Cory:
Unlock Your AI Assistant Now!
Drug-coated balloon (DCB) angioplasty for treating the side branch was proposed several years ago12. While still ensuring the delivery of an antiproliferative medication to mitigate reactive post-percutaneous coronary intervention (PCI) neointimal proliferation, in comparison with drug-eluting stents (DES), side branch DCB angioplasty avoids a permanent metallic implant and may potentially reduce major stent-related complications, such as restenosis and thrombosis, particularly at the side branch ostium. In addition, a DCB-based strategy may help preserve the original bifurcation anatomy and reduce main branch stent strut deformation at the carina. Nevertheless, only a few, small, randomised trials assessing DCB angioplasty for coronary bifurcation disease have been conducted thus far, and, in the available trials, DCB angioplasty was employed in the context of combined interventional strategies and tested against heterogeneous comparators (Table 1).
Over the past decade, an increasing number of DCBs have been introduced, featuring differences in antiproliferative medications, excipients, and balloon catheters1. Accumulated data on DCBs and head-to-head comparisons have revealed no class effect, with some devices proving more effective than others34. In this regard, the TRANSFORM and REFORM trials failed to show angiographic non-inferiority of sirolimus- and biolimus-based DCBs compared to paclitaxel-based DCBs, leaving the potential advantages in antirestenotic effectiveness, and especially safety, of limus-based DCBs largely unconfirmed34.
In this issue of EuroIntervention, Zhou and colleagues report the results of the randomised, multicentre SPACIOUS trial, in which 230 patients with non-left main true bifurcation disease who had undergone main branch stenting and successful side branch predilation were assigned to sirolimus- or paclitaxel-based DCB angioplasty for the side branch treatment5.
At 9-month angiography follow-up, the mean percentage diameter stenosis was 30.5±16.1% in the sirolimus-based DCB group and 33.5±16.2% in the paclitaxel-based DCB group, resulting in a least-square mean difference of −2.94%, with a 95% confidence interval −7.62% to 1.74%5. The upper limit of the 95% confidence interval of 1.74% was significantly below the prespecified non-inferiority margin of 15.0% (pnon-inferiority<0.01)5. The prevalence of post-DCB kissing balloon inflation and proximal optimisation technique did not significantly differ between groups5. The rate of non-adherence to the angiography follow-up was approximately 20%, but the investigators should be commended for having anticipated this potential issue and enrolling enough patients to preserve the original statistical power. Consistent with the primary outcome, late lumen loss was not significantly different between the sirolimus- and paclitaxel-based DCB groups (0.09 [interquartile range 0.02 to 0.24] vs 0.09 [interquartile range 0.00 to 0.20]; p=0.598)5. However, binary restenosis was significantly lower in the sirolimus-based DCB group compared with the paclitaxel-based DCB group (4.4% vs 12.8%; p=0.043)5. Interpreting this finding in the context of non-significant differences in percentage diameter stenosis and late lumen loss is challenging.
The important conclusions of the SPACIOUS trial enhance the amount of data on DCB angioplasty for coronary bifurcation disease and may support the use of DCBs for treating the side branch (Figure 1). Nevertheless, some questions surrounding the generalisability of these results remain unaddressed, and the evidence on DCBs for bifurcation coronary artery disease warrants further trials. In the early, small, randomised PEPCAD-BIF trial, DCB angioplasty demonstrated higher angiographic effectiveness compared with plain balloon angioplasty in the treatment of bifurcation lesions with variable side branch involvement and without proximal main branch disease6. More recently, in the larger, multicentre DCB-BIF trial, 784 patients with true coronary bifurcation disease and severe (≥70%) side branch involvement were randomly assigned after systematic main branch stenting to either DCB or plain balloon angioplasty for the treatment of the side branch7. At 1-year follow-up, the primary endpoint of cardiac death, target vessel myocardial infarction, or clinically driven target lesion revascularisation was lower in patients assigned to DCB angioplasty compared with those assigned to plain balloon angioplasty (7.2% vs 12.5%; hazard ratio 0.56, 95% confidence interval 0.35 to 0.88; p=0.013)7. However, this difference was essentially driven by periprocedural myocardial infarctions, making it uncertain whether there was a play of chance or an influence of superimposed conditions in the context of an open-label design7.
Although evidence from randomised trials and observational studies have generally shown no significant benefit from upfront 2-stent techniques and the European Bifurcation Club recommends using a stepwise provisional strategy for most bifurcations, in some true bifurcation patterns, the implantation of 2 stents remains necessary and is potentially beneficial891011. However, there is currently no comparison between DCBs and DES for the treatment of bifurcation disease. The recent ANDROMEDA study − an individual patient data meta-analysis of randomised trials comparing DCBs versus DES for the treatment of de novo small vessel coronary artery disease − indicated that, in a challenging setting traditionally associated with higher rates of restenosis after DES implantation, 3-year outcomes in patients assigned to DCBs were not significantly different or even improved compared with those in patients assigned to DES12. Whether these findings extend to the treatment of the side branch is uncertain.
Finally, the DCBs employed in the SPACIOUS trial have not been compared with established devices in previous studies and are not currently approved for use in Europe. In this context, the relative merits of the specific DCBs used in the SPACIOUS trial cannot be assessed against a validated reference, and the DCBs used in Europe may be associated with different – improved or worse – effectiveness and safety. While more data on DCBs for coronary bifurcations are needed, it is reasonable to assert that both sirolimus- and paclitaxel-based DCBs are valuable treatment options for side branch disease.
Table 1. Randomised trials with DCBs
| Trial name and reference | Patients | Comparison | Bifurcation type | DCB target site | Primary endpoint | Results |
|---|---|---|---|---|---|---|
| PEPCAD-BIF(2011) NCT01180517 | 32 vs 32 | DCB vs PB | 0,X,X(0,0,1; 0,1,0; 0,1,1) | SB | 9-month LLL | DCB was associated with lower LLL and BR |
| DEBIUT(2012)NCT00857441 | 40 vs 37 vs 40 | DCB (MB/SB)+BMS (MB)vsBMS (MB)+PB (SB)vsDES (MB)+PB (SB) | Any | MB/SB | 6-month LLL | The hybrid strategy including DCB-based predilation did not significantly improve LLL or other angiographic measures |
| BABILON(2014)NCT01278186 | 52 vs 56 | DCB (MB/SB)+BMS vs DES | Any | MB/SB | 9-month LLL | DCB+BMS was non-inferior to DES in terms of LLL;DCB+BMS was associated with higher 24-month TLR compared with DES |
| BEYOND (2020)NCT02325817 | 113 vs 109 | DCB vs PB | Any | SB | 9-month DS | DCB was associated with lower DS and LLL compared with PB |
| DCB-BIF(2024) NCT04242134 | 391 vs 393 | DCB vs PB | True(1,1,1; 0,1,1; 1,0,1) | SB | 1-year MACE | DCB was associated with a significant reduction in MACE, though this difference was driven by lower periprocedural events |
| SPACIOUS(2025) NCT04899583 | 114 vs 115 | Sirolimus-based DCBvspaclitaxel-based DCB | X,X,1 | SB | 9-month DS | Sirolimus-based DCB was non-inferior to paclitaxel-based DCB in terms of DS |
| BMS: bare metal stent; BR: binary restenosis; DCB: drug-coated balloon; DES: drug-eluting stent; DS: diameter stenosis; LLL: late lumen loss; MACE: major adverse cardiac events; MB: main branch; PB: plain balloon; SB: side branch; TLR: target lesion revascularisation | ||||||
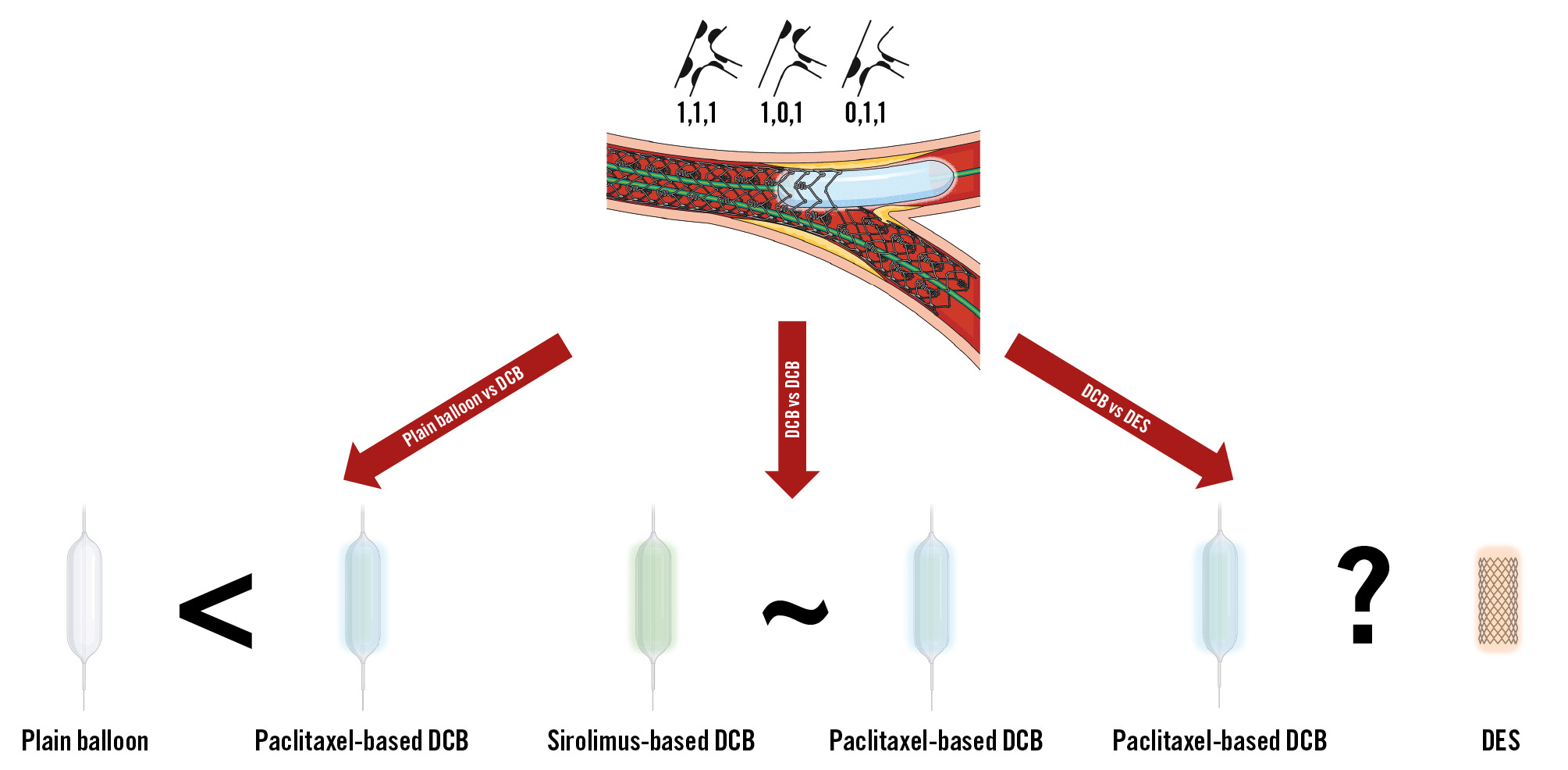
Figure 1. Current evidence on DCB angioplasty for the treatment of side branch disease. DCB: drug-coated balloon; DES: drug-eluting stent
Conflict of interest statement
D. Giacoppo has no conflicts of interest to declare.

