It seems extraordinary to say, but I can’t remember the last time I paid for goods or services with cash. If you had asked me five years ago whether I would have predicted that, the answer would have been a definitive no. One pandemic later and the introduction of universal contactless payment by card or smartphone, and the world is a very different place. Life, at times, moves forwards through disruption and revolution rather than iteration and evolution.
Looking forwards to five years in the future, might it be that we will have arrived at a point where we look back and think how strange it was that the default position for treating de novo coronary lesions requiring revascularisation was the implantation of permanent metallic stents? The current renewed enthusiasm for research and development in drug-coated balloon (DCB) angioplasty and the prospect of the availability of results from a number of well-designed, large-scale randomised clinical trials comparing the outcomes of patients treated with a drug-coated balloon angioplasty strategy versus a conventional stenting strategy make that prospect rather more likely than at any stage over the last three decades1.
For the moment, though, in most catheterisation laboratories, the main use for drug-coated balloon angioplasty will continue to be in patients with stent failure due to in-stent restenosis (ISR)2. Aggregate data from randomised clinical trials for this indication show a generally favourable risk-benefit profile for angioplasty with DCB compared with repeat stenting with drug-eluting stents3. Although there is some evidence of modest incremental gain in clinical efficacy with repeat stenting, this is not of an order of magnitude that would make a compelling argument in favour of adding extra metallic stent layers as a general approach. Accordingly, if the mechanical integrity of the restenosed stent is intact, angioplasty with DCB is the first-line approach for many operators2.
European clinical practice guidelines have long recommended angioplasty with DCB as an evidence-based approach to treat ISR45. Now, in 2024, for the first time, in the USA, a DCB catheter has received U.S. Food and Drug Administration approval for the treatment of coronary ISR. This was based on the primary results of AGENT IDE, an important randomised clinical trial which showed a favourable efficacy and safety profile in patients with ISR6, albeit against a rather unusual comparator (angioplasty with a plain uncoated balloon: multiple clinical trials have shown that plain balloon angioplasty is not an effective approach for treating stent failure due to ISR78). The availability of DCB catheters for coronary use in the USA adds important momentum to the field.
To date, all clinically successful DCB catheters have used a paclitaxel-based coating as opposed to coatings based on rapamycin/sirolimus analogues. The high lipophilicity of paclitaxel means that, if combined with an appropriate excipient or spacer to prevent clumping and adherence to the balloon surface, an effective balance can be achieved such that sufficient drug is retained on the balloon surface during transit to the lesion, and adequate local drug transmission to the vessel wall can be achieved with a relatively brief application using a 1:1-sized balloon for dilation9.
Recently, there has been renewed interest in investigating the efficacy of balloons coated with rapamycin analogues. Results, however, have been mixed10111213. As ever with clinical investigations of medical devices, it has taken randomised clinical trials to tease out the differences in performance between devices. Unfortunately, historically, the evidence base for most devices used in cardiovascular medicine in Europe is deficient in randomised trials14. Last year, the results from two clinical trial programmes investigating different sirolimus analogue-coated balloons released disappointing results.
In the REFORM trial, a balloon coated with biolimus A9 was clearly inferior to one coated with paclitaxel in treating patients with ISR10. Similarly, in the TRANSFORM I trial a sirolimus-coated balloon proved inferior to proven paclitaxel balloon technology in an ISR patient cohort13. Indeed, thus far, encouraging comparative efficacy data have only been seen with a single device, the sirolimus-coated SeQuent balloon (B. Braun), and these have been confirmed only in selected patients enrolled in modest-sized clinical trials11. Moreover, for reasons that are unclear, this device has not, as yet, been widely marketed.
One sirolimus-coated balloon being tested as part of an interesting clinical trial programme is the SELUTION SLR sirolimus-coated balloon (Cordis). This balloon is being used in the investigational arm of the large SELUTION DeNovo trial which is comparing a DCB angioplasty strategy to a conventional stenting strategy in over 3,300 patients15. The results of this trial will surely go a long way in determining the role of angioplasty with DCB as a part of routine clinical practice. However, it is a curious approach to test such an important question using a balloon which has no published randomised trial data to support its clinical performance. Whether the risk of this is justified remains to be seen, but clearly, the device evaluation strategy deviates from conventional best practice: demonstrate efficacy first in a surrogate endpoint randomised trial, then proceed to testing in a large-scale trial powered for clinical endpoints. High risk, potentially high reward.
In this issue of EuroIntervention, Chen et al report the results of the BIO ASCEND ISR randomised trial16. Here the focus is back on angioplasty for ISR. In 290 patients enrolled at 17 sites in China, the second-generation biolimus A9-coated balloon showed similar efficacy to the paclitaxel-coated balloon for the treatment of ISR using angiographic surveillance parameters as a yardstick of efficacy. The authors should be congratulated for executing and reporting this study. The results are of interest to the community and represent a potential additional step forward for DCB technology using rapamycin-derivative coatings in the context of previously published studies (Figure 1).
The main scientific interest relates to the positive (non-inferior) treatment effect versus a widely studied paclitaxel-coated balloon seen in the current BIO ASCEND ISR trial in comparison with the negative treatment effect seen in the REFORM trial. The two trials share much in common in terms of design and execution. The two investigational devices used in the trials are also similar, and both are coated with biolimus A9. There is some suggestion that the formulations differ in terms of their excipient and that this results in smaller crystal particle sizes with the second-generation balloon used in BIO ASCEND ISR; this may in turn lead to enhanced local drug transmission and explain the improved efficacy in comparison with the first-generation device. The key question for clinicians and regulators alike will be whether these results can be replicated in a non-East Asian population. This will be a matter for another day.
For now, the field of angioplasty with DCB continues to capture the interest of the interventional cardiology community. In the area of “leave nothing behind” angioplasty, we have experience of false dawns in the recent past, most notably with bioresorbable scaffolds. There were clear lessons that were reinforced from those experiences. Ultimately, results from well-designed, large-scale randomised trials are required to provide a clear answer to the question of whether the full promise of DCB technology for de novo disease will become an everyday clinical reality.
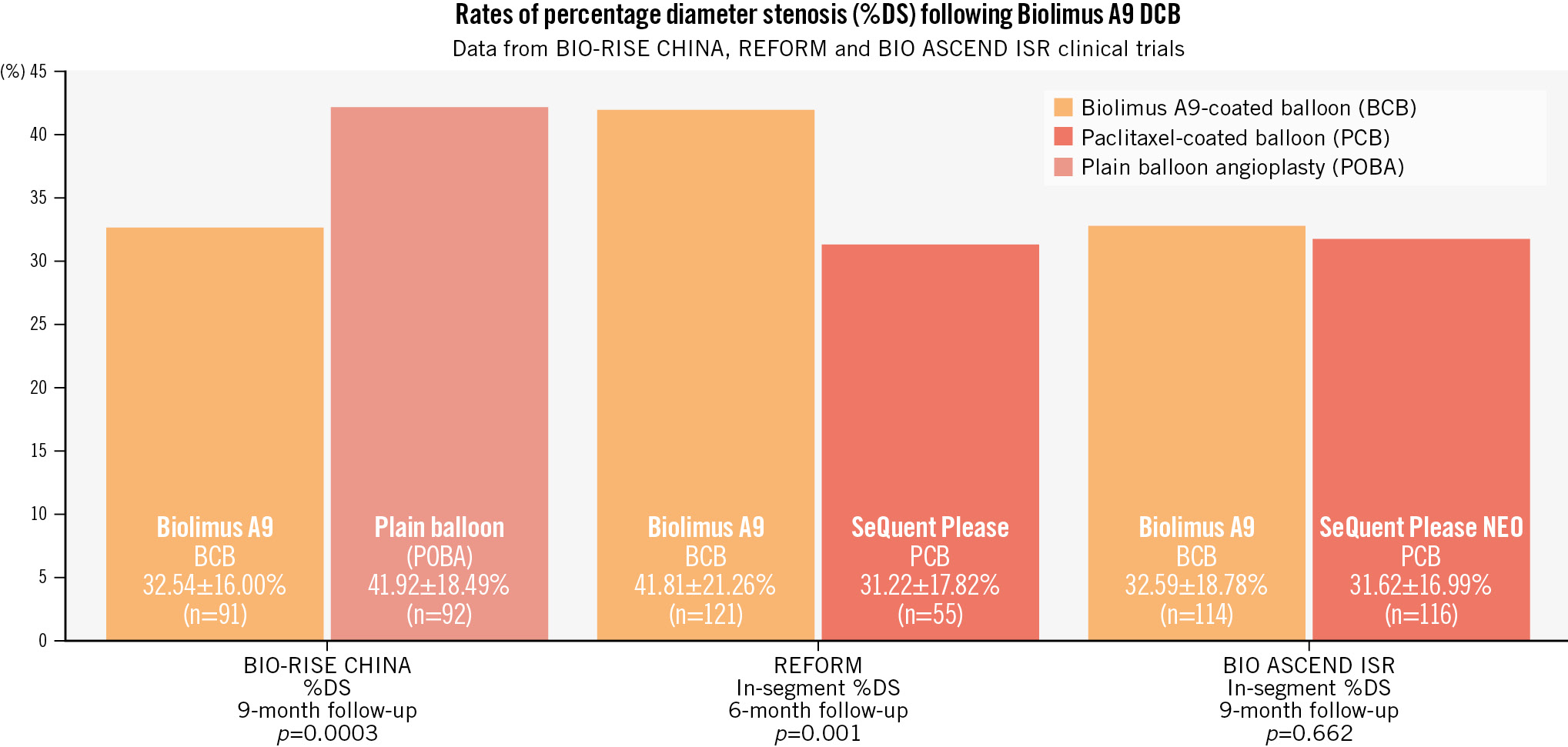
Figure 1. Comparative rates of percentage diameter stenosis at angiographic follow-up in randomised trials of biolimus A9-coated balloons versus control. Xu et al12 reported superior efficacy of a biolimus A9-coated balloon compared to plain balloon angioplasty in patients with small vessel coronary artery disease in the BIO-RISE CHINA study. The REFORM trial failed to show non-inferiority of a biolimus A9-coated balloon compared to a paclitaxel-coated balloon for percentage diameter stenosis at 6 months. The current BIO ASCEND ISR trial shows similar efficacy of a biolimus A9-coated balloon to a paclitaxel-coated balloon. DCB: drug-coated balloon
Conflict of interest statement
R.A. Byrne reports research grants received by the institutions of employment from Abbott, Biosensors, Boston Scientific, and Translumina, without impact on personal remuneration; he does not accept personal payments from the medical device or pharmaceutical industry. R. Durand has no conflicts of interest to declare in relation to this work.

