Compared to radial access, femoral access has been shown to increase bleeding and vascular complications in stable ischaemic heart disease and even mortality in acute coronary syndrome patients undergoing percutaneous coronary intervention (PCI)1. However, transfemoral access (TFA) remains necessary for larger-bore complex procedures and in cases of radial access failure2. Precise cannulation of the common femoral artery is essential, as punctures higher than the inguinal ligament increase the risk for retroperitoneal haemorrhage, while punctures below the femoral bifurcation increase the risk for pseudoaneurysms, arteriovenous fistulas and acute limb ischaemia2. Ultrasound (US) guidance has emerged as an effective adjunct to increase TFA safety and procedural efficacy. The recent Routine Ultrasound Guidance for Vascular Access for Cardiac Procedures: a Randomized Trial (UNIVERSAL) did not demonstrate a benefit of US guidance over no US guidance for TFA regarding access site complications3. However, a follow-up meta-analysis suggested a benefit based on the totality of the evidence4.
In this issue of EuroIntervention, Meijers et al report the results of the Ultrasound Guided Transfemoral Large-bore Trial (ULTRACOLOR), in which 554 patients undergoing complex PCI mandating large-bore (≥7 Fr) TFA were randomised to US guidance versus fluoroscopy guidance for the primary outcome of major bleeding (Bleeding Academic Research Consortium [BARC] 2, 3 or 5) or vascular complications during the index hospitalisation5. The median age was 71 years, 76% of participants were male, and 68% underwent a chronic total occlusion PCI. The authors demonstrated that US guidance did not decrease the incidence of the primary outcome in the US-guided TFA group as compared to the fluoroscopy-guided TFA group (43/274 [15.7%] vs 51/270 [18.9%]; p=0.32). However, US guidance was associated with a higher rate of first-pass success as compared to fluouroscopy guidance (251/274 [92%] vs 230/270 [85%]; p=0.02).
The authors must be commended for completing this well-conducted and informative trial in a high-risk and challenging patient population. Notably, most events counted in the primary composite endpoint were BARC 2 bleeding events. While these events are important to patients and physicians, they are most likely not associated with increased mortality6. However, completing an adequately powered trial with more serious bleeding would have been challenging. The sample size would have needed to be much larger because of the low frequency of events. Interestingly, the authors demonstrated a paradoxical increase in high punctures in the US arm. This was not detected in the UNIVERSAL trial but can be observed in routine clinical practice, given the frequent tendency to inadvertently tilt the US probe downwards3. This highlights the importance of meticulous real-time needle tracking to ensure proper common femoral cannulation and avoid high punctures2.
The ULTRACOLOR Trial was only powered for a 50% relative risk reduction, which was unlikely to be demonstrated given the individual participant-level meta-analysis findings of a smaller treatment effect47. ULTRACOLOR cannot exclude modest treatment effects with US. The individual participant-level meta-analysis, including four trials with a total of 2,441 participants undergoing coronary procedures, demonstrated that US-guided TFA decreased major vascular complications or major BARC 3 bleeding as compared to non-US-guided TFA (34/1,208 [2.8%] vs 55/1,233 [4.5%]; odds ratio [OR] 0.61, 95% confidence interval [CI]: 0.39-0.94; p=0.026) with a particular benefit in patients receiving a vascular closure device48. However, only 34.5% and 7.9% of participants underwent PCI and had a 7 Fr or greater femoral sheath.
When combining the ULTRACOLOR Trial with the previous coronary US TFA trials from the Femoral Ultrasound Trialist Collaboration in a two-stage fixed-effect meta-analysis, the totality of the data suggests a more modest benefit of US compared to no US in TFA for the outcome of major vascular complications or BARC 2 or 3 bleeding at the longest available follow-up (OR 0.72, 95% CI: 0.54-0.95; p=0.02; I2=2%) (Figure 1). All statistical analyses were performed using RevMan version 5.4 (Cochrane Community)4. Furthermore, the ULTRACOLOR effect estimate and 95% CI are nearly identical to those of the UNIVERSAL trial, suggesting a consistent effect throughout the range of complexity for coronary procedures3. ULTRACOLOR is a valuable addition to a series of multiple underpowered trials demonstrating a coherent, small, yet clinically relevant benefit, given the benign nature of US guidance.
While TFA remains a workhorse access for complex coronary procedures, the best way to avoid major vascular complications or bleeding is to avoid the femoral artery. The COLOR trial randomised 388 patients undergoing complex PCI to either 7 Fr transradial access (TRA) or 7 Fr TFA for the primary composite outcome of major vascular complications or major bleeding at discharge. The results were overwhelmingly in favour of radial access (7/194 [3.6%] vs 37/194 [19.1%]; p<0.001), with only a 3.6% crossover from radial to femoral access and similar procedural success rates9. Even with the most meticulous technique, US, and perhaps micropuncture needles, patients undergoing TFA will remain at higher risk than those undergoing TRA, given the anatomical characteristics that differentiate the two access sites1.
In summary, a TRA approach is the most effective way of preventing vascular access complications in coronary procedures. If this approach is not possible, the data support US guidance for TFA. The interventional cardiology community needs to focus on training the current and next generation of interventionalists with US-guided TFA.
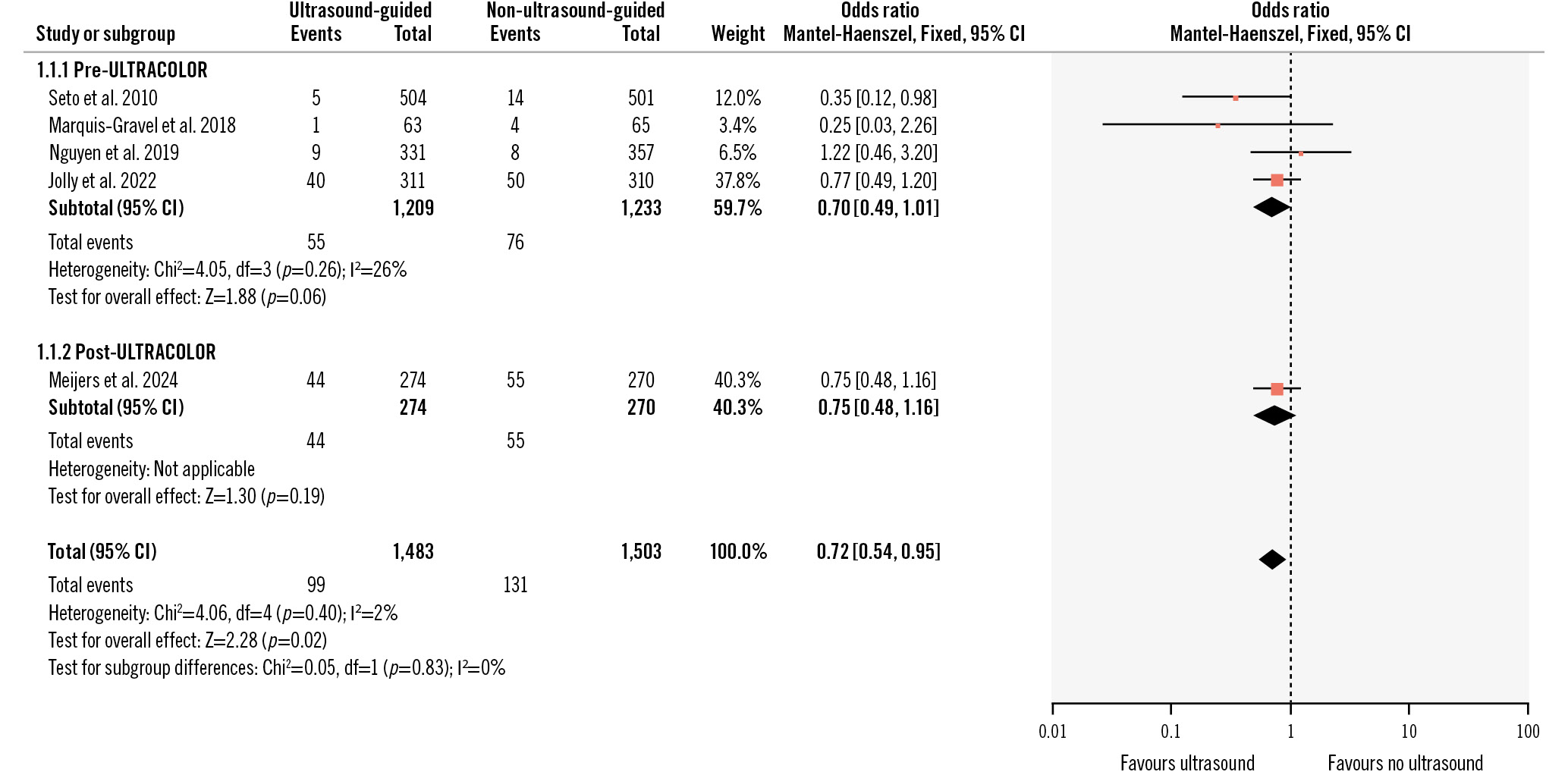
Figure 1. Fixed-effect two-stage meta-analysis for major vascular complications or BARC 2 or 3 bleeding at longest available follow-up. BARC: Bleeding Academic Research Consortium; CI: confidence interval
Conflict of interest statement
M-A. d’Entremont is a Canadian Institutes of Health Research Canada Graduate Scholarship awardee. S.S. Jolly has received grants or contracts from Boston Scientific; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Penumbra, Teleflex, and Abiomed.

