Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Paclitaxel-coated balloons (PCB) are a viable alternative to drug-eluting stents in the treatment of de novo coronary lesions. Whether sirolimus represents an alternative to paclitaxel for drug-coated balloons remains elusive.
Aims: This randomised, controlled, multicentre, non-inferiority trial investigated a novel sirolimus-coated balloon (SCB) with a crystalline coating versus a PCB in de novo coronary lesions.
Methods: To compare a novel SCB with a clinically proven PCB, 70 patients with de novo coronary lesions were enrolled at 4 centres in Germany and Switzerland. The primary endpoint was non-inferiority regarding angiographic late lumen loss (LLL) at 6 months, with a predefined margin of δ=0.35 mm. Secondary endpoints included procedural success, major adverse cardiac events, and individual clinical endpoints.
Results: Quantitative coronary angiography revealed no differences in baseline parameters. At 6 months, in-segment LLL was 0.04±0.39 mm in the PCB group versus 0.11±0.37 mm in the SCB group (non-significant), respectively. The mean difference between SCB and PCB was 0.07 mm (95% confidence interval: –0.12 to 0.26). Non-inferiority at the predefined margin of 0.35 was shown. Clinical event rates up to 12 months were not different between the groups (3 target lesion revascularisations in the PCB group versus 2 in the SCB group, no myocardial infarctions, no deaths).
Conclusions: The novel SCB showed similar angiographic outcomes in the treatment of de novo coronary disease as compared with a clinically proven PCB (ClinicalTrials.gov: NCT03908450).
Drug-eluting stents (DES) represent the standard treatment for percutaneous coronary intervention (PCI). In the case of DES, substances of the -limus family have proven to be safe and effective. Stents allow for a controlled local release of drugs for a defined period of time. In contrast, the majority of drug-coated balloons (DCB) are coated with paclitaxel as the active drug in combination with specific excipients defining the efficacy and safety of these devices12. Paclitaxel binds irreversibly to the microtubules, resulting in excellent persistence in vascular cells13, and has beneficial cell-specific effects4. A large meta-analysis has demonstrated a high level of safety for paclitaxel-coated balloons (PCB) for the treatment of coronary artery disease5.
Several alternatives to paclitaxel have been recently investigated67. In the case of sirolimus and its analogues, specific measures are required to facilitate a controlled drug release without having a stent platform8. Preclinical studies have shown varying presence of therapeutic tissue levels lasting from a few days to several weeks6910. To date, randomised trial data have been published only for a balloon coated with biolimus A9 versus conventional angioplasty in small coronary arteries11 and for the highly crystalline sirolimus coating using butylated hydroxytoluene as an excipient, also investigated herein8. This novel sirolimus-coated balloon (SCB; SeQuent SCB, 4 μg/mm² [B. Braun]) was compared with a clinically proven PCB (SeQuent Please NEO, 3 μg/mm² [B. Braun]) in two randomised trials, i.e., for the treatment of in-stent restenosis (ISR)1213 and de novo coronary lesions. The first-in-human trial for de novo coronary lesions showed non-inferiority of the SCB compared with the PCB in a Malaysian population14.
The present randomised controlled trial aimed to extend these findings by investigating this novel SCB in a Central European population.
Methods
STUDY DESIGN AND PATIENT POPULATION
The study design was comparable with another trial conducted in Malaysia14. Briefly, 70 patients were enrolled in a randomised, prospective, controlled, multicentre, non-inferiority trial investigating the efficacy and safety of a European Conformity (CE)-marked SCB (SeQuent Neo percutaneous transluminal coronary angioplasty balloon catheter [B. Braun], coated with 4 μg/mm² of sirolimus on the balloon surface and butylated hydroxytoluene as an excipient [InnoRa GmbH]) compared with a commercially available PCB (SeQuent Please NEO, 3 μg/mm² of paclitaxel on the balloon surface). The study was conducted at 4 cardiology departments in Germany and Switzerland (Protestant Hospital Paul Gerhardt Stift, Lutherstadt Wittenberg, Germany; University Hospital of Saarland, Homburg/Saar, Germany; Heart Center Dresden, Technische Universität Dresden, Dresden, Germany; University Hospital of Basel, Basel, Switzerland). The study was performed according to the Declaration of Helsinki and World Health Organization guidelines. All patients gave written informed consent. The Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) and the local ethics committees approved the study.
Patients of at least 18 years of age with clinical evidence of stable or unstable angina or a positive functional study, a significant de novo coronary stenosis (≥70% diameter stenosis or intermediate ≥50% to <70% diameter stenosis with a positive functional test or symptoms of ischaemia) were considered for enrolment. Major clinical exclusion criteria were myocardial infarction within the past 72 hours; intolerance and/or allergy to sirolimus, paclitaxel and/or the delivery matrix; patients with an ejection fraction of <30%; those with a reference vessel diameter (RVD) <2.5 mm; or patients with a contraindication for whichever necessary accompanying medication. Cardiac catheterisation and intervention were carried out according to local practice.
After confirmation of eligibility, patients were randomly assigned to undergo balloon angioplasty of the target lesion with either a paclitaxel-coated or a sirolimus-coated balloon catheter. Predilatation of the target lesion was mandatory, using a non-study uncoated balloon catheter with a diameter similar to the size of the RVD (1:1)15. The use of scoring or cutting balloons for lesion preparation was recommended. Successful lesion preparation (no flow-limiting dissection and a residual stenosis ≤30%) was a requirement for randomisation. The recommended study DCB inflation time was 30 to 60 seconds at nominal pressure. The patients were preloaded with a P2Y12 inhibitor and aspirin. Unfractionated heparin was given according to local standards, and the activated clotting time (ACT) was kept above 250 seconds. Vascular sheaths were removed according to usual practice.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiography was performed before and after all interventions and at angiographic follow-up using identical projections and analyses. Independent technicians who were blinded to patient allocation and clinical characteristics performed quantitative analysis of the coronary angiographic images (core lab in Homburg/Saar, Germany). The Medis Suite XA (Medis Medical Imaging Systems BV) was used for automated contour detection and quantification. Measurements were obtained in the balloon-treated area with measurements shoulder-to-shoulder (in-lesion), and in the total treated area plus 5 mm proximally and distally (in-segment). Restenosis was defined as ≥50% diameter stenosis at angiographic follow-up.
FOLLOW-UP AND ENDPOINTS
Dual antiplatelet therapy was continued orally for 1 month in stable patients or 12 months in the case of acute coronary syndrome, followed by treatment with aspirin or clopidogrel alone. Patients underwent follow-up angiography after 6 months (up to 9 months). Clinical follow-up was performed at 30±7 days, 6 months±4 weeks, and 12 months±4 weeks post-procedure. All clinical endpoints and adverse events were evaluated in consensus by the investigators. All events were crosschecked with medical records by external monitors. Due to the different packaging of the study balloon catheters, the investigators performing the study procedures were unblinded.
Angiographic late lumen loss (LLL; difference between the postprocedural and 6-month follow-up in-segment minimal lumen diameter, evaluated by quantitative coronary angiography) was defined as the primary endpoint. Secondary endpoints included procedural success (≤30% final stenosis, Thrombolysis in Myocardial Infarction [TIMI] 3 flow, no flow-limiting dissection, and the absence of in-hospital major adverse cardiac events [MACE]), MACE (occurrence of cardiac death, target vessel myocardial infarction, or clinically driven target lesion revascularisation) at 6 months and 12 months, as well as individual clinical endpoints at 6 and 12 months of follow-up (vessel thrombosis, cardiac death, target lesion myocardial infarction, clinically driven target lesion revascularisation, and angiographic binary restenosis). Vessel thrombosis, cardiac death, target lesion myocardial infarction, and clinically driven target lesion revascularisation were defined according to the Academic Research Consortium (ARC) consensus document16.
STATISTICAL ANALYSIS
This study was designed to prove the non-inferiority of SCB as compared to PCB in LLL. The primary endpoint was defined as LLL at 6 months (measured in mm) between groups. A non-inferiority margin of δ=0.35 mm was used to test the difference between the SCB and PCB groups. The study was regarded as successful if the 95% confidence interval (95% CI) was completely below δ.
Statistical analyses were conducted in the intention-to-treat (ITT) population. The primary endpoint, LLL, was analysed in all patients with angiographic follow-up. Binary and categorical variables such as clinical outcomes were summarised using counts and percentages. For continuous variables, percentiles, means and standard deviations were calculated. For clinical baseline data, the t-test and Fisher’s exact test as well as the chi-square test were utilised to assess differences between treatment groups.
Results
Between 25 February 2019 and 15 February 2022, a total of 70 patients with de novo coronary lesions were enrolled and randomly assigned to the SCB (n=35) or PCB group (n=35). The study flowchart is presented in Figure 1. Baseline characteristics of the patients were similar in the two groups (Table 1). The mean age was 67 years, and 81% of patients were male. More than one-third of the patients were diabetic (37%), and 80% had multivessel coronary artery disease. In the PCB group, 37 study lesions were treated versus 39 in the SCB group.
The mean study balloon inflation pressures were 8±2 atm and 7±2 atm (p=0.458) with mean inflation times of 53±11 seconds and 52±10 seconds (p=0.622) in the SCB and PCB groups, respectively.
One lesion (2.6%) in the SCB group and 2 lesions in the PCB group (5.4%) underwent bailout DES implantation following treatment with the study balloon. Table 2 summarises the procedural data.
Quantitative coronary angiography revealed no differences in baseline parameters. The mean vessel size was similar. After 6 months, in-segment LLL was 0.04±0.39 mm in the PCB group versus 0.11±0.37 mm in the SCB group (Table 3). The mean difference between SCB and PCB was 0.07 mm (95% CI: –0.12 to 0.26). Non-inferiority at a predefined margin of 0.35 was shown (Table 4, Central illustration). Figure 2 and Figure 3 present the cumulative frequency distribution of in-segment LLL and minimal lumen diameter, respectively.
Late lumen gain (negative LLL) was more frequent in the PCB group without reaching statistical significance (56% of lesions vs 44% in the SCB group; p=0.544) (Table 3, Figure 2, Figure 3). Clinical event rates up to 12 months of follow-up did not differ between the groups (Supplementary Table 1).
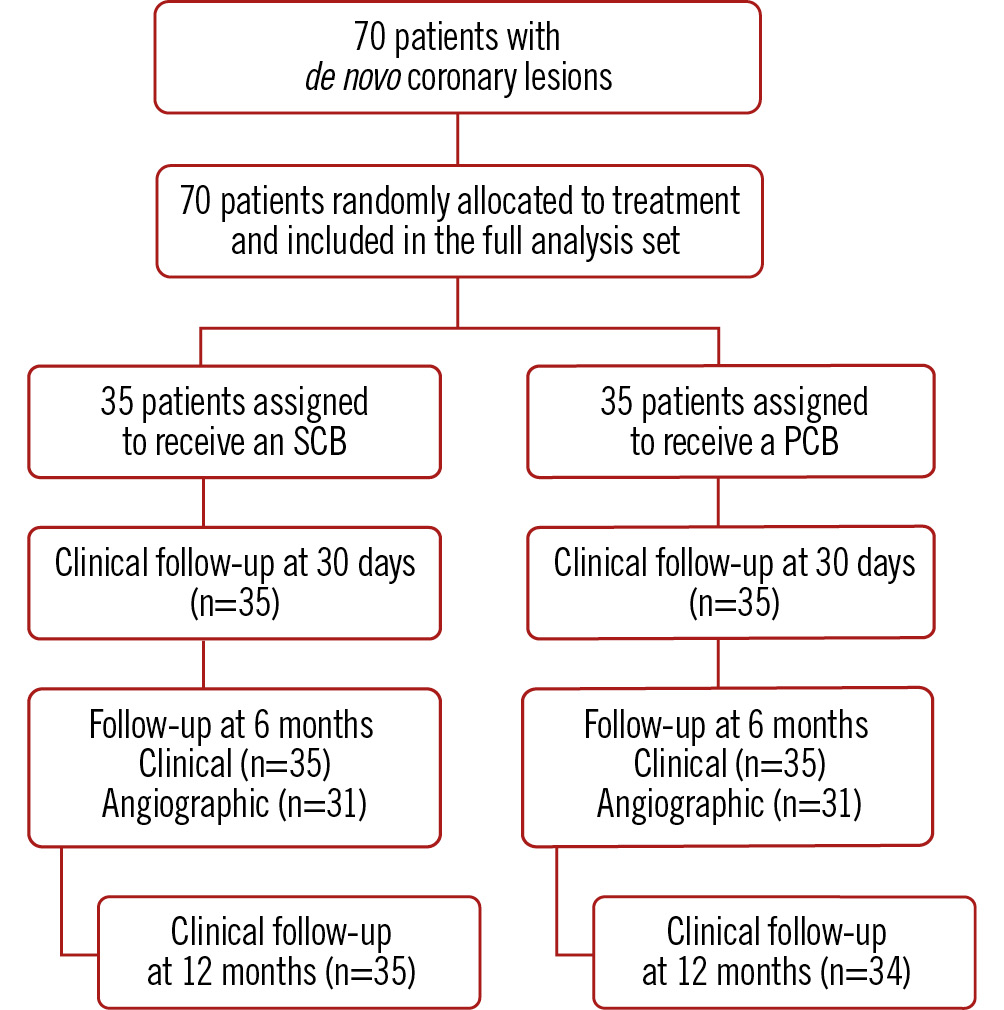
Figure 1. Flowchart of the study. PCB: paclitaxel-coated balloon; SCB: sirolimus-coated balloon
Table 1. Clinical baseline data (CRF data).
| Baseline characteristics | SCB N=35 | PCB N=35 | p-value |
|---|---|---|---|
| Age, years | 66±9 | 67±12 | 0.511 |
| Male | 30 (86) | 27 (77) | 0.357 |
| Height, cm | 172±9 | 172±11 | 0.962 |
| Weight, kg | 85±14 | 84±17 | 0.869 |
| Body mass index, kg/m2 | 29±4 | 28±4 | 0.751 |
| Angina pectoris status stable | 17 (49) | 18 (51) | 0.717 |
| Canadian Cardiac Society angina pectoris classification | 0.354 (Fisher) | ||
| None | 7 (20) | 6 (18) | |
| Class 1 | 7 (20) | 3 (9) | |
| Class 2 | 7 (20) | 10 (29) | |
| Class 3 | 7 (20) | 11 (31) | |
| Class 4 | 7 (20) | 5 (14) | |
| Prior PCI | 19 (54) | 18 (51) | 0.811 |
| Prior CABG | 2 (6) | 2 (6) | >0.99 (Fisher) |
| History of myocardial infarction | 13 (37) | 12 (34) | 0.803 |
| Hypertension | 29 (83) | 31 (89) | 0.495 |
| Prior stroke | 3 (9) | 3 (9) | >0.99 (Fisher) |
| Diabetes mellitus | 11 (31) | 15 (43) | 0.322 |
| Insulin | 5 (14) | 6 (16) | 0.781 |
| Hyperlipidaemia | 26 (77) | 24 (71) | 0.583 |
| Smoking | 8 (24) | 10 (30) | 0.580 |
| Data are presented as n (%) or mean±standard deviation. CABG: coronary artery bypass graft; CRF: case report form; PCI: percutaneous coronary intervention; PCB: paclitaxel-coated balloon; SCB: sirolimus-coated balloon | |||
Table 2. Procedural data.
| SCB N=35 | PCB N=35 | p-value | |
|---|---|---|---|
| Severity of CAD: number of diseased vessels | 0.484 | ||
| 1 | 6 (17) | 8 (23) | |
| 2 | 18 (51) | 13 (37) | |
| 3 | 11 (31) | 14 (40) | |
| LVEF, % | 55±11* | 54±10* | 0.894 |
| Missing data | 4 (11) | 4 (11) | |
| TIMI flow before procedure | 0.098 (Fisher) | ||
| 0 | 1 (3) | 0 | |
| 1 | 0 (0) | 1 (3) | |
| 2 | 2 (6) | 2 (6) | |
| 3 | 32 (91) | 32 (91) | |
| Missing data | 0 | 0 | |
| Stenosis as a visual estimation by operator, % | 84±9 | 85±10 | 0.732 |
| Predilatation | |||
| Predilatation carried out | 35 (100) | 35 (100) | - |
| Number of balloons used | 1.6±0.7 (1-4) | 1.6±0.8 (1-4) | 0.878 |
| Type of balloon used | NA | ||
| Scoring | 14 | 15 | |
| POBA | 39 | 42 | |
| Other | 3 | 0 | |
| Number of inflations | 2.6±1.7 (1-8) | 2.4±1.5 (1-7) | 0.553 |
| Highest pressure used, bar | 12.7±4.8 (4-24) | 12.7±4.4 (6-23) | 0.959 |
| Total duration of inflation, sec | 34.4±29.9 (6-130) | 44.4±37.1 (10-197) | 0.216 |
| Study intervention | |||
| Number of study balloons | 39 | 43 | - |
| Balloon diameter, mm | 2.95±0.39 | 2.85±0.36 | 0.264 |
| Balloon length per balloon, mm | 22.6±5.4 | 20.1±3.7 | 0.018 |
| Balloon pressure, bar | 8±2 | 7±2 | 0.458 |
| Balloon inflation time, sec | 53±11 | 52±10 | 0.622 |
| Final results | |||
| Bailout stenting | 1 (3) | 2 (6) | >0.99 |
| TIMI flow at end of procedure | >0.99 (Fisher) | ||
| 2 | 0 (0) | 1 (3) | |
| 3 | 35 (100) | 34 (97) | |
| Final diameter stenosis, % | 6±8 | 8±10 | 0.276 |
| Final dissection | >0.99 (Fisher) | ||
| Type A | 1 (3) | 1 (3) | |
| Type B | 0 (0) | 0 (0) | |
| Type C | 0 (0) | 1 (3) | |
| Data are presented as n (%) or mean±standard deviation (min-max), unless otherwise indicated. *Data available for 31 patients each. Fisher's exact test was used when the expected count was <5. CAD: coronary artery disease; LVEF: left ventricular ejection fraction; NA: not applicable; PCB: paclitaxel-coated balloon; POBA: plain old balloon angioplasty; SCB: sirolimus-coated balloon; TIMI: Thrombolysis in Myocardial Infarction | |||
Table 3. Quantitative coronary angiography intention-to-treat analysis.
| QCA | SCB | PCB | p-value |
|---|---|---|---|
| Number of patients/lesions | 35/39 | 35/37 | |
| Lesion length, pre-PCI, mm | 11.81±6.01 | 11.51±4.89 | 0.81 |
| RVD, pre-PCI, mm | 2.93±0.42 | 2.83±0.45 | 0.365 |
| In-lesion MLD, pre-PCI, mm | 0.88±0.38 | 0.85±0.37 | 0.778 |
| In-segment MLD, pre-PCI, mm | 0.85±0.37 | 0.83±0.40 | 0.852 |
| In-lesion diameter stenosis, pre-PCI, % | 67.4±12.5 | 66.4±13.2 | 0.746 |
| In-lesion area stenosis, pre-PCI, % | 87.8±8.8 | 87.0±9.7 | 0.7 |
| In-lesion MLD, post-predilatation, mm | 1.99±0.57 | 1.99±0.43 | 0.998 |
| In-lesion diameter stenosis, post-predilatation, % | 24.8±18.9 | 21.2±13.9 | 0.4 |
| In-lesion area stenosis, post-predilatation, % | 38.3±24.9 | 36.0±21.4 | 0.703 |
| In-lesion MLD, final, mm | 2.35±0.39 | 2.24±0.35 | 0.201 |
| In-segment MLD, final, mm | 2.19±0.57 | 2.17±0.35 | 0.854 |
| In-lesion diameter stenosis, final, % | 8.9±8.9 | 10.3±8.2 | 0.503 |
| In-lesion area stenosis, final, % | 16.0±15.1 | 18.8±14.2 | 0.419 |
| In-lesion acute gain, mm | 1.48±0.43 | 1.38±0.42 | 0.356 |
| Follow-up, days | 177.3±55.0 | 197.3±31.0 | 0.079 |
| Follow-up angiography | |||
| Number of patients | 31 (89) | 28 (80) | 0.536 |
| Number of lesions | 34 (87) | 32 (86) | |
| In-lesion LLL, mm | 0.13±0.39 | 0.03±0.41 | 0.331 |
| In-segment LLL, mm | 0.11±0.37 | 0.04±0.39 | 0.44 |
| RVD, FU, mm | 2.78±0.38 | 2.75±0.41 | 0.773 |
| In-lesion MLD, FU, mm | 2.19±0.42 | 2.16±0.59 | 0.817 |
| In-segment MLD, FU, mm | 2.15±0.42 | 2.13±0.56 | 0.876 |
| In-lesion diameter stenosis, FU, % | 12.0±13.4 | 14.3±14.6 | 0.498 |
| In-lesion area stenosis, FU, % | 19.2±20.7 | 24.6±21.3 | 0.313 |
| Late lumen enlargement, number of lesions | 15 (44.1) | 18 (56.3) | 0.544 |
| In-lesion binary restenosis, number of lesions | 2 (5.1) | 2 (5.4) | 0.536 |
| Data are presented as n (%) or mean±standard deviation. Fisher's exact test was used when the expected count was <5. FU: follow-up; LLL: late lumen loss; MLD: minimal lumen diameter; PCB: paclitaxel-coated balloon; PCI: percutaneous coronary intervention; QCA: quantitative coronary angiography; RVD: reference vessel diameter; SCB: sirolimus-coated balloon | |||
Table 4. Quantitative coronary angiography: primary endpoint LLL non-inferiority testing intention-to-treat analysis.
| QCA | SCB (N=33*) | PCB (N=32) | Mean difference (SCB−PCB) | Threshold for non-inferiority | |||
|---|---|---|---|---|---|---|---|
| LS-mean | 95% CI | LS-mean | 95% CI | Differ-ence | 95% CI | ||
| In-lesion LLL, mm | 0.13 | (−0.01 to 0.27) | 0.03 | (−0.11 to 0.17) | 0.1 | (−0.10 to 0.30) | <0.35 |
| In-segment LLL, mm | 0.11 | (−0.02 to 0.25) | 0.04 | (−0.10 to 0.17) | 0.07 | (−0.12 to 0.26) | <0.35 |
| *For LLL, only data from 33 lesions are available at lesion and segment level. CI: confidence interval; LLL: late lumen loss (primary efficacy endpoint); LS: least squares; PCB: paclitaxel-coated balloon; QCA: quantitative coronary angiography; SCB: sirolimus-coated balloon | |||||||
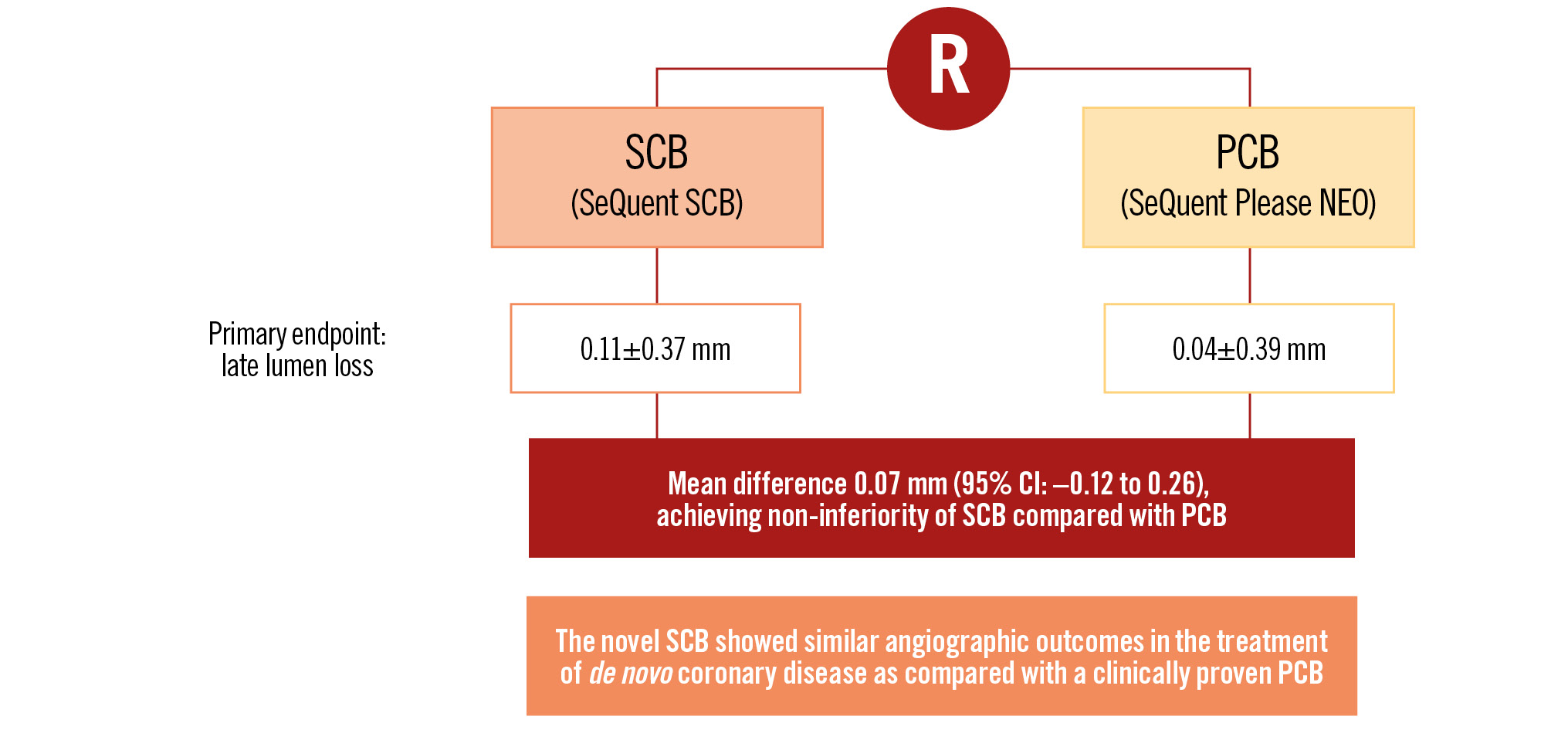
Central illustration. SCBDENOVO in Europe: a prospective, randomised, multicentre trial of sirolimus- versus paclitaxel-coated balloons for de novo coronary lesions. CI: confidence interval; PCB: paclitaxel-coated balloon; R: randomisation; SCB: sirolimus-coated balloon
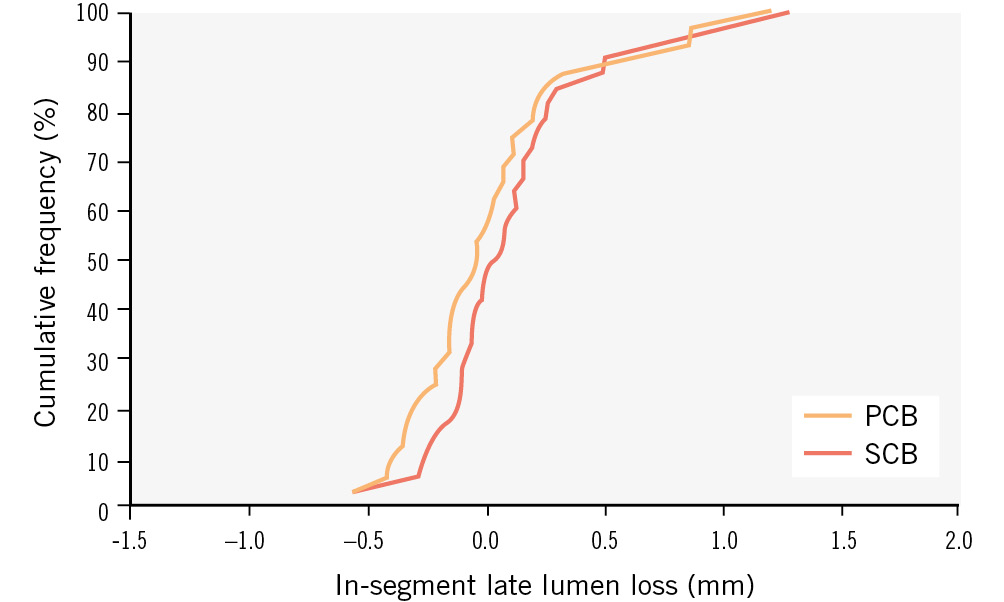
Figure 2. Angiographic patency: cumulative frequency distribution of in-segment late lumen loss determined by quantitative coronary angiography. Paclitaxel-coated balloon (PCB) versus sirolimus-coated balloon (SCB).
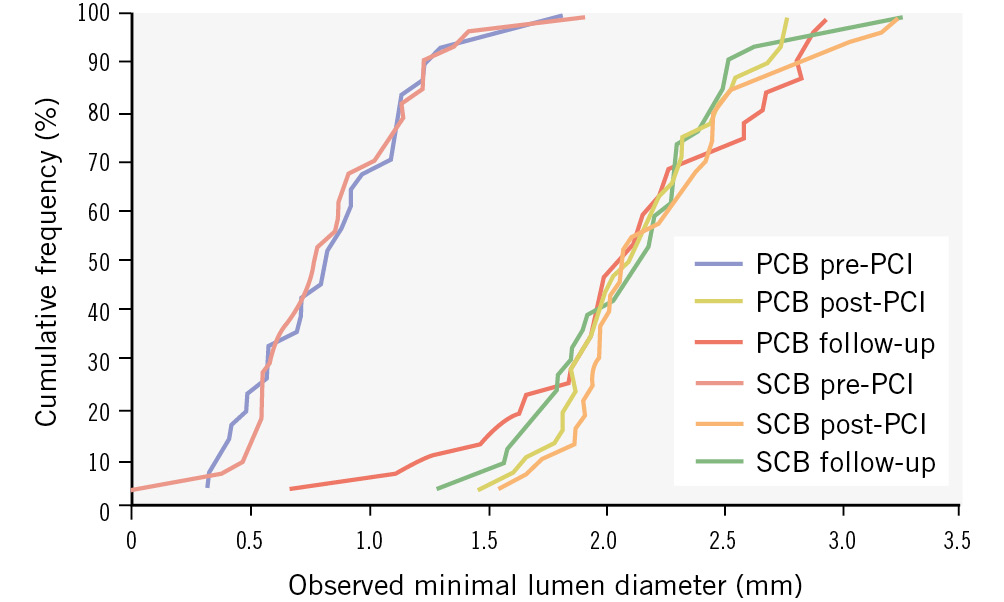
Figure 3. Angiographic patency: cumulative frequency distribution of in-segment minimal lumen diameter determined by quantitative coronary angiography. Paclitaxel-coated balloon (PCB) and sirolimus-coated balloon (SCB) before procedure (pre-PCI), post-procedure (post-PCI), and at 6 months (follow-up). PCI: percutaneous coronary intervention
Discussion
This randomised controlled study demonstrates non-inferiority of a novel SCB in terms of LLL against a clinically proven PCB in de novo coronary artery lesions.
Despite the very good results with current DES technologies, there remains a device-related event rate beyond the first year of approximately 2-4%1718. In contrast, treatment with a DCB without a permanent implant allows restoration of the physiological vasomotion1920. Furthermore, especially with a PCB, an improvement of the initial lumen due to positive remodelling and a reduction of plaque volume occurs over the course of months212223.
The safety and efficacy of the DCB-only approach has been demonstrated in several randomised trials52425262728 which predominantly utilised PCB. The preferred use of PCB is related to the different modes of action of paclitaxel and sirolimus. Treatment with DCB only allows for a single short-term contact with the vessel wall, during which the entire therapeutic drug amount has to be transferred. For long-term antirestenotic efficacy, irreversible binding of paclitaxel to microtubules is helpful. Sirolimus, on the other hand, acts reversibly via mammalian target of rapamycin (mTOR) inhibition which requires additional measures to ensure longer-term biological efficacy when used with DCB. The SCB studied herein used butylated hydroxytoluene as an excipient with sirolimus in a highly crystalline formulation, enabling therapeutic drug concentrations in the vessel wall over a long period of time8.
In the present trial conducted in Germany and Switzerland and in line with a previous study from Malaysia14, the non-inferiority of an SCB to a clinically proven PCB was demonstrated. After 6 months, the in-segment LLL was 0.04±0.39 mm in the PCB group versus 0.11±0.37 mm in the SCB group. These angiographic results are in accordance with the results of the above-mentioned trial conducted using a similar study protocol (LLL, PCB vs SCB: 0.01±0.33 mm vs 0.10±0.32 mm)14. This is indeed remarkable since the patients in Malaysia were younger, had lower body mass index and a significantly higher proportion of patients with diabetes14.
The concept of two studies, each with an identical protocol, was first implemented with the first-in-human trials PACCOCATH - ISR I29 and PACCOCATH - ISR II30 pioneering the field. Both studies were powered for the primary angiographic endpoint. The aim was to ensure the reproducibility of the results, at that time in a sequential manner. This concept was repeated for the current comparison of two different DCB. However, the reproducibility in different healthcare systems was also taken into account, in this case, Malaysia and Germany/Switzerland. The two previously published studies on the treatment of ISR1213 and the de novo sister trial14 also had a primary angiographic endpoint with the same non-inferiority margin used in the de novo trial presented in this manuscript.
The LLL, although numerically larger in the SCB groups in both studies, was not significantly different from that in the PCB groups. The Malaysia study showed a significant difference in the frequency of late lumen gain (negative LLL) between PCB and SCB (60% vs 32%; p=0.019)14. The present study could not confirm this finding with statistical significance, but numerically pointed in the same direction (56% PCB vs 44% SCB; p=0.544) (Supplementary Table 2). Interestingly, the comparison of both DCB in patients with ISR showed no numerical difference in LLL13. However, in the ISR trials, differences were found between the Asian and European patients. Thus, in the European ISR study, the non-inferiority of SCB versus PCB was not demonstrated13.
The present study was not designed to assess clinical endpoints but confirmed that DCB-only treatment of de novo coronary artery lesions was feasible and safe. Only 3 of 76 lesions treated with a PCB or an SCB required implantation of an additional DES. No cardiac death or target vessel myocardial infarction occurred in the first year.
In contrast to our findings, another commercially available sirolimus DCB, based on a nanocarrier technology of sirolimus encapsulated in a phospholipid bilayer, did not achieve the non-inferiority primary endpoint of net gain when compared to the paclitaxel DCB used in our trial. That SCB was in fact inferior to this specific device31. Finally, a biolimus DCB has also shown mixed results, with superiority against plain old balloon angioplasty in small vessel disease11, and in ISR indication, inferiority against a PCB in one trial32 and non-inferiority in another study33.
Limitations
The current trial replicates the sister trial conducted in an Asian population. It is a mechanistic trial and, thus, does not allow the investigation of hard clinical endpoints. The concept of conducting two identical trials may be questioned; however, it is in line with prior first-in-human trials from our group, paving the path for the basic concept of drug-coated balloons. Clinical events were defined by local investigators and not by an independent clinical events committee. The correlation between lesions and patients was disregarded because of the limited sample size. The non-inferiority margin of the primary angiographic endpoint was larger than in other trials. Furthermore, there is no information on functional assessment or intravascular imaging in this trial.
Conclusions
This novel SCB with a crystalline coating compared with a clinically proven PCB showed non-inferior angiographic outcomes in the treatment of de novo coronary disease.
Impact on daily practice
There is increasing clinical evidence to support the use of drug-coated balloons in the treatment of de novo coronary disease. Whether paclitaxel remains the drug of choice or whether sirolimus represents an alternative in analogy to drug-eluting stents remains elusive. Herein, the angiographic efficacy of a new sirolimus-coated balloon with a crystalline coating as compared with a paclitaxel-coated balloon in de novo lesions was demonstrated. The outcomes of this trial should be supplemented by additional studies with a larger sample size and longer clinical follow-up.
Funding
Study coordination, data management, on-site monitoring, and financial support were provided by InnoRa GmbH. Study devices were provided by B. Braun. Neither InnoRa nor B. Braun were involved in the analysis and interpretation of the data nor writing of the manuscript, nor did they participate in the decision to submit the manuscript for publication.
Conflict of interest statement
B. Scheller is a shareholder of InnoRa GmbH. N. Mangner has received personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed, and Boston Scientific, outside the submitted work. C. Schwenke is a consultant for InnoRa GmbH, on an honorary basis. R.V. Jeger received grants to the institution from Abbott, Amgen, AstraZeneca, Bayer, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cardionovum, Cordis, Daiichi Sankyo, Edwards Lifesciences, GE HealthCare, MCM Medsys, Medtronic, Novartis, Pfizer, Terumo, and Vascular Medical GmbH. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung; he has received scientific support from Ablative Solutions, Medtronic, and ReCor Medical; and speaker honoraria/consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. The other authors have no conflicts of interest to declare with regard to this study.
Supplementary data
To read the full content of this article, please download the PDF.

