Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The effectiveness of oral anticoagulation (OAC) or dual antiplatelet therapy (DAPT) in reducing subclinical brain infarcts after transcatheter aortic valve implantation (TAVI) remains unclear.
Aims: We aimed to compare the efficacy of DAPT versus OAC in preventing cerebral microembolism during the first 3 months post-TAVI, assessed by diffusion-weighted magnetic resonance imaging (DW-MRI).
Methods: Patients with aortic stenosis and no indication for OAC were randomly assigned to receive either OAC (acenocoumarol) or DAPT (aspirin+clopidogrel) for 3 months post-TAVI. Brain DW-MRI was performed at baseline (0-3 days pre-TAVI) and at 6 and 90 days post-TAVI. The primary objective was the proportion of patients with new cerebral emboli on DW-MRI at 6 and 90 days.
Results: Of the 123 patients included in the study, 3.3% had new cerebral emboli on the baseline MRI prior to TAVI. At 6 days post-TAVI, new cerebral emboli were observed in 81.4% of OAC patients versus 69.8% of DAPT patients (p=0.209), and at 90 days, in 8.0% versus 8.2%, respectively (p=0.879). However, DAPT patients had a lower mean total emboli volume at 6 days (265.9 mm³ vs 303.4 mm³; p=0.019) and cumulatively at 6+90 days (266.45 mm³ vs 331.10 mm³; p=0.008).
Conclusions: In patients without an indication for OAC, an OAC strategy for 3 months post-TAVI did not show any benefit over an antiplatelet strategy in preventing cerebral microembolism. Patients treated with DAPT showed a lower mean volume of brain damage on DW-MRI during the 90 days following TAVI compared to those treated with acenocoumarol.
Approximately 60-80% of patients undergoing transcatheter aortic valve implantation (TAVI) are in sinus rhythm, and antiplatelet therapy is recommended as the antithrombotic strategy thereafter1. However, the incidence of periprocedural thromboembolic and haemorrhagic complications after TAVI remains relevant and is associated with higher morbidity and mortality1. Although long-term results have shown a low risk of neurological complications, particularly stroke, after aortic bioprosthesis implantation, the first 3 postoperative months are considered a higher-risk period for thrombus formation due to the incomplete endothelialisation of the transcatheter heart valve components23. Diverse diffusion-weighted magnetic resonance imaging (DW-MRI) studies of the brain have demonstrated new silent cerebral lesions in most patients within days after TAVI45, which have subsequently been related to early cognitive decline6. Currently, TAVI patients without an indication for oral anticoagulation (OAC) are preferentially managed with lifelong single antiplatelet therapy (SAPT), with aspirin, post-TAVI789. Optionally, in patients with low bleeding risk, dual antiplatelet therapy (DAPT) with aspirin and clopidogrel or OAC with vitamin K antagonists (VKAs) during the first 3 months are guideline-referred as a reasonable option7. Recent trials testing three different non-vitamin K direct oral anticoagulants (DOACs) involving patients without an established indication for OAC have shown them to be more effective than DAPT with aspirin and clopidogrel in preventing subclinical leaflet thrombosis (SCLT)10111213, but at the expense of more thromboembolic complications, bleeding events, or deaths. Still, a comparison of DAPT versus VKAs in patients with no underlying indication for OAC to prevent cerebral microembolisation after TAVI is lacking.
Methods
Trial design and oversight
The AUREA trial is a prospective, multicentre, parallel-group, randomised, open-label study (with third-party blinded DW-MRI endpoint assessment) investigating the efficacy of DAPT versus acenocoumarol (a VKA) for 3 months in preventing cerebral microembolism post-TAVI, assessed by brain DW-MRI. Patients with severe symptomatic aortic stenosis (AS), without an indication for OAC, were randomised before TAVI to receive either DAPT (aspirin+clopidogrel) or acenocoumarol post-procedure. The study flowchart is shown in Figure 1, with design details in Supplementary Appendix 1.
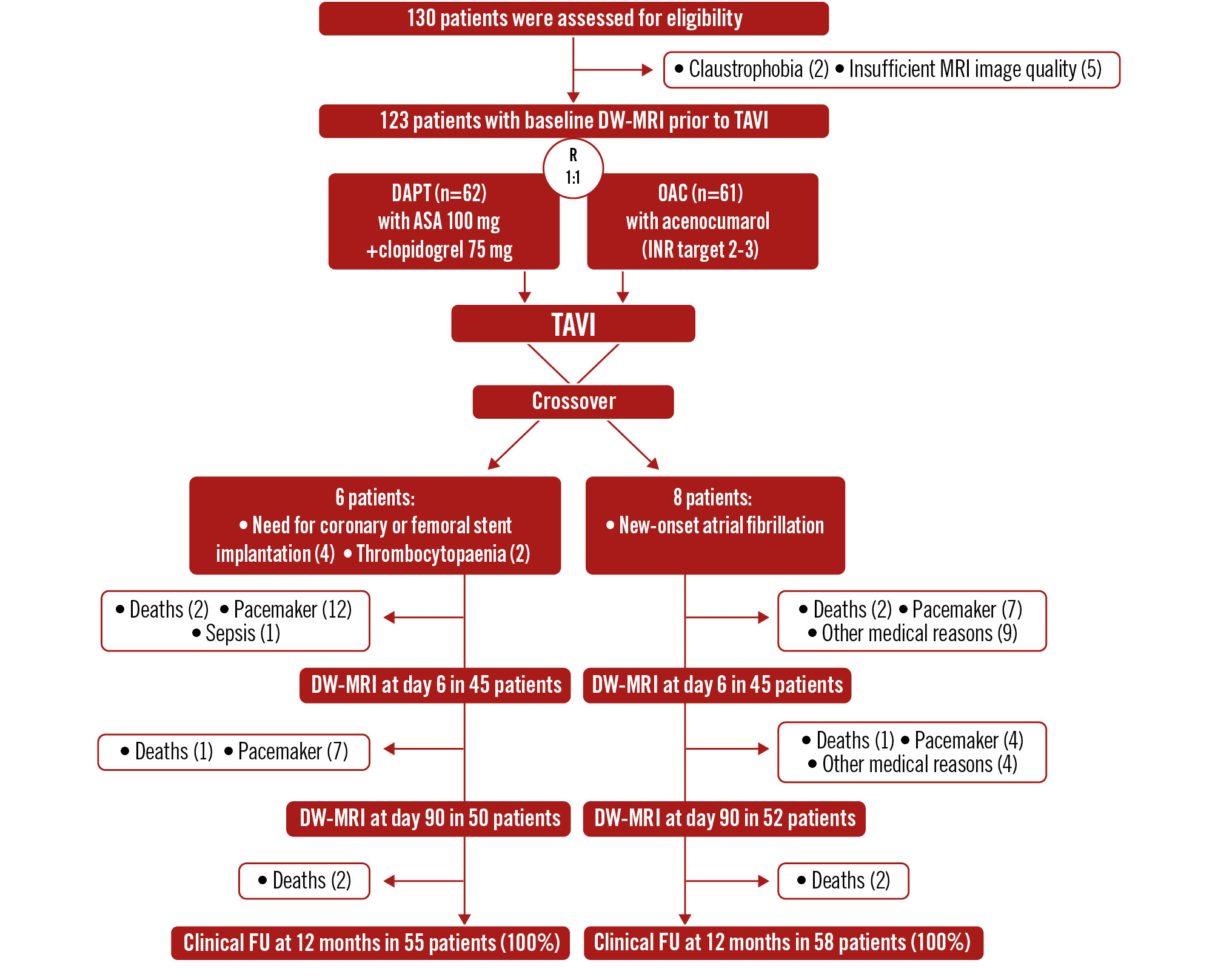
Figure 1. Study flowchart: screening, randomisation, and follow-up. ASA: aspirin; DAPT: dual antiplatelet therapy; DW-MRI: diffusion-weighted MRI; FU: follow-up; INR: international normalised ratio; MRI: magnetic resonance imaging; OAC: oral anticoagulation; R: randomisation; TAVI: transcatheter aortic valve implantation
Patient selection and randomisation
Patients eligible for transfemoral TAVI and without an OAC indication were considered for enrolment. Key exclusions included any need for long-term anticoagulation, an absolute DAPT indication, or a contraindication to DW-MRI. Patients were randomly assigned 1:1 to DAPT or OAC using an interactive web-response system. Full inclusion and exclusion criteria are detailed in Supplementary Appendix 1.
Trial treatment and follow-up
Patients in the OAC group received acenocoumarol for 3 months post-TAVI, targeting an international normalised ratio (INR) of 2-3. Treatment began after a successful TAVI without major complications, with the INR monitored by the Haematology department until OAC was stopped at 3 months. The DAPT group received aspirin 100 mg and clopidogrel 75 mg daily for 3 months. Patients who developed new-onset atrial fibrillation were switched to acenocoumarol or continued it if already on OAC. If a new DAPT indication arose, OAC patients were switched to aspirin and clopidogrel. After 3 months, all patients received aspirin 100 mg, unless they required extended DAPT or OAC. Follow-up occurred at 1, 3, 6, and 12 months.
Study procedures
MRI
Brain MRI was performed using a 1.5 T scanner at three timepoints: baseline (3±3 days pre-TAVI), 6±2 days post-TAVI, and 90±7 days post-TAVI. T2, FLAIR, and diffusion-weighted sequences were acquired, with all MRIs analysed by an independent core lab in a blinded fashion.
Serological biomarkers
Plasma levels of neuron-specific enolase (NSE) and S100 calcium-binding protein B (S100B) were measured before TAVI and at 1 hour and 24 hours post-TAVI as markers of periprocedural cerebral embolic events.
Neurological and neurocognitive assessments
A certified neurologist conducted physical examinations and standardised neurocognitive tests, including the National Institutes of Health Stroke Scale (NIHSS) and the 35-point Mini-Examen Cognoscitivo (MEC-35; the Spanish version of the Mini-Mental State Examination), in a blinded fashion before TAVI, immediately after TAVI during hospitalisation, and at 3 and 12 months post-TAVI. Any change in the NIHSS was considered clinically significant. MEC-35 scores ≤27 indicated cognitive impairment.
Antithrombotic management
Valve selection and TAVI procedures followed site-specific protocols. Unfractionated heparin was used during TAVI, targeting an activated clotting time >250 seconds. Protamine use was decided per local practice.
Clinical follow-up
Follow-ups at 1, 3, and 12 months included neurological exams. Transthoracic echocardiograms were performed 6-12 months post-TAVI. Data were collected during visits and from hospital records, then adjudicated by the clinical events committee. Study details are in Supplementary Appendix 1.
Outcome measures
The primary efficacy outcome was the presence of new areas of cerebral infarction by DW-MRI at 6 days and 3 months post-TAVI. Secondary MRI endpoints included the total volume of new cerebral emboli per patient and the total number of new cerebral emboli per patient. In addition, the incidence of major adverse cardiovascular events (MACE), a composite of all-cause death, myocardial infarction, transient ischaemic attack (TIA) or stroke; and net adverse clinical events (NACE), a composite of MACE and major bleeding, were classified according to the Valve Academic Research Consortium-3 (VARC-3) definitions.
Statistical analysis
The incidence of overt stroke in AS patients varies widely, around 7% per year, decreasing to approximately 0.7% with OAC and 2.5% with DAPT1415. Patients with bioprosthetic aortic valves have a thromboembolism rate of 1.9% per patient-year16. VKA use reduces stroke and mortality risk for up to 6 months without significantly increasing bleeding2. However, no studies have evaluated the reduction of post-TAVI silent stroke using pharmacological strategies assessed by MRI. The average incidence of post-TAVI brain embolic injuries detected by MRI is 84%45. We anticipated an ~60% incidence in the OAC group. Based on a 95% confidence level, 80% power, two-sided test, and a 1:1 ratio, the required sample size was 52 patients per group. To account for a 20% dropout rate, a total of 124 patients were to be recruited.
Descriptive analysis and univariate comparisons were performed using the Student’s t-test and χ² test for means and proportions. MRI lesion rates were evaluated with the χ² test. Descriptive statistics include mean±standard deviation (SD) or median with interquartile range (IQR) for continuous variables and numbers/percentages for categorical variables. Univariate analysis applied Fisher’s exact test, χ² test, the Student’s t-test, or Mann-Whitney U test as appropriate. Time-to-event data were assessed using the Kaplan-Meier method and compared with the log-rank test. Multivariate Cox proportional hazards models, adjusting for baseline covariates, included variables with p<0.10 in univariate analysis. All analyses followed the intention-to-treat principle using SPSS software, v22.0 (IBM).
Results
Patient population
From November 2013 to June 2019, 130 patients were enrolled. MRI before TAVI was not performed in 2 patients (claustrophobia) and had poor image quality in 5. Therefore, a total of 123 patients with evaluable DW-MRI before TAVI were randomised: 62 to DAPT and 61 to OAC. Within 48 hours post-TAVI, 14 patients crossed over (8 DAPT, 6 OAC) due to new-onset atrial fibrillation (8), coronary/femoral stent implantation (4), or thrombocytopaenia (2). Baseline characteristics (Table 1) show a moderately high-risk elderly population with comparable sex representation (51.2% female). OAC patients were significantly older (84.0±4.3 years vs 82.0±6.1 years; p=0.033), with no cerebral protection devices used. Other clinical profiles were comparable. Acenocoumarol was initiated on the day of TAVI, achieving a therapeutic INR within 3±2 days, and maintained in 91% of OAC patients during follow-up. Procedural, clinical, and echocardiographic data are in Table 2.
Baseline brain MRI occurred at 3±3 days before TAVI. Post-TAVI MRI was conducted at day 6±2 (90 patients: 45 per group) and at day 90±7 (102 patients: 52 OAC, 50 DAPT) (Figure 1).
Table 1. Baseline characteristics of the population.
| OAC group (n=61) | DAPT group (n=62) | p-value | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age, years | 84.0±4.3 | 82.0±6.1 | 0.033 |
| Female | 35 (55.6) | 28 (46.7) | 0.324 |
| NYHA Class II or III | 62 (98) | 52 (86) | 0.12 |
| STS-PROM score, %* | 4.4±2.1 | 4.8±3.2 | 0.457 |
| Hypertension | 55 (87.3) | 51 (85.0) | 0.712 |
| Diabetes mellitus | 18 (28.6) | 20 (33.3) | 0.568 |
| Chronic kidney disease | 15 (23.8) | 16 (26.7) | 0.715 |
| Peripheral artery disease | 6 (9.5) | 11 (18.3) | 0.261 |
| Chronic obstructive pulmonary disease | 6 (9.5) | 9 (15.0) | 0.354 |
| Previous myocardial infarction | 7 (11.1) | 7 (11.7) | 0.923 |
| Previous PCI | 11 (17.5) | 14 (23.3) | 0.418 |
| Previous CABG | 4 (6.3) | 7 (11.7) | 0.342 |
| Previous stroke | 3 (4.8) | 3 (5.0) | 1 |
| Permanent pacemaker | 0 (0) | 0 (0) | 1 |
| Pre-TAVI echocardiographic characteristics | |||
| Aortic valve area, cm2 | 0.71±0.22 | 0.72±0.25 | 0.696 |
| Maximum aortic valve gradient, mmHg | 84.5±22.1 | 78.4±19.4 | 0.147 |
| Mean aortic valve gradient, mmHg | 50.9±15.1 | 47.0±12.4 | 0.143 |
| Left ventricular ejection fraction, % | 53.9±10.8 | 54.7±11.5 | 0.708 |
| Aortic regurgitation | 0.243 | ||
| Mild | 26 (44.1) | 17 (29.8) | |
| Moderate | 4 (6.8) | 6 (10.5) | |
| Severe | 2 (3.4) | 5 (8.8) | |
| Values are n (%) or mean±standard deviation. The data are shown according to the intention-to-treat principle. *STS-PROM scores measure patient risk at the time of cardiovascular surgery and are calculated by means of logistic regression equations. A score of greater than 8% indicates high risk, 3% to 8% intermediate risk, and less than 3% low risk. CABG: coronary artery bypass grafting; DAPT: dual antiplatelet therapy; NYHA: New York Heart Association; OAC: oral anticoagulation; PCI: percutaneous coronary intervention; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation | |||
Table 2. Procedural characteristics and echocardiographic findings post-TAVI.
| OAC group (n=61) | DAPT group (n=62) | p-value | |
|---|---|---|---|
| Procedural characteristics | |||
| Conscious sedation | 58 (92.1) | 54 (90.0) | 0.689 |
| Transfemoral access | 63 (100) | 59 (98.3) | 0.488 |
| Subclavian access | 0 (0) | 1 (1.7) | 0.472 |
| Valve type | 0.338 | ||
| Balloon-expandable | 17 (27.0) | 12 (20.3) | |
| Self-expanding | 46 (73.0) | 47 (79.7) | |
| Other* | 0 (0) | 1 (1.7) | |
| Mean valve diameter, mm | 27.3±3.5 | 27.8±3.1 | 0.351 |
| Ballon predilatation | 34 (56.7) | 27 (46.6) | 0.272 |
| Ballon post-dilatation | 19 (31.1) | 15 (26.8) | 0.604 |
| 2 ProGlide technique for vascular closure | 51 (83.6) | 40 (72.7) | 0.171 |
| Full heparin antagonisation with protamine | 5 (8.1) | 4 (8.0) | 0.889 |
| Procedural success | 63 (100) | 57 (95) | 0.113 |
| Mean residual AV pressure gradient, mmHg | 4.1±3.4 | 3.4±2.8 | 0.33 |
| ≥Moderate residual aortic regurgitation | 3 (4.7) | 7 (11.6) | 0.716 |
| Need for a second valve | 0 (0) | 0 (0) | 1 |
| Need for open-heart surgery | 0 (0) | 0 (0) | 1 |
| Need for permanent pacemaker implantation | 12 (19.0) | 10 (16.7) | 0.731 |
| Post-TAVI echocardiographic findings | |||
| Aortic valve area, cm2 | 1.5±0.08 | 1.4±0.07 | 0.667 |
| Maximum aortic valve gradient, mmHg | 14.7±7.6 | 13.8±5.3 | 0.212 |
| Mean aortic valve gradient, mmHg | 7.1±3.5 | 4.8±3.5 | 0.148 |
| Left ventricular ejection fraction, % | 54.2±10.0 | 55.7±11.9 | 0.594 |
| Paravalvular aortic regurgitation | 0.261 | ||
| None | 43 (71.6) | 34 (59.6) | |
| Mild | 13 (21.6) | 16 (28.0) | |
| Moderate | 4 (6.6) | 6 (9.6) | |
| Severe | 0 (0) | 0 (0) | |
| Values are n (%) or mean±standard deviation. The data are shown according to the intention-to-treat principle. *Mechanically expandable valve. DAPT: dual antiplatelet therapy; OAC: oral anticoagulation; TAVI: transcatheter aortic valve implantation | |||
Primary DW-MRI outcomes
The incidence of ≥1 new preprocedural cerebral lesion on baseline DW-MRI before TAVI was 3.3%, occurring exclusively in the DAPT group (6.5%; p=0.119) (Table 3), predominantly in the middle cerebral artery territory. At 6 days post-TAVI, 75.6% of patients had developed ≥1 new cerebral lesion, with a higher, though not statistically significant, rate in the OAC group (81.4% vs 69.8%; p=0.209). These lesions affected multiple brain territories. At 90 days post-TAVI, 8.1% had new lesions, distributed equally between groups (8.0% vs 8.2%; p=0.879), primarily in the posterior territory. Figure 2 illustrates the new cerebral lesions observed on brain MRI before and after TAVI, along with a brain reconstruction showing all post-TAVI lesions for each treatment group.
Table 3. DW-MRI outcomes pre-TAVI and post-TAVI.
| Endpoints | Total (n=123) | OAC group (n=61) | DAPT group (n=62) | p-value |
|---|---|---|---|---|
| Pre-TAVI | ||||
| Patients with ≥1 new preprocedural cerebral emboli on DW-MRI | 4 (3.3) | 0 (0) | 4 (6.5) | 0.119 |
| Total number of new lesions | 15 (100) | 0 (0) | 15 (100) | 0.39 |
| Total volume of all lesions, mm3 | 824.94 | - | 824.94 | NA |
| Lesion volume per patient, mm3 | 206.23±58.30 | - | 206.23±58.30 | NA |
| Day 6 post-TAVI | ||||
| Patients with ≥1 new preprocedural cerebral emboli on MRI | 90 (75.6) | 37 (82.2) | 31 (68.8) | 0.209 |
| Total number of new lesions | 315 (100) | 149 (47.3) | 166 (52.7) | 0.61 |
| Number of new lesions per patient | 3.65±5.09 | 4.25±5.24 | 3.07±5.05 | 0.288 |
| Total volume of all lesions, mm3 | 21,104.94 | 11,529.21 | 9,575.73 | 0.017 |
| Lesion volume per patient, mm3 | 285.17±329.49 | 303.40±330.69 | 265.99±331.79 | 0.019 |
| Lesion size on DW-MRI | 90 (75.6) | 0.737 | ||
| ≥1,000 mm3 | 1 (2.3) | 1 (2.2) | ||
| 500-999 mm3 | 2 (4.5) | 1 (2.2) | ||
| <500 mm3 | 33 (75.0) | 30 (66.7) | ||
| 3 months post-TAVI | ||||
| Patients with ≥1 new preprocedural cerebral emboli on MRI | 8 (8.1) | 4 (8.0) | 4 (8.2) | 0.879 |
| Total number of new lesions | 11 (100) | 6 (54.5) | 5 (45.5) | 0.3 |
| Number of new lesions per patient | 0.12±0.41 | 0.16±0.55 | 0.08±0.28 | 0.498 |
| Total volume of all lesions, mm3 | 1,601.73 | 1,052.46 | 549.27 | 0.033 |
| Lesion volume per patient, mm3 | 178.22±86.54 | 210.58±105.54 | 137.80±35.82 | 0.327 |
| Lesion size on DW-MRI | 8 (7.8) | 0.783 | ||
| ≥1,000 mm3 | 0 (0) | 0 (0) | ||
| 500-999 mm3 | 0 (0) | 0 (0) | ||
| <500 mm3 | 5 (10.0) | 4 (8.2) | ||
| Combined MRI endpoints | ||||
| Total number of new lesions (6 days+3 months post-TAVI) | 326 (100) | 155 (47.5) | 171 (52.4) | 0.145 |
| Mean total volume per lesion post-TAVI (6 days+3 months post-TAVI), mm3 | 298.77±333.29 | 331.10±343.85 | 266.45±323.72 | 0.008 |
| Patients with ≥1 new preprocedural cerebral emboli on MRI (6 days+3 months post-TAVI) | 98 (51.0) | 39 (41.0) | 34 (35.0) | 0.409 |
| Total volume of all lesions (6 days+3 months post-TAVI), mm3 | 22,706.67 | 12,581.67 | 10,125.00 | 0.046 |
| Values are n (%) or mean±standard deviation. The data are shown according to the intention-to-treat principle. DAPT: dual antiplatelet therapy; DW-MRI: diffusion-weighted MRI; MRI: magnetic resonance imaging; NA: not applicable; OAC: oral anticoagulation; TAVI: transcatheter aortic valve implantation | ||||
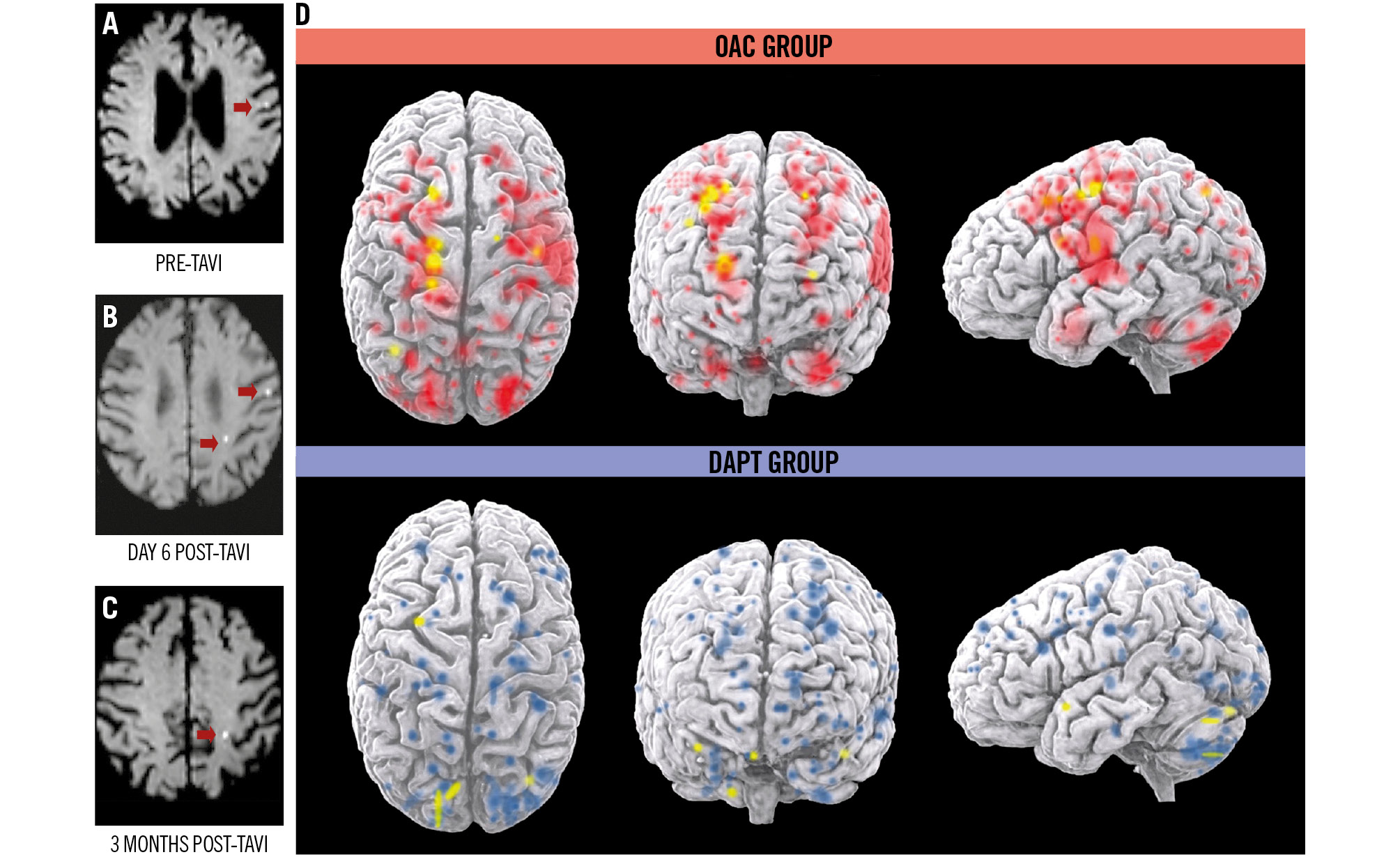
Figure 2. DW-MRI images showing new cerebral lesions before and after TAVI and 3D rendering of the topographic size of brain lesions and their distribution in all patients. DW-MRI of new lesions pre-TAVI (A), at 6 days post-TAVI (B) and at 3 months post-TAVI (C). Red arrows indicate areas of restricted diffusion. D) 3D rendering of brain lesions in both treatment arms at 6 days (red and blue) and 3 months (yellow) after TAVI (intention-to-treat population). Coloured brain areas indicate diffusion-restricted areas on DW-MRI. 3D: three-dimensional; DAPT: dual antiplatelet therapy; DW-MRI: diffusion-weighted magnetic resonance imaging; OAC: oral anticoagulation; TAVI: transcatheter aortic valve implantation
Secondary endpoints on MRI and serological biomarkers of brain injury
A total of 15 new preprocedural cerebral lesions were detected before TAVI, all in the DAPT group (p=0.390). Post-TAVI, 326 new lesions were identified – 155 in the OAC group and 171 in the DAPT group (p=0.145). On day 6, 315 lesions were detected (149 in OAC, 166 in DAPT; p=0.610), and on day 90, 11 lesions were identified (6 in OAC, 5 in DAPT; p=0.300).
The mean lesion volume per patient was higher in the OAC group compared to DAPT on both days 6 and 90 (day 6: 303.4±330.6 mm³ vs 265.9±331.7 mm³; p=0.019; day 90: 210.5 ± 105.5 mm³ vs 137.8±35.8 mm³; p=0.327). The total volume of affected brain tissue was also lower in the DAPT group (10,125.00 mm³ vs 12,581.67 mm³; p=0.046). Detailed MRI outcomes are shown in Table 3. Plasma levels of NSE and S100B increased significantly at 1 hour and 24 hours post-TAVI in both groups, with no significant differences between them (Supplementary Table 1, Supplementary Figure 1, Supplementary Figure 2).
Clinical outcomes
The incidence of clinical stroke and major bleeding at 6 and 90 days after TAVI did not significantly differ between groups (Table 4, Figure 3). All patients with overt stroke had new emboli on MRI. No differences in embolisation by hemisphere or vascular territory were observed between groups (Supplementary Table 2). Multivariate analyses showed no significant interactions suggesting differential effects between treatments (Supplementary Figure 3).
At the 90-day follow-up, all-cause mortality was 4.9%, with equal distribution between groups (Table 4). Clinical stroke occurred in 7.3% of patients overall, with 9.8% in the OAC group and 4.8% in the DAPT group (p=0.323) (Figure 3). There were no significant differences in major bleeding (11.5% vs 11.3%; p=0.974) (Figure 3) or clinical valve thrombosis (1.6% vs 1.6%; p=1.00) between the groups at 90 days. All-cause death, MACE, and NACE at 90 days and 1 year were comparable between groups (Table 4, Supplementary Figure 4, Supplementary Figure 5). OAC did not significantly reduce new “silent” brain lesions on day 6 (relative risk 0.53; 95% confidence interval [CI]: 0.19-1.44; p=0.209) or day 90 (relative risk 1.02; 95% CI: 0.24-4.33; p=1.00) compared to DAPT. OAC also showed no significant differences in major bleeding on day 6 (relative risk 0.47; 95% CI: 0.11-1.95; p=0.323) or day 90 (relative risk 0.98; 95% CI: 0.32-2.99; p=0.974) according to VARC-3 criteria. Three-dimensional visualisations of brain lesion size and distribution for all patients in the OAC (Supplementary Figure 6) and DAPT (Supplementary Figure 7) groups are available in Supplementary Appendix 1, showing lesions across the three DW-MRI scans (3 days before TAVI, on day 6, and on day 90 post-TAVI).
Seven out of 123 patients (5.7%) met the VARC-3 criteria for periprocedural TIA or stroke, with 4 in the OAC group and 3 in the DAPT group. Of these, 2 patients suffered from significant neurological deficits (NIHSS 14 and 11) immediately after the procedure, one in each group. Three additional patients experienced mild neurological deficits during hospitalisation, 2 in the OAC group and 1 in the DAPT group.
Consecutive NIHSS assessments were performed on all 123 patients pre- and post-TAVI, and on 90% (111 patients) at 90 days, and 83% (102 patients) at the 1-year follow-up. Excluding the 7 patients with clinical periprocedural stroke, NIHSS worsening was detected in 19% of patients at discharge, 8% at 90 days, and 6% at 1-year follow-up (Supplementary Table 3).
Consecutive MEC-35 assessments were performed on all 123 patients pre-TAVI, on 115 patients (93.5%) post-TAVI, on 102 patients (82.9%) at 90 days, and on 95 patients (77.2%) at the 1-year follow-up. The mean MEC-35 score was 26.2±3.2 (scale 0 to 35; higher scores indicate better cognitive function, and scores ≤27 denote cognitive deficits) in the entire population at baseline, showing progressive deterioration throughout the 1-year clinical follow-up (24.9±2.7 at hospital discharge, 23.9±3.4 at 90 days, and 22.1±2.9 at the 1-year follow-up) (Supplementary Table 3).
Table 4. Clinical outcomes at 6-day, 3-month and 1-year follow-up.
| Endpoints | Total (n=123) | OAC group (n=61) | DAPT group (n=62) | Risk ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| Outcomes at 6-day follow-up | |||||
| Major bleeding (BARC Type ≥3b or VARC-3) | 9 (7.3) | 6 (9.8) | 3 (4.8) | 0.47 (0.11-1.95) | 0.323 |
| Transient ischaemic attack or stroke | 7 (5.7) | 4 (6.6) | 3 (4.8) | 0.72 (0.15-3.39) | 0.717 |
| Outcomes at 3-month follow-up | |||||
| Major bleeding (BARC Type ≥3b or VARC-3) | 14 (11.4) | 7 (11.5) | 7 (11.3) | 0.98 (0.32-2.99) | 0.974 |
| Major adverse cardiovascular events | 14 (11.4) | 8 (13.1) | 6 (9.7) | 0.71 (0.23-2.18) | 0.548 |
| Net adverse clinical events | 25 (20.3) | 13 (21.3) | 12 (19.4) | 0.89 (0.37-2.13) | 0.787 |
| All-cause death | 6 (4.9) | 3 (4.9) | 3 (4.8) | 0.98 (0.19-5.07) | 1.000 |
| Transient ischaemic attack or stroke | 9 (7.3) | 6 (9.8) | 3 (4.8) | 0.46 (0.11-1.95) | 0.323 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| Outcomes at 1-year follow-up | |||||
| Major bleeding (BARC Type ≥3b or VARC-3) | 15 (12.2) | 8 (13.1) | 7 (11.3) | 0.84 (0.29-2.49) | 0.757 |
| Major adverse cardiovascular events | 19 (15.4) | 8 (13.1) | 11 (17.7) | 1.43 (0.53-3.84) | 0.348 |
| Net adverse clinical events | 30 (24.4) | 14 (23.0) | 16 (25.8) | 1.17 (0.51-2.66) | 0.712 |
| All-cause death | 10 (8.1) | 3 (4.9) | 7 (11.3) | 2.46 (0.60-9.99) | 0.323 |
| Transient ischaemic attack or stroke | 11 (8.9) | 6 (9.8) | 5 (8.1) | 0.80 (0.23-2.79) | 0.731 |
| Myocardial infarction | 0 (0) | 0 (0) | 1 (1.6) | NA | NA |
| Values are n (%) unless otherwise indicated. The data are shown according to the intention-to-treat principle. BARC: Bleeding Academic Research Consortium; CI: confidence interval; DAPT: dual antiplatelet therapy; NA: not applicable; OAC: oral anticoagulation; VARC-3: Valve Academic Research Consortium-3 | |||||
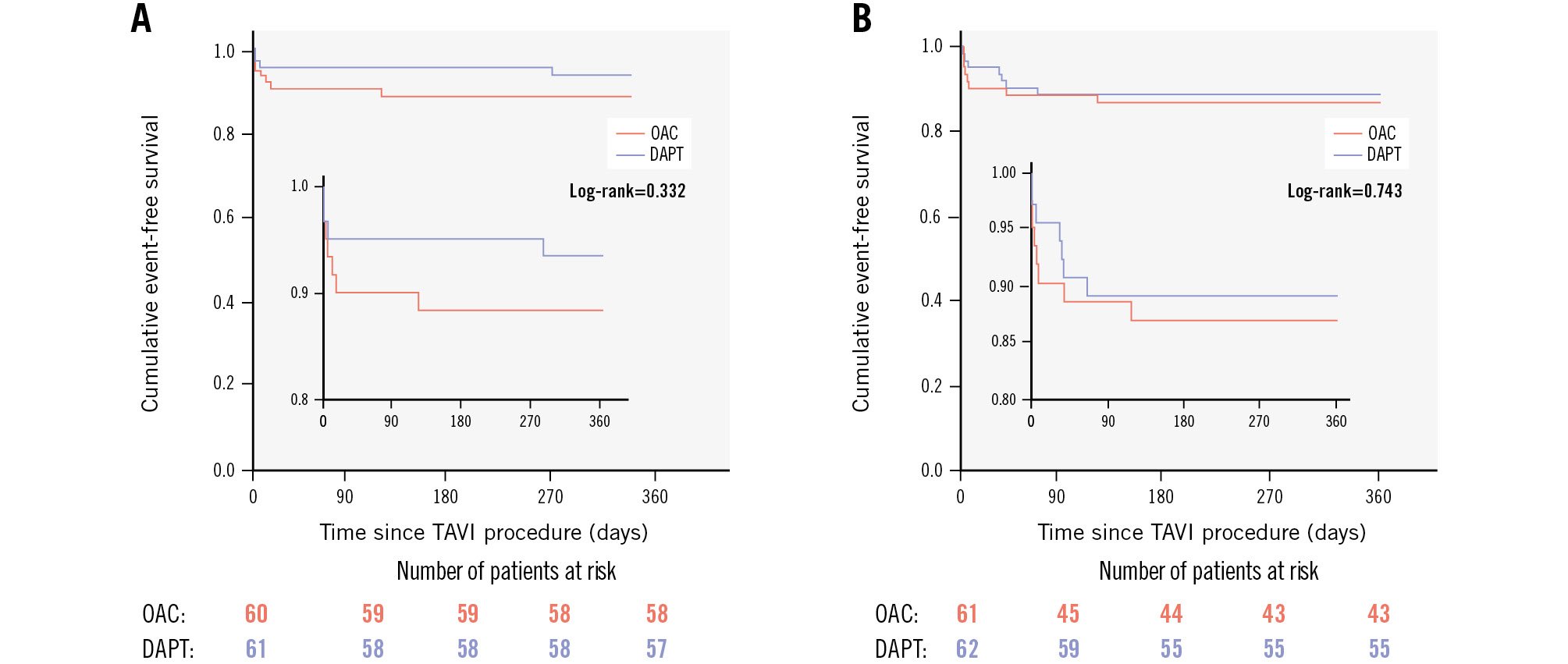
Figure 3. Kaplan-Meier event curves for stroke or transient ischaemic attack and major bleeding events. A) Kaplan-Meier curves for stroke or transient ischaemic attack, defined according to the VARC-3 definitions. B) Kaplan-Meier curves for major bleeding events, defined according to the VARC-3 definitions or BARC Type ≥3b. BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; OAC: oral anticoagulation; TAVI: transcatheter aortic valve implantation; VARC-3: Valve Academic Research Consortium 3
Discussion
The main findings can be summarised as follows: first, cerebral embolism detected by MRI post-TAVI is very high, affecting 75% of recipients, consistent with prior reports45. These embolic phenomena persist for at least 3 months after TAVI, regardless of the antithrombotic regimen. Second, OAC does not offer more protection against acute minor stroke compared to DAPT. Third, DAPT patients had a lower volume of embolic brain lesions on MRI after TAVI than OAC patients. Fourth, serological biomarkers of brain injury increase significantly in all patients after TAVI, indicating some degree of procedure-induced brain damage. Finally, no differences in MACE, major stroke, haemorrhagic complications, or clinical valve thrombosis were found between the treatment groups (Central illustration). Interestingly, cerebral microembolism was detected in 3.3% of patients a few days before TAVI, likely related to underlying AS rather than the treatments tested in this study.
Antiplatelets are essential for preventing thrombotic events in coronary artery disease, but their effectiveness in stroke prevention is less clear compared to anticoagulants. OAC is often expected to reduce stroke risk more than DAPT. Kosmidou et al found that OAC did not prevent cerebral thromboembolic events post-TAVI in atrial fibrillation patients. Conversely, antiplatelet therapy, whether alone or combined with OAC, significantly reduced stroke risk at 6 months and up to 2 years17. The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry18 found no association between OAC therapy and reduced stroke risk within the first 30 days, nor was there a significant difference in 30-day stroke rates between DAPT and OAC patients.
Three DOACs were evaluated in TAVI patients without atrial fibrillation. The GALILEO trial13 found higher risks of death, thromboembolic complications, and bleeding with rivaroxaban plus aspirin compared to DAPT, though it had fewer SCLT incidents at 90 days10. The ATLANTIS stratum 2 trial reported increased risks of death, stroke, TIA, or systemic embolism with apixaban versus antiplatelet regimens (SAPT or DAPT) at 1 year19. A subanalysis noted lower SCLT from 3 to 6 months with apixaban but higher non-cardiovascular mortality11. The ADAPT-TAVR trial observed a numerically lower incidence of SCLT with edoxaban compared to DAPT but similar rates of cerebral lesions on MRI and new neurological or neurocognitive dysfunction12. Fewer patients had new MRI cerebral lesions with DAPT (20.2%) compared to edoxaban (25.0%). In our trial, MRI-detected new brain embolic lesions post-TAVI were similar between groups, but the mean lesion volume within 3 months was lower with DAPT than with acenocoumarol, aligning with ADAPT-TAVR findings.
Patients with AS are at higher risk for thrombotic cerebral events compared to age- and sex-matched controls, even before aortic valve replacement20. This increased risk may result from heightened platelet activation and microthrombosis associated with stenotic aortic valves21. Contributing factors2223 include the following: (1) high gradient causing shear stress and platelet activation/adhesion; (2) activation of prothrombotic factors (e.g., von Willebrand factor, factor VIII, tissue factor, thrombin) and exposure to subendothelial material; and (3) inadequate antiplatelet effects. These factors may, to some extent, explain the brain lesions detected on pre-TAVI MRI.
The bioprosthesis implantation procedure can exacerbate vascular and endothelial damage both mechanically and biologically. Mechanical changes involve alterations in the native sinus shape and valve frame geometry, retaining the unexcised calcified native leaflets and creating a neosinus with unphysiological and assorted flow patterns that may promote thrombus formation. Biologically, shear stress releases proinflammatory and prothrombotic microparticles, enhancing inflammatory cell activation, platelet aggregation, and thrombin generation. This process also affects diseased native leaflets post-TAVI, serving as a reservoir for prothrombotic elements and inducing thrombus formation in both native and bioprosthetic valves2223. Additionally, intense platelet reactivity has been observed after TAVI24, related to various clinical features25 and independent of the type of transcatheter heart valve used26.
Platelets play a key role in thrombosis and inflammation early in AS, and this activity intensifies post-TAVI. Thrombin generation represents the culmination of the process at the final stage272829. Thus, antiplatelet therapy during this period of heightened platelet activation is crucial and warrants further investigation.
NSE and S100B are established biomarkers of cerebral injury, particularly related to embolic stroke and hypoxia. In our study, plasma levels of both biomarkers increased during the first 24 hours after TAVI, reflecting a significant cerebral microembolic load generated during the procedure, which can induce neuronal and glial injury. Although no significant differences were observed between the antithrombotic therapies studied – primarily due to a mismatch between the timing of biomarker measurement and the initiation of antithrombotic therapy – these findings reinforce evidence of cerebral microembolic injury during TAVI, as observed through neuroimaging.
The analysis of neurological (NIHSS) outcomes suggests a slightly higher early neurological impact in DAPT patients after TAVI. However, this impact decreased in both groups over time, with potentially better recovery at 12 months in the DAPT group compared to the OAC group. Baseline (pre-TAVI) neurocognitive function (MEC-35) was slightly better in the OAC group than in the DAPT group. However, a progressive decline in MEC-35 scores was observed in both groups, with the OAC group exhibiting a steeper decline at 12 months. Larger studies designed to evaluate these outcomes should confirm these differences and assess their clinical relevance.
Our findings of 75% of patients developing new cerebral lesions post-TAVI and nearly 9% experiencing strokes with neurological deficits align with other MRI studies45 but are higher compared to some reports18. The true incidence of neurocognitive impairments from “silent” emboli may be underestimated due to non-standardised definitions, limited post-procedure imaging, and routine neurocognitive assessments by stroke specialists.
Although OAC is considered appropriate for stroke prevention in the first 3-6 months after aortic bioprosthesis implantation7, it raises concerns about increased bleeding risk in elderly and frail patients. Recent trends favour SAPT to reduce bleeding risk in TAVI patients30. The major bleeding incidence with DAPT in our trial was comparable to the POPular-TAVI study, which lacked brain imaging data (ClinicalTrials.gov: NCT02247128). Further research is needed to evaluate the effectiveness of low-dose aspirin in mitigating the prothrombotic environment post-TAVI and to explore enhanced antiplatelet protection in high-risk populations (e.g., diabetics, patients with coronary or peripheral artery disease) and younger patients with low bleeding risk (REAC-TAVI2; ClinicalTrials.gov: NCT05283356).
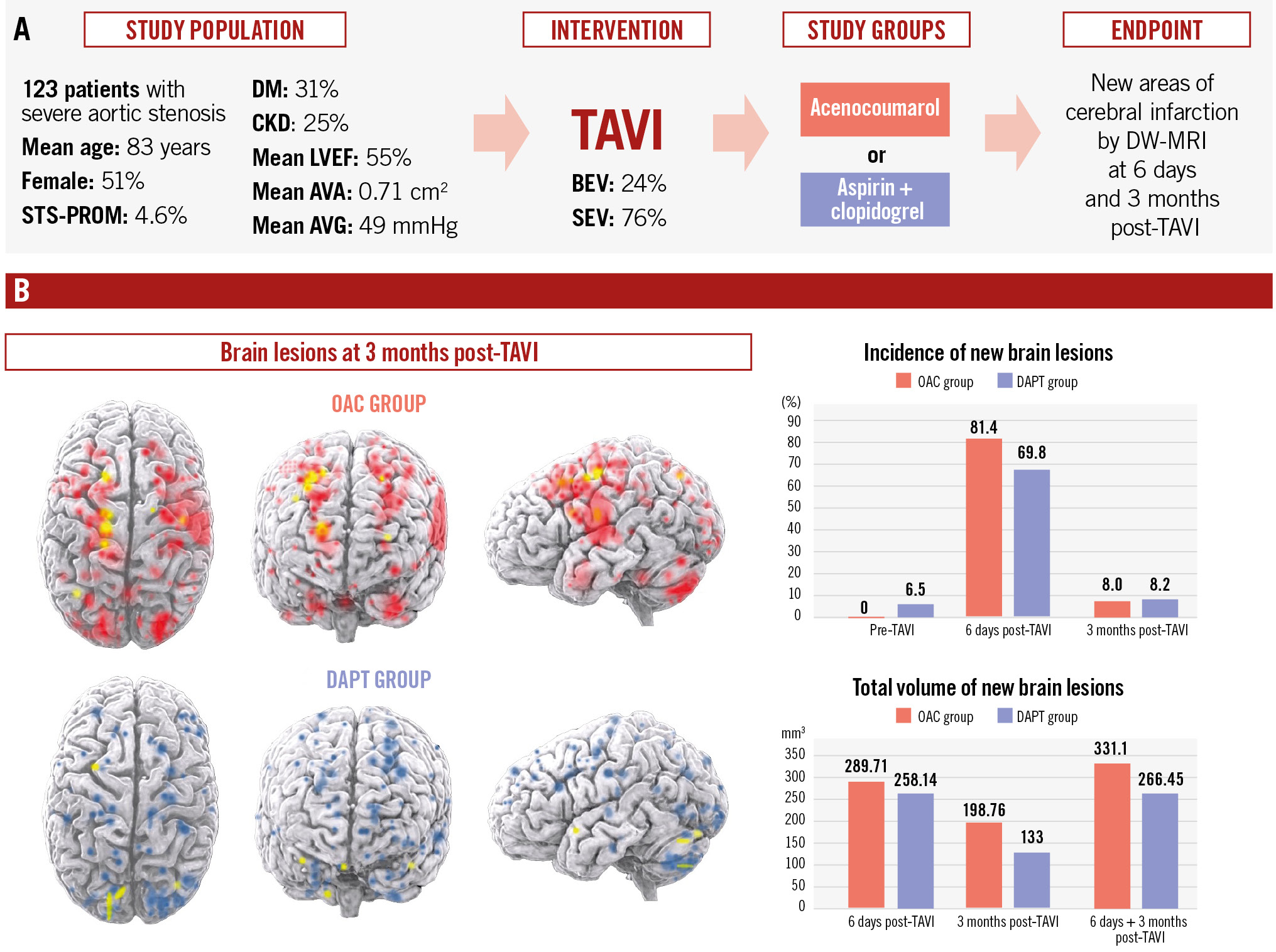
Central illustration. Dual antiplatelet therapy versus oral anticoagulation with vitamin K antagonist to prevent cerebral embolism after TAVI. AVA: aortic valve area; AVG: aortic valve gradient; BEV: balloon-expandable valve; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; DM: diabetes mellitus; DW-MRI: diffusion-weighted magnetic resonance imaging; LVEF: left ventricular ejection fraction; OAC: oral anticoagulation; SEV: self-expanding valve; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation
Limitations
The AUREA trial compared two antithrombotic strategies with pre- and post-TAVI neuroimaging but lacked sufficient statistical power to assess clinical outcomes. The study included patients in sinus rhythm without an indication for OAC, and therefore, its findings do not apply to patients requiring chronic oral anticoagulation for any indication. MRIs were independently reviewed, and clinical outcomes were assessed by a blinded committee. However, potential biases remain because of the open-label design and the lack of platelet reactivity evaluation. Current guidelines and trends favour SAPT over DAPT post-TAVI, particularly in elderly patients with a high risk of bleeding. As a result, DAPT is no longer the standard treatment for most post-TAVI patients without a specific indication for OAC. As TAVI expands to include younger patients with a lower bleeding risk, a more comprehensive antithrombotic strategy may become necessary. Furthermore, complex procedures such as transcatheter aortic valve (TAV)-in-TAV and Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) increase the risk of thrombosis. Our results, viewed in the context of the current and future TAVI population, highlight the importance of optimising antithrombotic regimens and ensuring effective long-term management.
Conclusions
The AUREA trial confirms the presence of “silent” cerebral microembolism in aortic stenosis patients undergoing TAVI, persisting from the preprocedure phase to at least 3 months post-TAVI and affecting various cerebral territories. A 3-month acenocoumarol-based strategy did not demonstrate superiority over DAPT with aspirin and clopidogrel in preventing cerebral microembolism. Compared to the OAC group, patients in the DAPT group exhibited reduced brain lesion volumes at 90 days and appeared to experience milder cognitive decline at 1 year. Therefore, exploring new pharmacological approaches for the optimal post-TAVI antithrombotic therapy is crucial, especially as TAVI expands towards a younger demographic.
Impact on daily practice
Cerebral embolic lesions are a common occurrence after transcatheter aortic valve implantation (TAVI) and may have implications for long-term neurocognitive outcomes. In patients without an indication for oral anticoagulation, acenocoumarol did not reduce brain lesions compared to dual antiplatelet therapy (DAPT). Notably, patients in the DAPT group exhibited smaller lesion volumes on diffusion-weighted magnetic resonance imaging. As TAVI is increasingly performed in younger, lower-risk patients undergoing more complex procedures, the need for comprehensive pharmacological strategies to prevent stroke is becoming more urgent. Developing targeted therapies is essential to optimise protection in this evolving patient population, particularly given their lower bleeding risk.
Funding
The trial is sponsored by the Spanish Ministry of Health, Consumer Affairs and Social Welfare (call for innovative medicines 2012, EC11-193) and complemented by the Galician Innovation Agency (Axencia Galega de Innovación - GAIN) through the programme code IN607B-2021/18. Neither sponsor had any role in the design or execution of the trial nor in the analysis of the data. There is no industry involvement in the trial. (ClinicalTrials.gov: NCT01642134. EudraCT: 2011-005784-24)
Conflict of interest statement
The authors have no conflicts of interest to declare regarding this manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

