Cory:
Unlock Your AI Assistant Now!
Traditionally, lower-limb endovascular interventions have used transfemoral or transbrachial access. Alternative approaches such as transradial and distal foot artery (DFA) access are now, however, increasingly adopted12. DFA access (distal anterior tibial/dorsalis pedis, distal posterior tibial, and distal peroneal/perforator arteries) offers a smaller-calibre, superficial, and easily compressible artery, lowering access site bleeding complications2. Given the DFA’s small size, intravascular closure devices cannot be used; haemostasis relies on external compression. The standard method is manual compression, but dedicated devices are often employed for convenience. Two devices are commonly used: a balloon compression device (TR Band [Terumo]) originally designed for radial artery haemostasis3, and a topical haemostatic patch (StatSeal [Biolife]). StatSeal utilises a hydrophilic polymer that dehydrates blood and absorbs exudate, while its potassium ferrate-induced low pH aggregates proteins and promotes seal formation. StatSeal has demonstrated efficacy in reducing transradial access haemostasis time4. The PED-PRESS trial presented herein compared DFA access site complications utilising these two closure devices.
This prospective, randomised trial enrolled 150 patients. The procedures used ultrasound-guided DFA access. Patients were randomised to TR Band or StatSeal closure devices post-sheath removal. If retrograde crossing failed, proximal femoral access was used. The primary endpoints were major (requiring surgical/interventional treatment, e.g., large haematoma needing transfusion, pseudoaneurysm needing thrombin injection, or access site occlusion) and minor (self-limiting bleeding or haematoma <5 cm requiring no therapy)5. Group comparisons used chi-square or Fisher’s exact tests for categorical variables, with p<0.05 considered significant.
Patients classified in Rutherford categories 2-5 (from claudication to chronic limb-threatening ischaemia, e.g., ischaemic rest pain, crural ulcer, pedal gangrene) were included. Those in Rutherford categories 0-1 (asymptomatic to mild claudication) were excluded.
Inaccessible DFA arteries (e.g., complete occlusion, severe calcification, anatomical variations), non-viable lower limbs, contraindications to dual antiplatelet therapy for ≥1 month, heart failure (ejection fraction <35%), significant valvular disease, age >85 years, severe renal dysfunction (glomerular filtration rate <30 mL/kg/min), ongoing sepsis, or life expectancy <3 years. Of the screened patients, 30% were excluded, mainly due to non-viable limbs (25 patients), antiplatelet contraindications (20 patients), or severe comorbidities (15 patients).
A postoperative vascular ultrasound assessed DFA artery patency and puncture-related haematomas on day 1.
Patients received preprocedural aspirin (325 mg) and clopidogrel (300 mg), with dual antiplatelet therapy (aspirin 100 mg, clopidogrel 75 mg) for two months after stenting or lifelong aspirin after balloon angioplasty. Heparin (100 IU/kg) and nitroglycerine (250 mcg) were administered via the DFA sheath.
For access, a 4 Fr Terumo transradial sheath, a HI-TORQUE PROGRESS 40 0.14” guidewire (Abbott) and CXI Support 0.35” catheter (Cook Medical) were used. Stenting was performed for flow-limiting dissections, with sheath upsizing to 6 Fr in 66% of cases.
Percutaneous transluminal angioplasty was performed in all 150 patients using DFA access. Secondary femoral access was required in 89 patients (59.3%) due to retrograde crossing failure. Access sites comprised the anterior tibial/dorsalis pedis arteries in 115/150 (76.7%), the distal posterior tibial artery in 21/150 (14.0%), and the peroneal artery in 14/150 (9.3%). Baseline characteristics were balanced between groups (Supplementary Table 1), and procedural characteristics are provided in Supplementary Table 2.
Major DFA access-site complications occurred in 6.7% (5/75) of patients in the TR Band group versus 5.3% (4/75) with StatSeal (p=1.00). Minor complications occurred in 4/75 (5.3%) versus 3/75 (4.0%), for TR Band and StatSeal, respectively (p=1.00). Combined DFA access site complications (major and minor) occurred in 9/75 (12.0%) TR Band patients versus 6/75 (8.0%) StatSeal patients (p=0.60). Component events were the following, for TR Band and StatSeal patients, respectively: haematomas <5 cm: 4/75 (5.3%) versus 3/75 (4.0%); major bleeding: 1/75 (1.3%) versus 0/75 (0%); pseudoaneurysm: 1/75 (1.3%) versus 1/75 (1.3%); arteriovenous fistula 1/75 (1.3%) versus 0/75 (0%); and tibial occlusions 1/75 (1.3%) versus 1/75 (1.3%). Per-artery, per-device data are shown in Supplementary Table 3. No infections, acute limb ischaemia, nor compartment syndrome occurred. Next-day vascular ultrasound confirmed DFA patency was 74/75 (98.6%) in TR Band vs 72/75 (96.0%) in StatSeal (p=1.00). Figure 1 summarises DFA access site complication rates.
This is the first randomised trial comparing TR Band and StatSeal for DFA access site closure after endovascular intervention. Complication rates were similar (any: 12.0% TR Band vs 8.0% StatSeal; p=0.60; major: 6.7% TR Band vs 5.3% StatSeal; p=1.00; minor: 5.3% TR Band vs 4.0% StatSeal; p=1.00). The study was not powered to detect small between-group differences; therefore, numerical differences should be interpreted cautiously. Compared to prior studies, our results align with the low bleeding complication rates reported for DFA access34.
Both devices provided reliable DFA haemostasis. Limitations include the modest sample size and absence of a manual compression arm, of patient-reported outcomes, and of cost analyses. Peroneal access (~10% of cases) had one event overall (7.1%; StatSeal) and was not analysed separately due to low counts. Larger trials are warranted.
Distal foot artery access for lower limb interventions has low access site complication rates. Both TR Band and StatSeal closure devices are safe and effective, with no significant differences in access site complication rates. While closure device choice may not significantly impact overall success and complication rates, further research is needed to optimise closure strategies.
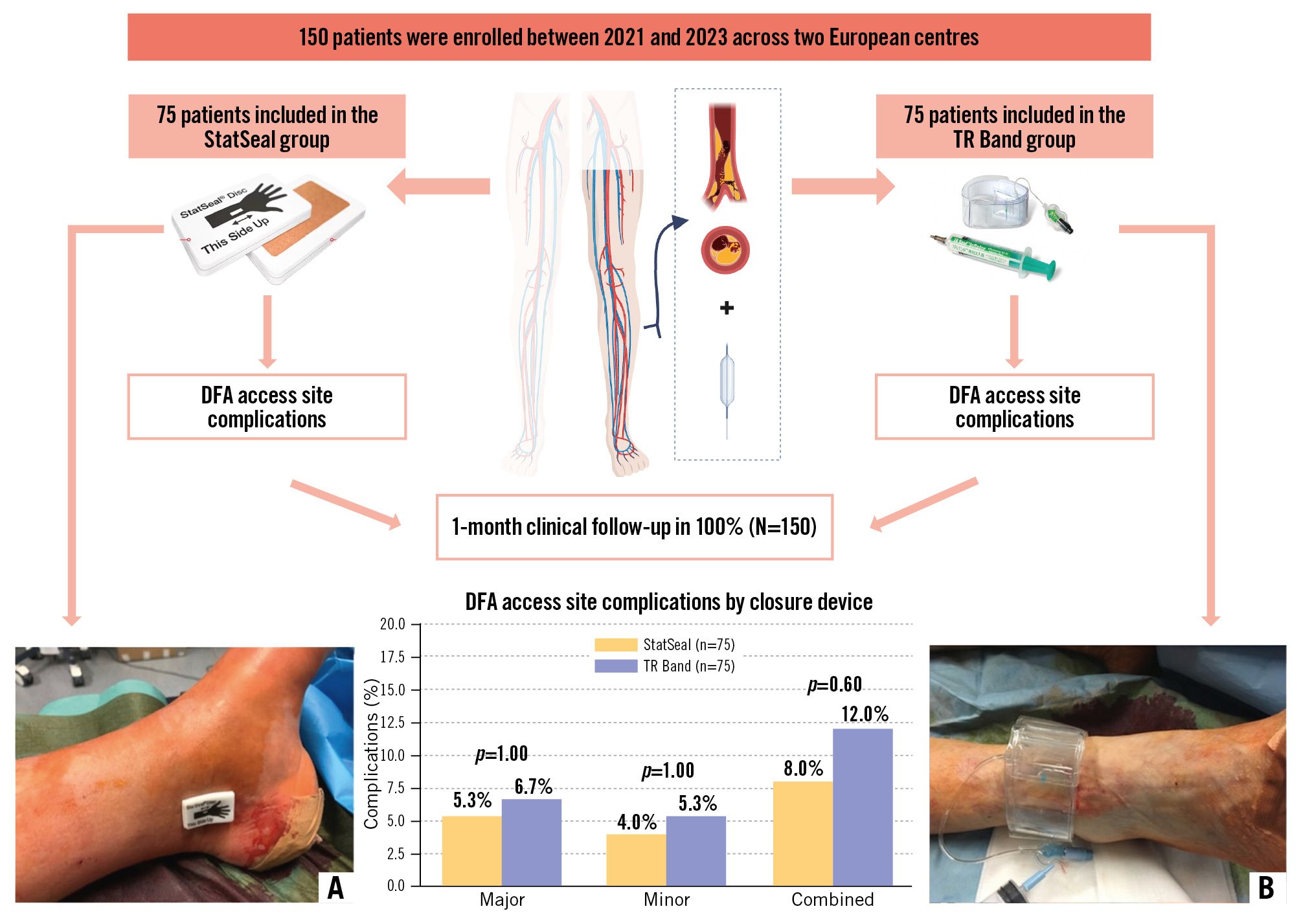
Figure 1. PED-PRESS study design and outcomes. Study design showing 150 patients enrolled (2021-2023) across two European centres, randomised to StatSeal (n=75) or TR Band (n=75) for distal foot artery access site closure. The bar chart displays distal foot artery access site complications: major (6.7% TR Band vs 5.3% StatSeal; p=1.00), minor (5.3% vs 4.0%; p=1.00), and combined (12.0% vs 8.0%; p=0.60). An illustration of device applications is provided for (A) StatSeal and (B) TR Band. Created with BioRender.com.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

