Despite multiple refinements in drug-eluting stent (DES) technology and implantation techniques, percutaneous coronary intervention (PCI) with DES is complicated by the continuous accrual of clinical events due to thrombosis and in-stent restenosis (ISR). Drug-coated balloon (DCB) angioplasty offers advantages related to drug elution from the balloon surface and the absence of permanent struts, and it yields non-inferior clinical outcomes in patients with ISR and de novo small vessel disease1. Historically eluting paclitaxel, DCBs with newer antiproliferative drugs, including sirolimus, have been developed and tested for DCB PCI23. To date, large-scale evidence on safety and efficacy beyond 1 year after sirolimus-coated balloon (SCB) PCI is limited.
EASTBOURNE (ClinicalTrials.gov: NCT03085823) is a prospective, multicentre, investigator-driven cohort study evaluating the performance of the MagicTouch SCB (Concept Medical) for PCI in all-comer patients enrolled at 38 European and Asian centres between September 2016 and November 2020. The study design and 1-year results have been previously reported3. Briefly, real-world patients with any clinical indication for PCI with DCB, i.e., de novo small vessel disease and ISR, were included. Among the exclusion criteria were (i) unsuccessful predilation of the target lesion with persisting residual stenosis higher than 50%, (ii) severe calcification of the target vessel (at the lesion site or proximal to it), (iii) a highly tortuous target vessel and (iv) visible thrombus at the lesion site, not treatable with manual aspiration (Supplementary Appendix 1). The 2-year primary endpoint assessment consisted of clinically indicated target lesion revascularisation (TLR), defined as reintervention of the target lesion after demonstrating at least 70% narrowing and the presence of objective evidence of ischaemia by stress test or functional assessment.
The baseline characteristics of included patients have been published previously (Supplementary Table 1). Compared to patients with ISR, those treated for de novo disease were less often diabetic (38% vs 45%; p=0.002), had smaller reference vessel diameters (2.3 mm vs 3.0 mm; p<0.001), underwent predilation less often (89% vs 95%; p<0.001) and tended to have stents implanted more often (9% vs 6%; p=0.059).
Among 2,123 patients, 2-year clinical follow-up was available for 90% (n=1,907) of patients. The cumulative incidence of the primary endpoint, clinically indicated TLR, was 7% (n=163) at 2-year follow-up, with 36 events (1%) accruing between the 1- and 2-year follow-up (Central illustration). Between years 1 and 2, the incidence of major adverse cardiac events (MACE; a composite of cardiac death, spontaneous myocardial infarction [MI] and TLR) increased by 1.3% to 11.2% (n=234), cardiac death by 0.1% to 1.6% (n=34) and spontaneous MI by 1.7% to 4.1% (n=86). Death from any cause was observed in 5.3% (n=111) of cases, TVR in 6.8% (n=141) and Bleeding Academic Research Consortium (BARC) 2-5 bleeding in 1.3% (n=28). The incidence of TLR was lower in patients with de novo lesions than in those with ISR (2.6% vs 12.3%; p<0.001); this was confirmed in the time-to-event analysis, including a landmark analysis at 1-year follow-up (plog-rank<0.001 for all comparisons) (Supplementary Figure 1). Similarly, compared to patients with ISR, those with de novo lesions experienced less MACE (5.5% vs 18.6%; p<0.001), death (3.5% vs 7.7%; p<0.001), cardiac death (0.6% vs 3.0%; p<0.001), spontaneous MI (2.6% vs 6.1%; p<0.001) and TVR (2.6% vs 12.2%; p<0.001). No difference in BARC 2-5 bleeding was found between the groups (0.9% vs 1.7%; p=0.153). After multivariable adjustment, the risk for undergoing clinically indicated TLR at the 24-month follow-up was higher in patients with ISR, females, patients with smaller visually estimated minimal lumen diameters, and those with larger SCB diameters, and when more than one lesion was treated during the index procedure (Supplementary Table 2).
The main findings of our study assessing 2-year follow-up outcomes of patients undergoing SCB PCI suggest MagicTouch SCB angioplasty is effective, with a low incidence of TLR, safe, as demonstrated by the low incidence of MACE, including cardiac death and MI, and might yield more favourable outcomes when treating de novo lesions than when treating ISR.
Notwithstanding the fact that only DES ISR patients were included in EASTBOURNE, our results in patients with ISR treated with SCBs are promising when compared to those of paclitaxel-coated balloons (PCBs), with a reported accrual of TLR events between 1- and 2-year follow-up (1.8%) lower than that reported in the DAEDALUS study (4.4%)4. In addition, despite a non-contemporary lesion predilation rate (only 89%) among de novo lesions, we report 2-year outcomes within a sample of lesions with a mean reference vessel diameter of 2.3 mm that compare favourably with those reported in randomised controlled trials of PCBs5. Nonetheless, failure to demonstrate the non-inferiority of the MagicTouch SCB for angiographic net lumen gain at 6 months compared to a PCB should be acknowledged2. Overall, these findings stress the need to raise awareness of the possible absence of a class effect in the antirestenotic performance of DCB technologies due to different drugs, excipients and catheter properties.
Selection bias and residual confounding bias when comparing de novo disease and ISR, notwithstanding multivariable adjustment, cannot be excluded because of the observational nature of our study. Underreporting or missing follow-up data in 10% of the study population need to be acknowledged. Also, core laboratory angiographic evaluation and quantitative coronary angiography were not available. The single-arm, open-label design of the study should be noted, though event adjudication was performed by an independent clinical events committee based on prespecified criteria after revision of the source documents. The fact that the results in the de novo lesions cohort represent mostly small coronary vessels needs to be highlighted, and the results might not be directly extrapolated to large vessels. Finally, the external validity of our study is limited by the fact that our results cannot be extrapolated to centres who have limited experience with DCB PCI.
EASTBOURNE − a multicentre, observational, prospective, investigator-driven study, including 2,123 all-comer patients with coronary artery disease − suggests MagicTouch SCB PCI is safe and effective, with low rates of clinically relevant adverse events at 2-year follow-up.
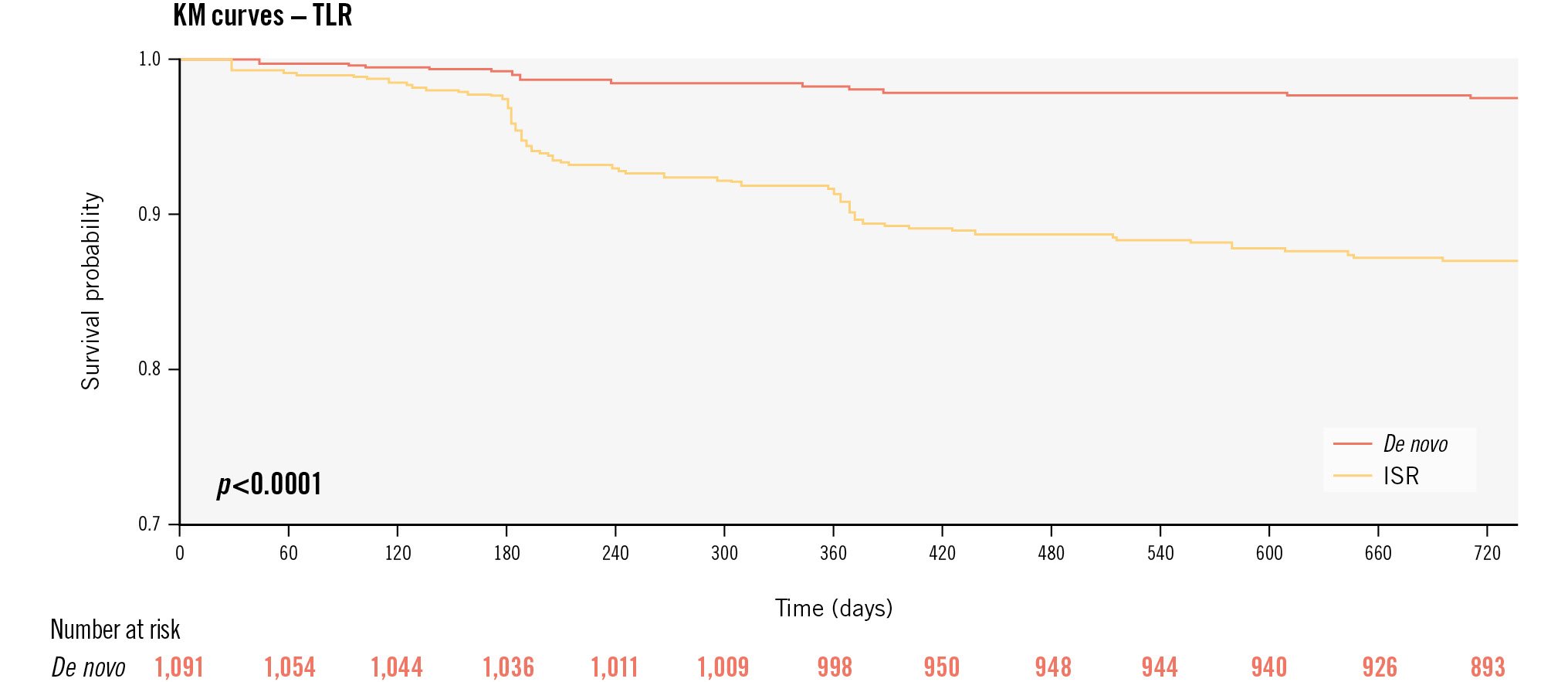
Central illustration. Kaplan-Meier estimates of the primary endpoint up to 2-year follow-up according to the type of lesion treated. ISR: in-stent restenosis; KM: Kaplan-Meier; TLR: target lesion revascularisation
Funding
The study was an independent, investigator-driven study which received an unrestricted research grant for statistical analysis from Concept Medical. This society had no role in the protocol definition, selection of centres, conduction of the study or interpretation of the results.
Conflict of interest statement
B. Cortese has served as an advisory board member for Envision Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

