Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The optimal strategy to treat coronary bifurcation lesions (CBL) has been a long-debated topic. The combination of a stent in the main vessel (MV) and a drug-coated balloon (DCB) in the side branch (SB) seems promising, but the evidence is limited.
Aims: This study aims to investigate a novel sirolimus-coated balloon in the treatment of non-left main CBL compared with a paclitaxel-coated balloon.
Methods: The SPACIOUS trial is a prospective, non-inferiority, multicentre trial. A total of 230 patients were randomised to the sirolimus DCB or the paclitaxel DCB group in a 1:1 ratio. Angiographic and clinical follow-ups were planned at 9 months and 1 year, respectively. The primary endpoint was diameter stenosis (DS) in the SB at 9 months.
Results: At 9 months, DS in the sirolimus group was 30.5±16.1% compared with 33.5±16.2% in the paclitaxel group (difference −2.94%; 95% confidence interval: −7.62% to 1.74%; p for non-inferiority<0.01). The incidence of binary restenosis was significantly lower in the sirolimus group compared to the paclitaxel group (4.4% vs 12.8%; p=0.043). Secondary angiographic endpoints, including late lumen loss and net lumen gain, and 1-year clinical outcomes were not significantly different between groups.
Conclusions: In de novo non-left main CBL treatment, MV stenting accompanied by SB dilation with the sirolimus DCB was non-inferior to the paclitaxel DCB.
Percutaneous coronary intervention (PCI) has been routinely used in patients with coronary artery disease. Coronary bifurcation lesions (CBL) are encountered in approximately 20% of PCI and are associated with a high rate of adverse cardiac events12. Despite emerging randomised clinical trials, the optimal PCI technique to treat CBL remains a matter of debate345. In 2024, the European Bifurcation Club recommended a provisional stenting strategy in most cases, aiming for simpler procedures and reduced stent use6. Drug-coated balloons (DCB) are able to deliver medication to the vessel wall without stent implantation. Stenting in the main vessel (MV) with DCB dilation in the side branch (SB) seems a promising strategy to treat CBL7. However, to our knowledge, it has not been validated in large-scale research.
For the past years, paclitaxel-coated balloons (PCB) have represented the best-in-class DCB. Bifurcation treatment using paclitaxel DCB and stent deployment has been proved to be efficacious8. Compared with a regular balloon, SB dilation with a paclitaxel-coated balloon after stenting in the MV may significantly reduce late lumen loss (LLL)910. With the advent of technique innovations, limus-based drugs have emerged as potential alternative options for balloon coatings1112. Early studies have shown favourable results for sirolimus-coated balloon (SCB) treatment in de novo lesions and intrastent restenosis131415. Nevertheless, clinical evidence about the safety and efficacy of SCB in the treatment of CBL is lacking.
In the present study, a novel SCB, which used magnesium stearate and butylated hydroxytoluene as excipients, demonstrating improved drug delivery and bioavailability, was compared with a commercially available PCB9. We sought to investigate the role of this novel SCB in the treatment of bifurcation lesions, aiming to generate evidence for the improvement of DCB therapy in the future.
Methods
Study design and patient population
The sirolimus-coated versus paclitaxel-coated balloons for bifurcated coronary lesions in the side branch (SPACIOUS) study is a prospective, multicentre, randomised, non-inferiority study conducted at 14 hospitals in China. This study was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov: NCT04899583. The study protocol was approved by independent ethics committees responsible for each participating centre. All patients gave written informed consent as soon as the diagnostic catherisation and lesion preparation were qualified for enrolment. This manuscript adheres to the CONSORT guidelines for reporting (Supplementary Appendix 1, Supplementary Table 1).
Patients at least 18 years old with de novo non-left main true bifurcation lesions (Medina bifurcation classification 1,1,1, 1,0,1, or 0,1,1) and stable angina, acute coronary syndrome, or asymptomatic myocardial ischaemia were considered for enrolment (Figure 1, Central illustration). Patients with SB stenosis >70% after MV stenting were included, and lesion preparation was mandatory before enrolment. The key exclusion criteria included ST-segment elevation myocardial infarction, New York Heart Association Class IV, intolerance of antiplatelet medication, and SB predilation failure (Thrombolysis in Myocardial Infarction [TIMI] flow grade ≤2 or National Heart, Lung, and Blood Institute [NHLBI] more than type B dissection). All patients were treated by MV stenting first under SB protection with a jailed guidewire or balloon. Then, the SB was rewired and predilated with regular balloons, which was mandatory. A total of 43 patients were not eligible because of predilation failure; out of these 43, 31 had NHLBI more than type B dissection, and 12 had TIMI flow grade ≤2 due to severe residual stenosis. After successful target lesion preparation, central randomisation was conducted with a computer-generated allocation sequence. Patients were consecutively enrolled from October 2021 to October 2022 and randomised (1:1) to treatment with a novel SCB (4 μg of sirolimus/mm² with magnesium stearate and butylated hydroxytoluene as excipients [Acotec]) or a commercially available PCB (3 μg/mm2 paclitaxel incorporated in a matrix of iohexol; Bingo [Yinyi Biotech]).
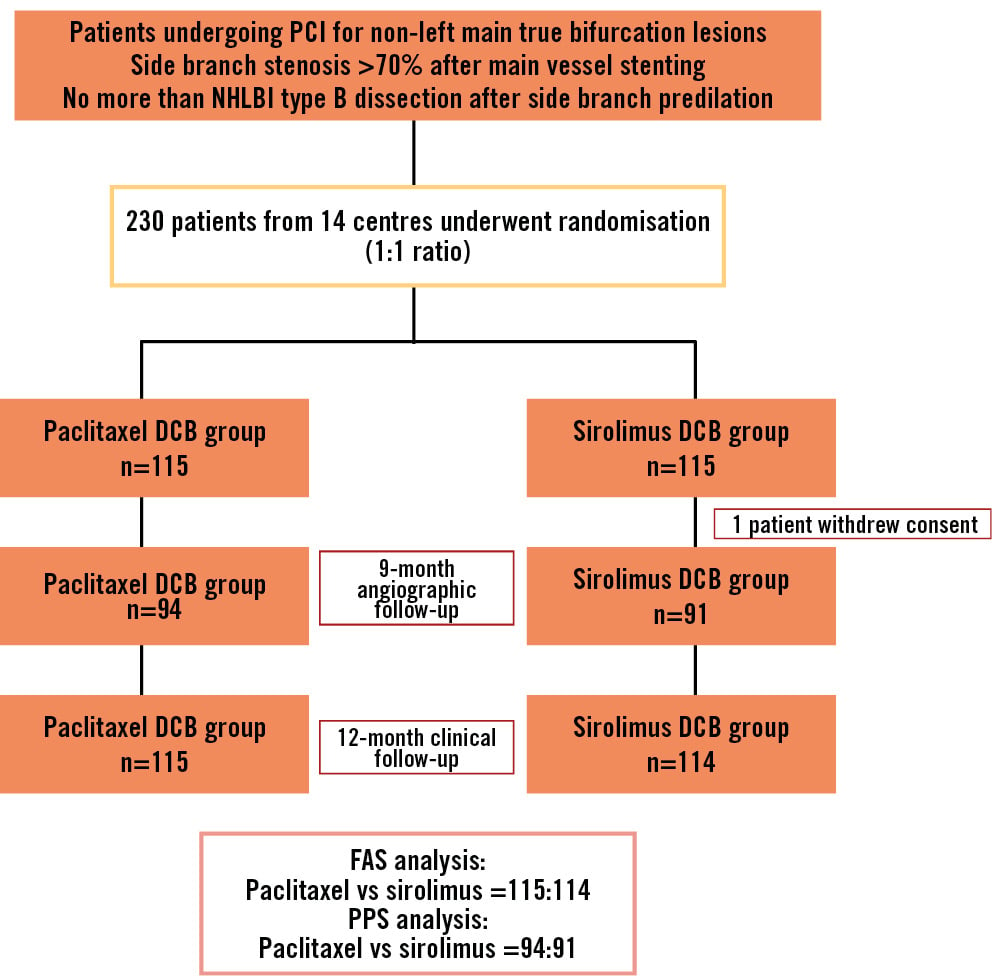
Figure 1. Flowchart of the study. DCB: drug-coated balloon; FAS: full analysis set; NHLBI: National Heart, Lung, and Blood Institute; PCI: percutaneous coronary intervention; PPS: per-protocol set
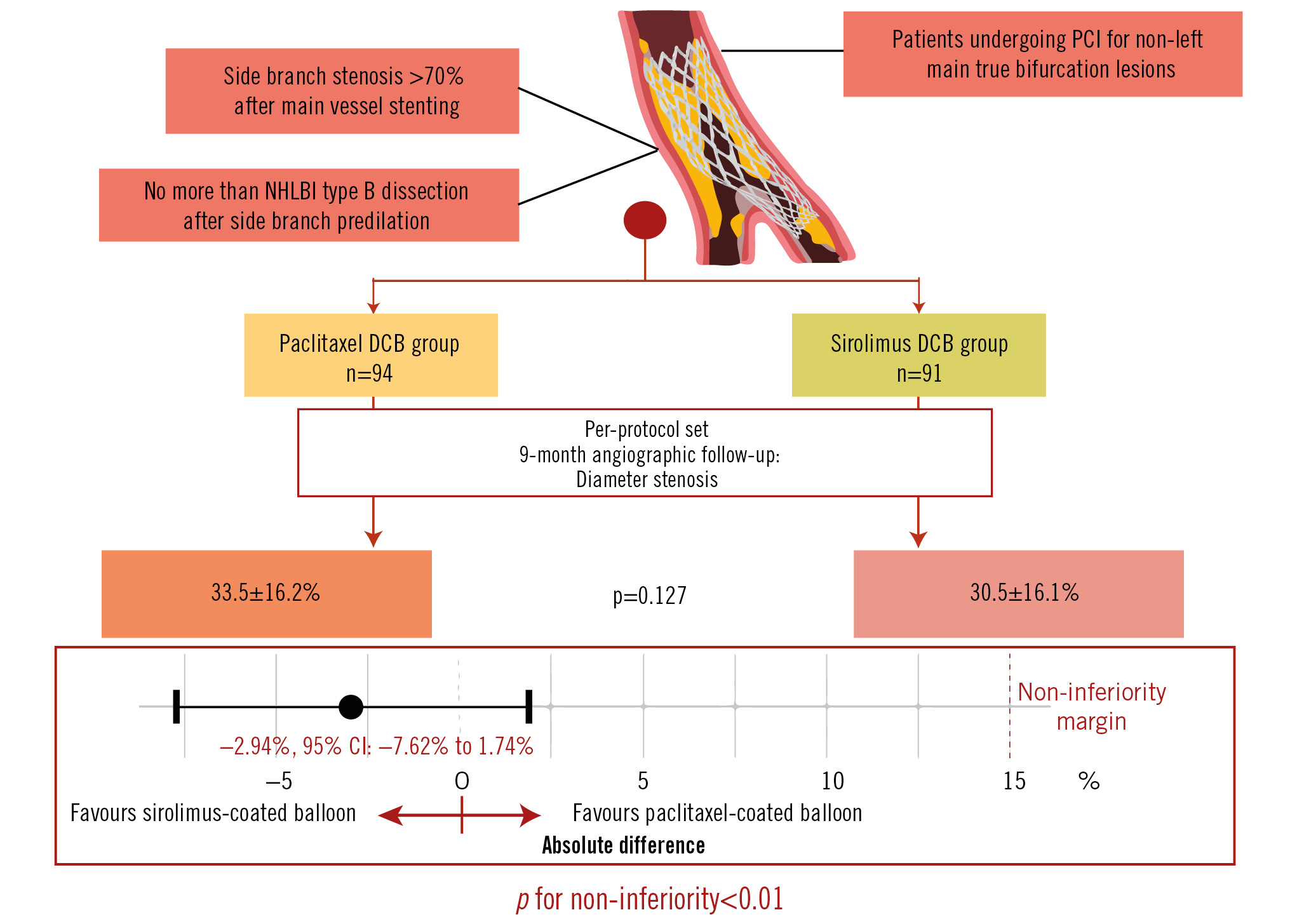
Central illustration. The SPACIOUS trial: sirolimus-coated versus paclitaxel-coated balloons for bifurcated coronary lesions in the side branch. DCB: drug-coated balloon; NHLBI: National Heart, Lung, and Blood Institute; PCI: percutaneous coronary intervention
PCI procedures
Coronary angiography and intervention were performed according to usual hospital practice. All patients were on dual antiplatelet therapy at the time of PCI. Procedural anticoagulation was achieved with unfractionated heparin (70 to 100 U/kg intravenous bolus with dose adjustment to maintain an activated clotting time of about 300 s). The study DCB was inflated for 60 to 90 s at nominal pressure, according to the characteristics of the lesion. After DCB dilation, kissing balloon inflation (KBI) and proximal optimisation techniques (POT) were carried out at the operators’ discretion. After the procedure, all patients received dual antiplatelet therapy (aspirin [or indobufen] and a P2Y12 inhibitor [clopidogrel or ticagrelor]) for at least 12 months.
Quantitative coronary angiography
Pre-PCI, post-PCI, and follow-up angiograms were analysed by personnel blinded to patient assignment at the central core laboratory (CoreMed, Beijing, China). Angiographic measurements were taken along the entire length of the study device at the target lesion site. At least 2 orthographic views were required for each lesion before the intervention. Accurate DCB location angiograms were obtained before DCB dilation. Two postprocedural and follow-up angiograms were obtained with similar projection angles to the predilation angiograms. Quantitative coronary angiography (QCA) was performed under the same standard conditions using the QAngio XA system 7.3 (Medis Medical Imaging Systems). The following parameters were obtained: reference diameters of the MV and SB; minimal lumen diameters (MLD) of the MV and SB; and percentage diameter stenosis (DS, defined as [1–MLD/reference vessel diameter]×100%) of the MV and SB.
Follow-up and endpoints
Angiographic and clinical follow-ups were planned at 9 months and 1 year post-procedure, respectively. Clinical endpoints and adverse events were evaluated in consensus by the investigators. The primary endpoint was DS in the SB at 9 months. Secondary endpoints included (1) device success (defined as successful delivery, inflation and withdrawal of the study DCB with residual stenosis ≤30% and TIMI flow grade 3 in the target lesion); (2) procedural success (defined as lesion success with the absence of cardiac death, target vessel-related myocardial infarction and target lesion revascularisation during hospitalisation); (3) LLL (the difference between postprocedural and follow-up in-device MLD); (4) net lumen gain (NLG, defined as the follow-up MLD minus the postprocedural MLD); (5) binary restenosis (>50% DS); (6) the device-oriented composite endpoint (DoCE; a composite of cardiac death, target vessel-related myocardial infarction and ischaemia-driven target lesion revascularisation); and (7) the patient-oriented composite endpoint (PoCE; a composite of all-cause death, myocardial infarction and ischaemia-driven revascularisation).
Statistical analysis
This study aims to show non-inferiority of the SCB in comparison with the PCB in terms of DS. Based on previous studies and animal experiments, the study’s sample size was determined based on the assumption that DS at 9 months would be 34.7% and 28.7% in the SCB and PCB groups, respectively, with a common standard error of 21%91617. The least-square mean difference was computed with a 95% confidence interval (CI). A non-inferiority margin of 15% was used to test the mean difference between the SCB and PCB groups. At least 86 patients per group would need to be enrolled in the study to have an alpha error of 0.025 and power of 80%. To compensate for possible failure or dropout (about 25% of the cohort), the sample size was increased to 115 patients per group, i.e., a total sample size of 230 patients. The full analysis set (FAS) and per-protocol set (PPS) were used for further analysis. The FAS is the main population evaluated in clinical outcomes reporting. The PPS was meant for angiographic analysis excluding patients who violated the protocol, were lost to follow-up, or did not meet the primary endpoint.
Continuous variables are expressed as the mean±standard deviation or median (interquartile range [IQR]). Discrete variables are presented as counts (percentages). Continuous variables were compared by the Student’s t-test or Mann-Whitney U test accordingly. Chi-square tests or Fisher’s exact test were used to compare discrete variables when appropriate. The incidence of clinical adverse events over time was analysed using Kaplan-Meier analysis and compared with log-rank tests. The impact of the study DCB on clinical prognosis was assessed with the Cox regression model. Analysis was carried out using the open-source software R, version 4.2.0 (R Foundation for Statistical Computing) and SPSS, version 25.0 (IBM). A 2-sided p<0.05 was defined to be statistically significant.
Results
Patient and procedural characteristics
A total of 230 patients were enrolled between 2021 and 2022 and randomly allocated in a 1:1 ratio to the SCB and PCB groups. One patient in the SCB group withdrew consent one day after the procedure and was excluded from the FAS population. The median age of the patients was 66 (IQR 58-71) years. Overall, 171 (74.7%) patients were male, and 68.6% presented with acute coronary syndrome. More than one-half of the patients had hypertension (65.1%), and 32.3% had diabetes mellitus. The PPS population comprised 91 patients in the SCB group and 94 patients in the PCB group, all of whom completed the 9-month angiographic follow-up. The study flowchart is shown in Figure 1. Demographic (Table 1) and procedural (Table 2) characteristics are presented for the FAS and PPS populations. Baseline parameters were similar between the SCB and PCB groups, except that more patients in the SCB group had undergone prior PCI (p<0.05).
One DCB per lesion was utilised in all cases. The comparisons of stent diameter, stent length, DCB diameter, length, and inflation parameters showed no significant differences (allp>0.05). The prevalence of KBI and POT did not differ between the groups. TIMI flow grade 3 was observed in all vessels. No patient required bailout stent implantation. QCA analysis revealed no significant differences in pre- or postprocedural SB stenosis. Both groups had similar reference vessel diameters, and pre- and postprocedural MLD for the MV and the SB (allp>0.05) (Figure 2).
Table 1. Demographics and clinical characteristics.
| FAS | PPS | |||||
|---|---|---|---|---|---|---|
| Paclitaxel DCB group (n=115) | Sirolimus DCB group (n=114) | p-value | Paclitaxel DCB group (n=94) | Sirolimus DCB group (n=91) | p-value | |
| Age, years | 64 (57-69) | 66 (60-71) | 0.055 | 65 (57-69) | 66 (60-71) | 0.080 |
| Male | 86 (74.8) | 85 (74.6) | 0.969 | 69 (73.4) | 69 (75.8) | 0.705 |
| Hypertension | 72 (62.6) | 77 (67.5) | 0.433 | 59 (62.8) | 60 (65.9) | 0.653 |
| Diabetes mellitus | 36 (31.3) | 38 (33.3) | 0.743 | 30 (31.9) | 28 (30.8) | 0.867 |
| Dyslipidaemia | 34 (29.6) | 34 (29.8) | 0.966 | 31 (33.0) | 26 (29.6) | 0.516 |
| Smoking history | 61 (53.0) | 49 (43.0) | 0.129 | 47 (50.0) | 41 (46.6) | 0.501 |
| Body mass index, kg/m2 | 24.2 (22.4-26.6) | 24.4 (22.8-26.9) | 0.239 | 24.2 (22.2-26.6) | 24.4 (22.7-26.3) | 0.288 |
| Prior myocardial infarction | 9 (7.8) | 14 (12.3) | 0.262 | 3 (3.2) | 12 (13.2) | 0.013* |
| Prior PCI | 27 (23.5) | 44 (38.6) | 0.013* | 18 (19.1) | 33 (36.3) | 0.009* |
| Prior CABG | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Prior stroke | 11 (9.6) | 6 (5.3) | 0.214 | 11 (11.7) | 4 (4.4) | 0.069 |
| Presentation | ||||||
| Stable angina pectoris | 33 (28.7) | 39 (34.2) | 0.369 | 25 (26.6) | 33 (36.3) | 0.156 |
| Unstable angina pectoris | 72 (62.6) | 65 (57.0) | 0.388 | 60 (63.8) | 50 (54.9) | 0.219 |
| NSTEMI | 10 (8.7) | 10 (8.8) | 0.984 | 9 (9.6) | 8 (8.8) | 0.854 |
| STEMI | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| NYHA/Killip | ||||||
| Class I | 69 (60.0) | 69 (60.5) | 0.935 | 59 (62.8) | 56 (61.5) | 0.863 |
| Class II | 40 (34.8) | 40 (35.1) | 0.961 | 29 (30.9) | 32 (35.2) | 0.533 |
| Class III | 6 (5.2) | 5 (4.4) | 0.769 | 6 (6.4) | 3 (3.3) | 0.497 |
| Class IV | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| White blood cell, x10^9/L | 6.10 (5.27-7.56) | 6.49 (5.45-7.65) | 0.293 | 6.16 (5.23-7.83) | 6.40 (5.39-7.57) | 0.920 |
| Platelet, x10^9/L | 214 (179-250) | 201 (168-243) | 0.300 | 215 (180-249) | 200 (162-234) | 0.127 |
| Haemoglobin, g/L | 138 (127-148) | 138 (129-148) | 0.674 | 139 (127-148) | 138 (127-149) | 0.968 |
| CK-MB, U/L | 11 (6-15) | 11 (3-15) | 0.700 | 11 (2-15) | 11 (3-15) | 0.683 |
| Creatine, µmol/L | 71 (61-84) | 76 (64-89) | 0.219 | 72 (60-84) | 77 (67-88) | 0.068 |
| Data are shown as median (interquartile range) or n (%). *Indicates statistical significance. CABG: coronary artery bypass grafting; CK-MB: creatine kinase-myocardial band; DCB: drug-coated balloon; FAS: full analysis set; NA: not applicable; NSTEMI: non-ST-segment elevation myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PPS: per-protocol set; STEMI: ST-segment elevation myocardial infarction | ||||||
Table 2. Lesion and procedural features.
| FAS | PPS | |||||
|---|---|---|---|---|---|---|
| Paclitaxel DCB group (n=115) | Sirolimus DCB group (n=114) | p-value | Paclitaxel DCB group (n=94) | Sirolimus DCB group (n=91) | p-value | |
| Medina classification of lesions | ||||||
| 1,1,1 | 105 (91.3) | 99 (86.8) | 0.279 | 86 (91.5) | 78 (85.7) | 0.157 |
| 1,0,1 | 3 (2.6) | 2 (1.8) | 1.000 | 3 (3.2) | 2 (2.2) | 1.000 |
| 0,1,1 | 7 (6.1) | 12 (11.4) | 0.223 | 5 (5.3) | 11 (12.1) | 0.102 |
| Target vessel | ||||||
| LAD-D | 102 (88.7) | 99 (86.8) | 0.669 | 84 (89.4) | 81 (89.0) | 0.939 |
| LCx-OM | 8 (7.0) | 8 (7.0) | 0.986 | 6 (6.4) | 5 (5.5) | 0.798 |
| RCA-PL/PDA | 2 (1.7) | 6 (5.3) | 0.146 | 2 (2.1) | 4 (4.4) | 0.439 |
| Others | 3 (2.6) | 1 (0.9) | 0.622 | 2 (2.1) | 1 (1.1) | 1.000 |
| Main vessel stenosis, % | 66.2±11.4 | 66.8±10.9 | 0.625 | 65.3±10.7 | 66.5±10.8 | 0.426 |
| Stent length, mm | 33 (25-46) | 33 (24-43) | 0.893 | 32 (24-43) | 34 (25-44) | 0.379 |
| Average stent diameter, mm | 3.00 (2.75-3.06) | 3.00 (2.75-3.12) | 0.557 | 3.00 (2.75-3.00) | 3.00 (2.75-3.12) | 0.348 |
| Side branch stenosis, % | 58.6±15.2 | 56.6±17.1 | 0.629 | 57.3±16.0 | 55.2±17.4 | 0.522 |
| Side branch stenosis after main vessel stenting, % | 84.7±8.1 | 83.2±8.5 | 0.224 | 84.6±8.1 | 83.2±8.6 | 0.295 |
| Side branch lesion length, mm | 13.2±4.4 | 14.0±4.5 | 0.174 | 13.2±4.5 | 13.8±4.5 | 0.564 |
| Procedural characteristics | ||||||
| Diameter of DCB, mm | 2.00 (2.00-2.50) | 2.00 (2.00-2.50) | 0.668 | 2.00 (2.00-2.50) | 2.00 (2.00-2.50) | 0.755 |
| Length of DCB, mm | 20 (15-20) | 20 (20-20) | 0.074 | 20 (15-20) | 20 (20-20) | 0.083 |
| Maximal inflation pressure with DCB, atm | 10 (8-10) | 8 (7.5-10) | 0.086 | 10 (8-10) | 8 (7-10) | 0.053 |
| Duration of inflation with DCB, s | 62.2±9.2 | 61.2±6.0 | 0.795 | 62.8±10.0 | 61.6±6.7 | 0.640 |
| Kissing balloon inflation | 48 (41.7) | 55 (48.2) | 0.322 | 35 (37.2) | 44 (48.4) | 0.126 |
| Proximal optimisation technique | 61 (53.0) | 52 (45.6) | 0.261 | 53 (56.4) | 39 (42.9) | 0.066 |
| Bailout stenting | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Post-procedure | ||||||
| Residual stenosis in side branch, % | 19.2±8.2 | 17.2±8.9 | 0.282 | 20.3±7.6 | 17.1±9.0 | 0.474 |
| Residual dissection in side branch (NHLBI) | ||||||
| Type A | 3 (2.6) | 4 (3.5) | 0.722 | 3 (3.2) | 3 (3.3) | 1.000 |
| Type B | 0 (0) | 1 (0.9) | 1.000 | 0 (0) | 0 (0) | NA |
| TIMI flow grade III | 115 (100) | 114 (100) | NA | 94 (100) | 91 (100) | NA |
| Lesion success | 115 (100) | 114 (100) | NA | 94 (100) | 91 (100) | NA |
| Procedural success | 115 (100) | 113 (99.1) | 0.498 | 94 (100) | 90 (98.9) | 0.492 |
| Data are shown as mean±standard deviation, median (interquartile range) or n (%). D: diagonal branch; DCB: drug-coated balloon; FAS: full analysis set; LAD: left anterior descending artery; LCx: left circumflex artery; NA: not applicable; NHLBI: National Heart, Lung, and Blood Institute; OM: obtuse marginal branch; PDA: posterior descending artery; PL: posterolateral; PPS: per-protocol set; RCA: right coronary artery; TIMI: Thrombolysis in Myocardial Infarction | ||||||
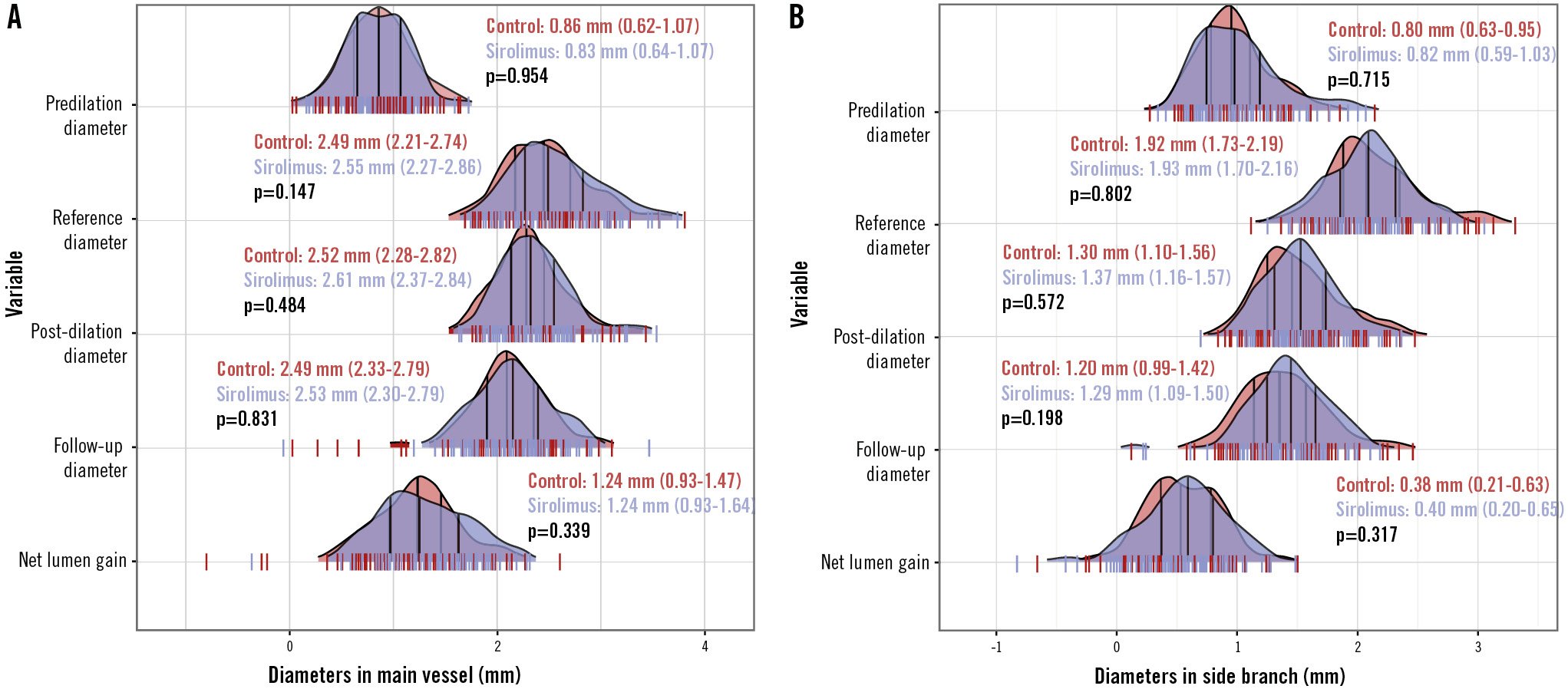
Figure 2. Ridge plot of the angiographic parameters. The distribution of baseline and follow-up angiographic parameters in the main vessel (A) and side branch (B). Vertical lines within the ridge plot represent the median and interquartile range.
Angiographic outcomes (PPS)
As presented in Figure 2, the measurements of MLD and NLG were all comparable at 9 months regarding both the MV and the SB in the PPS population (allp>0.05). The percentage DS was 30.5±16.1% in the SCB group versus 33.5±16.2% in the PCB group (p=0.127) (Figure 3A). The mean difference between the SCB and PCB groups was found to be −2.94% with 95% CI of −7.62% to 1.74%. The upper limit of the 95% CI was within the predefined margin of 15%, hence the result met the criteria for non-inferiority in the primary endpoint (p<0.01) (Central illustration). LLL was not significantly different between the groups (0.09 vs 0.09 mm; p=0.598) (Figure 3B). Of note, the incidence of binary stenosis was significantly lower in the sirolimus group compared with the paclitaxel group (4.4% vs 12.8%; p=0.043) (Figure 3C).
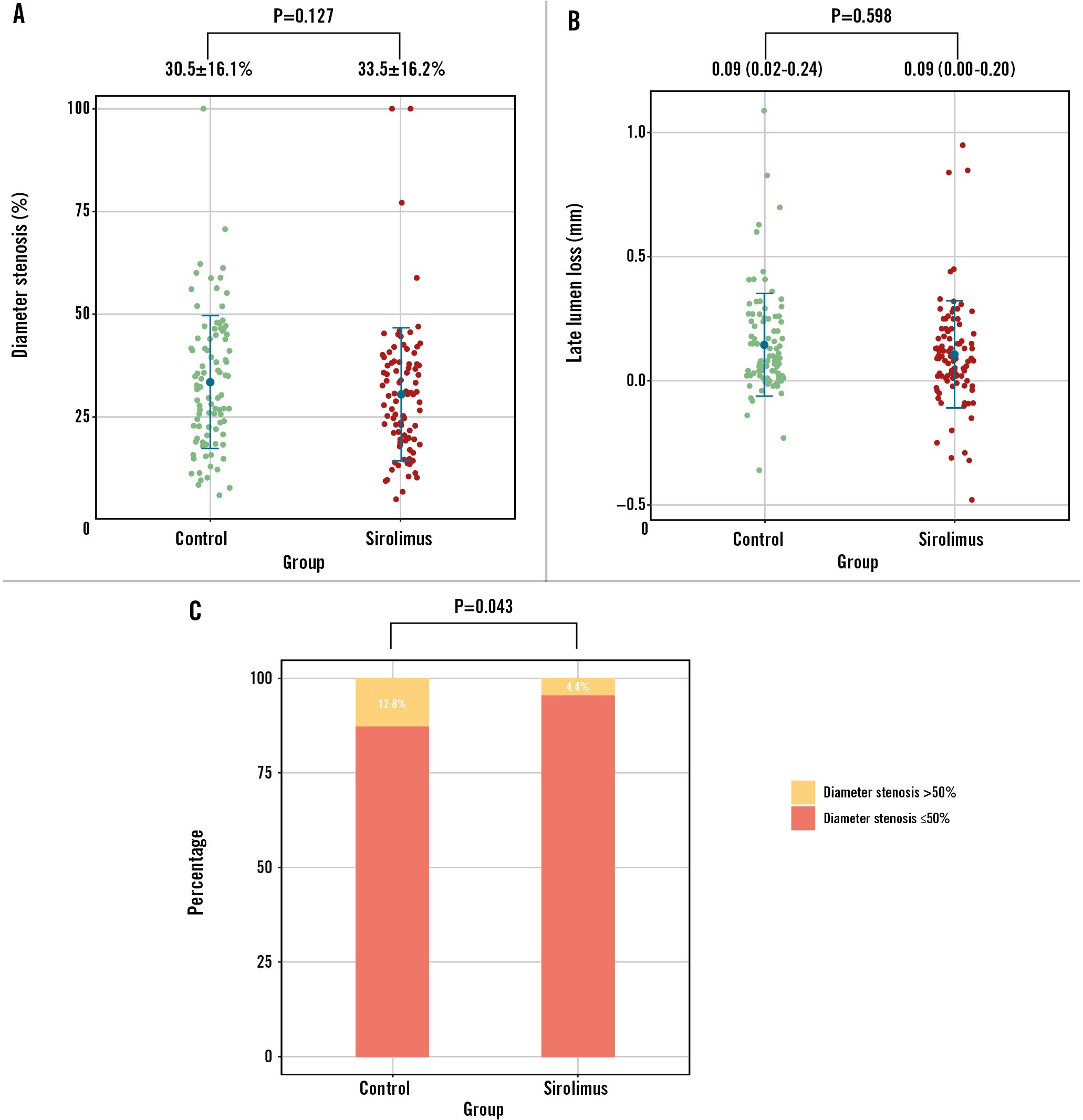
Figure 3. Angiographic endpoints at 9-month follow-up. The percentage of diameter stenosis (A), late lumen loss (B), and binary restenosis (C) at follow-up were plotted. The primary endpoint met the criteria for non-inferiority. The incidence of binary stenosis was significantly lower in the sirolimus group in comparison with the paclitaxel group.

Central illustration. The SPACIOUS trial: sirolimus-coated versus paclitaxel-coated balloons for bifurcated coronary lesions in the side branch. DCB: drug-coated balloon; NHLBI: National Heart, Lung, and Blood Institute; PCI: percutaneous coronary intervention
Clinical endpoints (FAS)
Device success was achieved in all cases. The procedure was successful in all patients in the PCB group, and in the SCB group, only one patient experienced procedural failure: target vessel-related myocardial infarction requiring revascularisation. There were no cardiac deaths in either group. The rates of clinical endpoints, including death, myocardial infarction and revascularisation, were similar between the groups (Table 3). Cox regression analysis demonstrated that, compared with PCB, SCB did not increase the risks of adverse clinical events (allp>0.05). Figure 4 shows the cumulative incidence of DoCE and PoCE in both groups, and the differences were not statistically significant in Kaplan-Meier analysis.
Table 3. Clinical outcomes in the full analysis set at 9-month follow-up.
| Total (n=229) | Control (n=115) | Sirolimus (n=114) | Hazard ratio | p-value | |
|---|---|---|---|---|---|
| All-cause death | 2 (0.9) | 0 (0) | 2 (1.8) | 66.2 | 0.470 |
| Cardiac death | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| Myocardial infarction | 3 (1.3) | 2 (1.7) | 1 (0.9) | 0.51 | 0.578 |
| Target vessel-related myocardial infarction | 2 (0.9) | 1 (0.9) | 1 (0.9) | 1.01 | 0.993 |
| Revascularisation | 35 (15.3) | 22 (19.1) | 12 (11.4) | 0.59 | 0.124 |
| Target lesion-related revascularisation | 9 (3.9) | 6 (5.2) | 3 (2.6) | 0.50 | 0.321 |
| DoCE* | 10 (4.4) | 7 (6.1) | 3 (2.6) | 0.43 | 0.220 |
| PoCE† | 37 (16.2) | 22 (19.1) | 15 (13.2) | 0.69 | 0.267 |
| Data are shown as n (%). *A composite of cardiac death, target vessel-related myocardial infarction and target lesion revascularisation. †A composite of all-cause death, myocardial infarction and revascularisation. DoCE: device-oriented composite endpoint; NA: not applicable; PoCE: patient-oriented composite endpoint | |||||
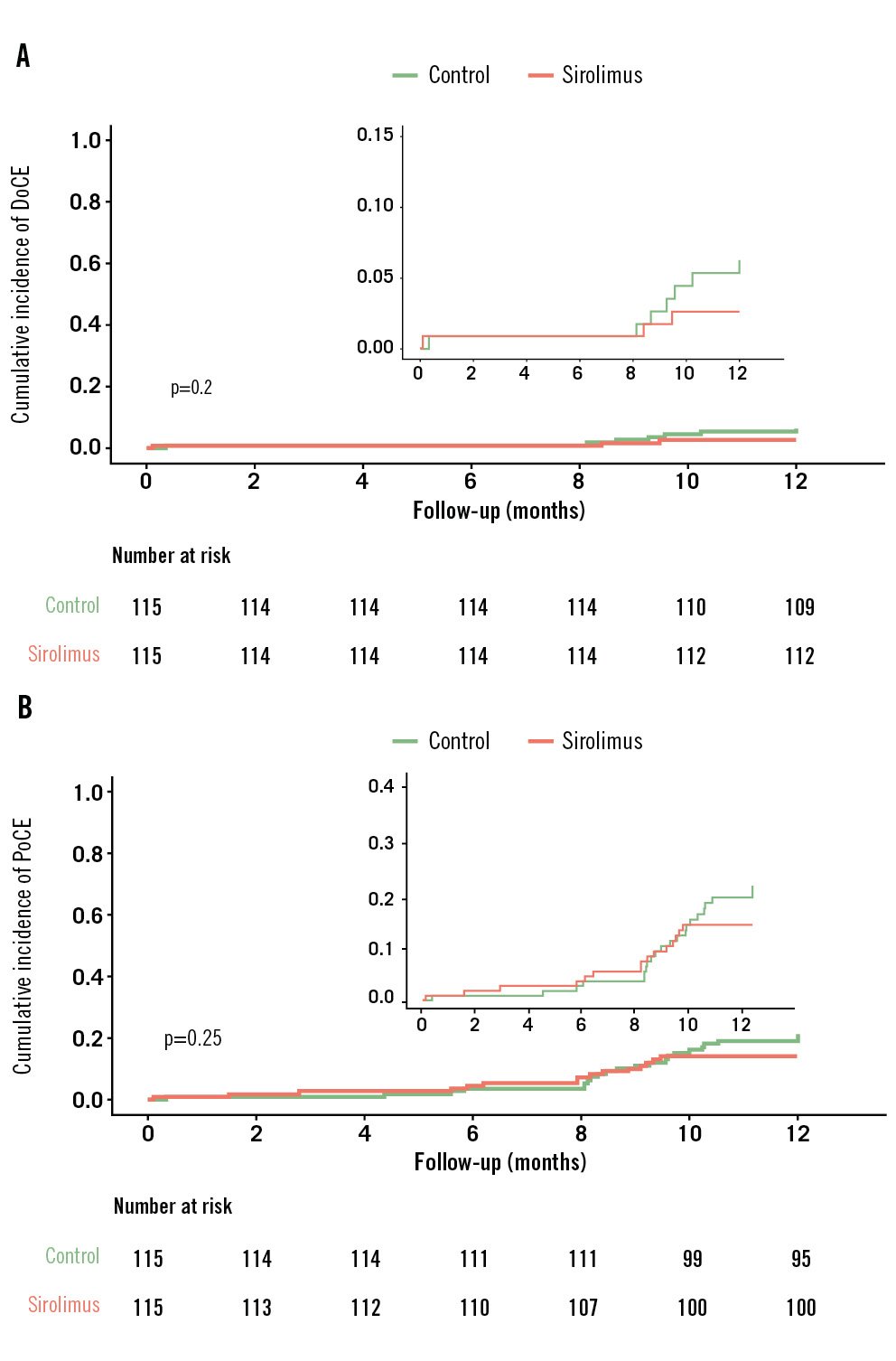
Figure 4. Time-to-event curve for clinical endpoints in the full analysis set population. The cumulative incidence of the clinical composite endpoints, DoCE (A) and PoCE (B), in the sirolimus- and paclitaxel-coated balloon groups at 1 year. The event rates were calculated using Kaplan-Meier methodology and compared with the log-rank test. DoCE: device-oriented composite endpoint; PoCE: patient-oriented composite endpoint
Discussion
The present SPACIOUS trial demonstrated that in the treatment of de novo non-left main bifurcated lesions, the novel sirolimus DCB was non-inferior to the paclitaxel DCB in terms of 9-month DS. Besides, the incidence of adverse clinical events was comparable between both treatment groups up to 1 year, indicating a safe profile of the study SCB.
CBL, commonly encountered in up to 20% of PCI, remain one of the most challenging lesion subsets in terms of technical complexity and long-term clinical outcomes18. Generally, MV-only stenting with provisional SB stenting is the recommended approach for most bifurcation lesions5619. A DCB-only strategy has also been attempted in CBL intervention. In de novo bifurcation lesions (Medina classification 0,X,X), the DCB-only strategy demonstrated feasibility with low rates of restenosis; this approach may be preferable to plain balloon angioplasty for SB or distal main branch lesions, taking into account LLL in the treated area20. In patients with high bleeding risk, complex vascular anatomy that is unsuitable for stent deployment or critical conditions that cannot tolerate a prolonged operation, DCB could be a promising approach in CBL treatment. However, DCB dilation without stenting needs to be conducted in the absence of severe complications, such as flow-limiting dissection and prominent residual stenosis. More evidence is required to precisely identify patients who could benefit from this strategy.
Paclitaxel and limus-based drug-eluting stents have been widely investigated in clinical trials. However, MV stenting in combination with SB DCB dilation, with non-paclitaxel DCB in particular, for the treatment of bifurcation lesions has not been broadly validated yet. Besides, it is unclear whether sirolimus represents an alternative to paclitaxel for DCB coating. The studied SCB innovatively used magnesium stearate and butylated hydroxytoluene as excipients, which carried high drug concentrations and improved drug bioavailability. In this setting, our study provided important insights in two respects: (1) compared with the previous studies using 1- or 2-stent techniques, MV stenting plus SB DCB did not significantly increase the incidence of procedural complications or adverse events; therefore, this strategy seems to be a safe and effective alternative option in CBL treatment32122; and (2) although late luminal gain was frequently observed after PCB treatment13, improved coating technology could enable sirolimus or other limus-based drugs to be competitive substitutes. However, large-scale studies are needed to draw firm conclusions.
Although LLL and diameter stenosis were not significantly different between the groups, we noticed the mean values of these parameters were slightly higher in the PCB group. In addition, although not significant either, the side branch stenosis before DCB inflation was slightly higher in the PCB group. The numerically inferior performance of PCB may be partially due to a more severe baseline side branch stenosis in patients randomised to paclitaxel-coated balloons. As a result, the patients in the PCB group may have been more likely to have restenosis >50%. Binary restenosis only roughly defined the patients by a preset cutoff point and could not fairly represent the efficacy of the studied SCB.
In CBL treatment, routine SB dilation is not recommended, except in the cases such as severe stenosis and when there is calcified plaque in the SB. As the study flowchart shows, only patients with SB stenosis >70% after MV stenting were included, and lesion preparation was mandatory before enrolment. Each lesion was predilated according to the operators’ preferences, using plain, cutting, or scoring balloons. Probably due to the relatively small size of the SB (median diameter of 1.9 mm), almost all predilation was performed with plain balloons in this study. Hence, we could not draw conclusions on the impact of the type of predilation balloon based on the present findings. However, adequate lesion preparation with proper techniques should be considered to improve the safety and efficacy of DCB. It was reported that lesion preparation with non-compliant, super non-compliant or cutting balloons before SCB dilation was feasible23. In addition, the potential influence of variability in lesion preparation on drug uptake and outcomes of the studied SCB needs to be verified in larger cohorts with longer follow-up.
The PEPCAD-BIF trial indicated the use of DCB is a sound strategy in bifurcation lesions with type A or B dissection according to the NHLBI classification20. After predilation, patients with NHLBI more than type B dissection in the SB were excluded from this study. Besides, only a few patients had residual dissection after DCB treatment. The low rate of residual dissection, on the one hand, could contribute to the low risk of abrupt closure of the target vessel and high procedural success rate. On the other hand, it raises a doubt as to whether the lesions were adequately dilated. Since this is the first-in-human study of this novel SCB, the operators might have been more prudent during the procedure to avoid balloon overexpansion. Patients with high-risk lesions, such as calcified and tortuous lesions which would be prone to dissection, may not have been amply enrolled. In previous studies focusing on paclitaxel-coated balloons, a non-flow-limiting larger dissection immediately after dilation was strongly associated with late lumen enlargement2425. It remains unclear whether luminal enlargement also occurs with sirolimus. Future studies investigating sirolimus DCB with more rigorous treatment strategies combined with intravascular imaging, such as intravascular ultrasound and optical coherence tomography, are warranted.
KBI and POT were performed in approximately half of the cases in the present study. POT was conducted mostly following KBI. The percentage of patients who underwent either KBI or POT was about 60%; this was comparable between the groups. About 10% of patients were not suitable for POT due to a short stent length proximal to the SB ostium (data not shown). In the treatment of CBL, routine KBI after provisional stenting did not provide clear clinical benefits1182627. It should be acknowledged that the balloons used in KBI were not restricted in this study. Hence, whether semicompliant or non-compliant balloons were used could be a confounding factor, considering the impact of KBI on the prognosis. Although not mandatory, POT was generally recommended in CBL treatment628. It has also been proposed that a “POT-SB dilation-POT” strategy would provide better circular geometry2930. A non-uniform optimisation strategy after DCB dilation may influence angiographic and clinical outcomes. However, this study was not intended to compare different optimisation techniques. Since the rates of KBI and POT were comparable between the groups, it is unlikely that these techniques significantly impacted the conclusion that SCB were not inferior to PCB in treating SB bifurcation lesions. Subgroup analysis of patients receiving optimisation techniques after DCB dilation (KBI or POT), or not, revealed comparable angiographic and clinical outcomes (data not shown). However, this study was not powered to draw solid conclusions. Studies are needed in the future with uniform regulations of optimisation techniques.
The PCB in this study is officially approved and widely used in China but has not been directly compared with other well-known PCB in other populations. Hence, different results might have been achieved with other PCB in different populations. Besides, it should be acknowledged that our findings cannot be extended to all SCB with different sirolimus formulations, since there is no class effect for either PCB or SCB.
Limitations
Some potential limitations should be considered. First, this is a moderate-sized trial with relatively short follow-up. However, this is comparable to previous studies. Although some patients dropped out, angiographic follow-up was achieved in 80% of patients, which is in line with similar studies. Real-world evidence is required to confirm the safety and efficacy of the studied DCB. Second, considering this studied novel SCB was used for the first time in humans, very high-risk patients were excluded from this study, such as those with ST-segment elevation myocardial infarction or left main bifurcation lesions. Therefore, the findings may not be transferred to these scenarios. Third, in comparison with previous studies, the median diameter of the SB is relatively small. As such, these vessels probably supply a small amount of myocardium and may not have been clinically relevant. This may partially be due to the fact that this is pioneering in-human research on the novel SCB. Considering DCB were officially established as a therapeutic option for the treatment of in-stent restenosis and small-vessel disease, patients with critically large SB may not have been adequately enrolled. Fourth, although ischaemia evidence (symptoms or physiological assessment) was required before revascularisation, the potential impact of the oculostenotic reflex during angiographic follow-up could not be completely eliminated, which may have influenced the clinical outcomes. Fifth, as we mentioned above, intravascular imaging was absent, and its incorporation should be considered in future studies.
Conclusions
The novel sirolimus DCB showed non-inferior angiographic and clinical outcomes compared with the paclitaxel DCB in de novo non-left main true bifurcated lesions.
Impact on daily practice
Drug-coated balloons are emerging as a promising strategy to treat the side branch in bifurcated coronary lesions. In the treatment of de novo non-left main bifurcated lesions, the studied sirolimus-coated balloons were non-inferior to the paclitaxel-coated balloons in terms of 9-month diameter stenosis. Limus-coated balloons are an attractive alternative to paclitaxel-coated balloons.
Acknowledgements
The authors thank the dedicated efforts of the clinical research collaborators in the SPACIOUS study organisation and the contributions of the participating centres.
Funding
This study was funded by the National Natural Science Foundation of China (Grant no: 82370261) and Acotec, China. The funding parties were not involved in either the analysis or interpretation of the data nor writing of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest pertaining to this submission to declare.
Supplementary data
To read the full content of this article, please download the PDF.

