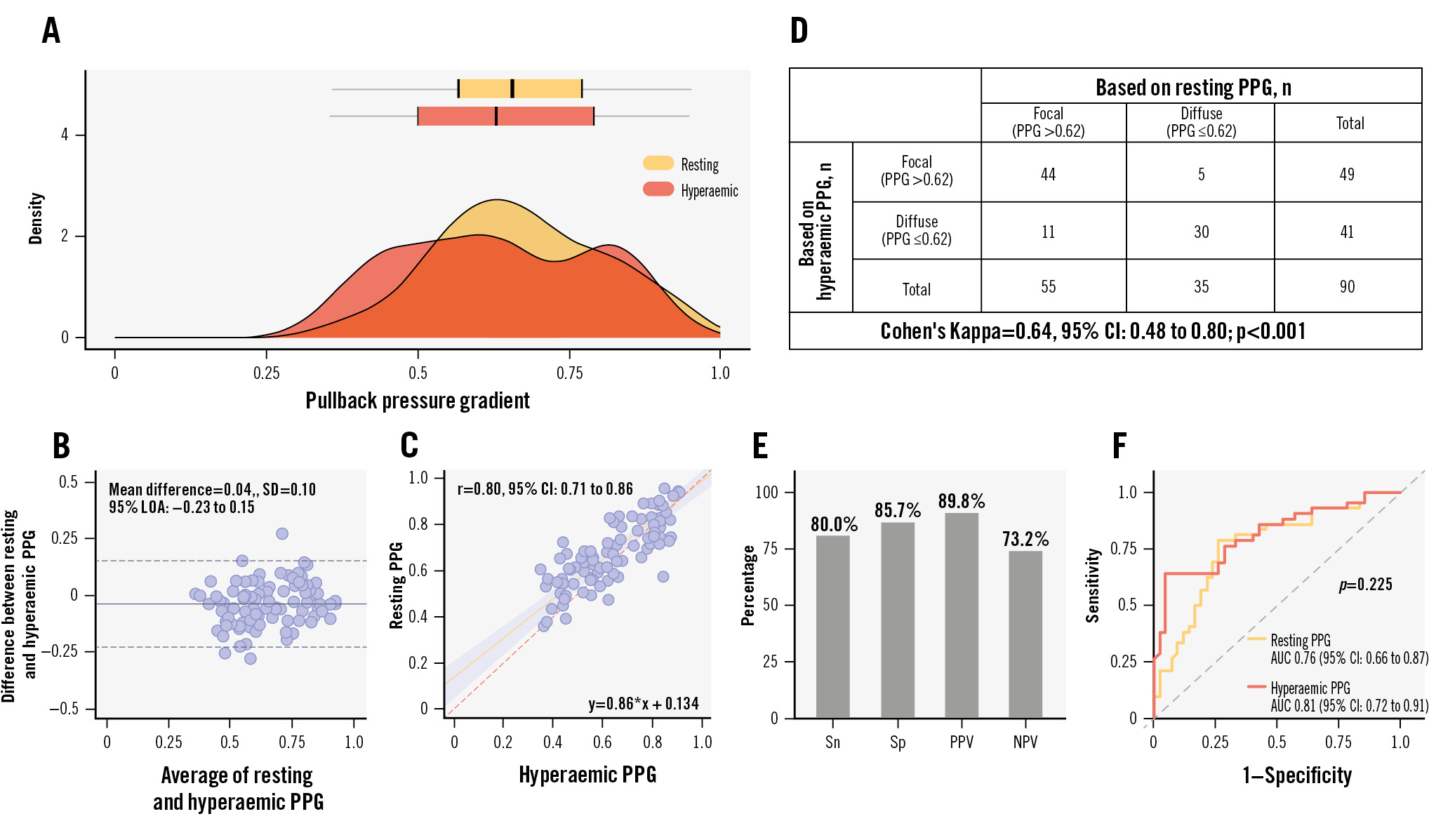
Figure 1. Distribution, correlation, agreement, diagnostic performance, and predictive capacity for optimal PCI outcomes between resting and hyperaemic PPG. A) Distribution of resting and hyperaemic PPG. B) Bland-Altman analysis comparing resting and hyperaemic PPG. C) Correlation between resting and hyperaemic PPG. D) The Cohen's Kappa analysis demonstrated a substantial agreement in CAD pattern classification between resting and hyperaemic PPG. E) Diagnostic performance of resting PPG, including sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV). F) Comparison of the predictive capacity of resting PPG (orange) and hyperaemic PPG (red) for achieving optimal PCI (post-PCI FFR >0.88). AUC: area under the curve; CAD: coronary artery disease; CI: confidence interval; LOA: limits of agreement; PCI: percutaneous coronary intervention; PPG: pullback pressure gradient; SD: standard deviation
Funding
The study was sponsored by the Cardiac Research Institute Aalst with a research grant from Abbott Vascular.
Conflict of interest statement
T. Mizukami reports receiving research grants from Boston Scientific; and speaker fees from Abbott, CathWorks, and Boston Scientific. H. Matsuo has received consulting fees from Kaneka and Zeon; and speaker fees from Abbott, Boston Scientific, Philips, and Amgen. B. Ko has received consulting fees from Canon Medical, Abbott, and Medtronic. D. Perera has received research grant support from Abbott, HeartFlow, and Philips. S. Biscaglia received research grants provided by Sahajanand Medical Technologies, Medis Medical Imaging Systems, Eukon S.r.l., Siemens Healthineers, GE HealthCare, and Insight Lifetech. A.M. Leone reports receiving consultancy fees from Abbott; and honoraria for sponsored symposiums from Abbott, Medtronic, and Abiomed. J. Escaned is supported by the Intensification of Research Activity project INT22/00088 from Spanish Instituto de Salud Carlos III; and received speaker and advisory board member fees from Abbott and Philips. G. Campo reported receiving grants from Sahajanand Medical Technologies, GE HealthCare, Siemens Healthineers, Insight Lifetech, Abbott, and Amgen outside the submitted work. T. Amano reports receiving lecture fees from Astellas Pharma, AstraZeneca, Bayer, Daiichi Sankyo, and Bristol-Myers Squibb. T. Shinke received personal fees and research grants from Abbott. Z. Ali reports institutional grant support from Abbott, Abiomed, Acist, Amgen, Boston Scientific, CathWorks, Canon, Conavi, HeartFlow, Inari, Medtronic, National Institute of Health, Nipro, Opsens Medical, Medis Medical Imaging, Philips, Shockwave Medical, Siemens Healthineers, SpectraWAVE, and Teleflex; consulting fees from Abiomed, AstraZeneca, Boston Scientific, CathWorks, Opsens, Philips, Shockwave Medical; and equity in Elucid, Lifelink, SpectraWAVE, Shockwave Medical, and VitalConnect. B. De Bruyne reports receiving consultancy fees from Boston Scientific and Abbott; and research grants from Coroventis Research, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens Healthineers, HeartFlow, and Abbott. N.P. Johnson received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE-FLOW, NCT02328820) for other studies using intracoronary pressure and flow sensors; has an institutional licensing agreement with Boston Scientific for the smart-minimum FFR algorithm (now commercialised under 510(k) K191008); and has patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters. C. Collet reports receiving research grants from Biosensors, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens Healthineers, HeartFlow, and Abbott; and consultancy fees from HeartFlow, Opsens, Abbott, and Philips/Volcano. A. Jeremias has received consulting fees from Canon, Artrya Medical, and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

