Cory:
Unlock Your AI Assistant Now!
The optimal strategy for patients with left main (LM) coronary disease and an indication for revascularisation is uncertain. Previous studies, including the EXCEL and NOBLE trials, have shown that percutaneous coronary intervention (PCI) is an acceptable alternative to coronary artery bypass graft (CABG) surgery, with comparable outcomes. However, the optimal PCI strategy has yet to be determined12. The IDEAL-LM trial randomised 818 patients undergoing LM PCI in a 1:1 ratio to treatment with either a durable-polymer (DP) drug-eluting stent (DES) and 12 months of dual antiplatelet therapy (DAPT) or a biodegradable-polymer (BP) DES with 4 months of DAPT. After 2 years of follow-up, clinical outcomes were similar in both groups3. Here, we report the final 5-year outcomes.
In brief, the IDEAL-LM trial was an investigator-initiated, international, multicentre, open-label, randomised clinical trial. The primary objective was to establish non-inferiority of the BP platinum-chromium everolimus-eluting stent (BP-PtCr-EES; SYNERGY [Boston Scientific]) group to the DP cobalt- chromium everolimus-eluting stent (DP-CoCr-EES; XIENCE [Abbott]) group for the composite endpoint of major adverse cardiovascular events (MACE) − defined as all-cause death, myocardial infarction (MI), and ischaemia-driven target vessel revascularisation (TVR) at 2 years after PCI. Secondary endpoints included the individual components of the primary endpoint, a device-oriented composite endpoint (DOCE) defined as cardiac death, MI not clearly attributable to a non-treated vessel and clinically-indicated target lesion revascularisation (TLR), a patient-oriented composite endpoint (POCE) defined as a composite endpoint of all-cause death, any stroke, and any MI, and any revascularisation, stent thrombosis, and bleeding as per the Bleeding Academic Research Consortium (BARC) criteria. The final study results were assessed at 5 years after PCI.
The 5-year results are summarised in the Central illustration. From December 2014 to October 2016, 818 patients were enrolled (BP-PtCr-EES: n=410; DP-CoCr-EES: n=408). Baseline characteristics are summarised in Supplementary Table 1. The original protocol mandated follow-up for 5 years, but a combination of the COVID-19 pandemic and limited funding meant this was not possible in some patients. Ultimately, 4-year follow-up data were available for 382 (93.0%) patients in the BP-PtCr-EES group and 379 (92.9%) patients in the DP-CoCr-EES group, while 5-year follow-up data were available for 183 (44.6%) patients and 178 (43.6%) patients in the respective groups (Supplementary Figure 1).
At 5 years, the primary endpoint of MACE occurred in 24.1% (89 events) of the BP-PtCr-EES group and in 22.5% (81 events) of the DP-CoCr-EES group (Kaplan-Meier estimates; p=0.50) (Supplementary Figure 2). Landmark analysis showed no significant differences between groups either up to 1 year or from 1 year to the end of follow-up (Supplementary Figure 3). There were no significant differences between groups in any of the secondary endpoints, including TVR (Supplementary Figure 4). Landmark analysis at 1 year demonstrated a higher incidence of TVR in the BP-PtCr-EES group compared to the control group (5.5% vs 2.3%; p=0.02); however, this difference was not observed during the 1- to 5-year follow-up period (Supplementary Figure 5). The incidence of definite/probable stent thrombosis between the BP-PtCr-EES and DP-CoCr-EES groups was not significantly different over the entire period of follow-up (3.3% vs 1.8%; p=0.18) nor in the landmark analysis (0-12 months: 1.7% vs 1.2%; p=0.57; 12-60 months: 1.6% vs 0.5%; p=0.16) (Supplementary Figure 6, Supplementary Figure 7). There was no significant difference between groups with respect to BARC 3 or 5 bleeding (3.8% vs 1.6%; p=0.05) (Supplementary Table 2). There was no significant subgroup difference in the association between treatment strategy and MACE at 5 years (Supplementary Figure 8).
At the time this study was designed, the standard of care after LM PCI was 12 months of DAPT, except in patients at high bleeding risk. Accordingly, the experimental arm in our study, utilising BP-PtCr-EES with only 4 months of DAPT, was considered highly novel. Since then, as evidence has accumulated, the use of shorter-duration DAPT, even after complex PCI, has increased. As a result, the 2024 European Society of Cardiology guidelines on the management of chronic coronary syndromes recommend 3-6 months of DAPT in patients undergoing high thrombotic risk PCI, which includes LM PCI4. In addition, no convincing data have emerged to support the hypothesis that BP-DES are inherently safer than DP-DES, particularly in the context of short-duration DAPT. The results of IDEAL-LM support this conclusion. Despite the difference in DAPT duration between the two groups in IDEAL-LM, there was no difference in major bleeding. This may reflect the fact that we did not study an exclusively high bleeding risk population, and further studies are required to assess strategies utilising very short (1 month) durations of DAPT in patients undergoing LM PCI. Overall, both groups in this study showed excellent long-term outcomes with respect to cardiac death, MI and TVR, comparable to the 5-year results from the CABG arms in major clinical trials of PCI versus CABG56. Although a significant number of patients (approximately half) were lost to follow-up between years 4 and 5, this was, to some extent, mitigated by the use of Kaplan-Meier methodology to estimate event rates.
In conclusion, the use of BP-PtCr-EES followed by 4 months of DAPT in patients undergoing LM coronary artery PCI did not confer any advantages over treatment with a durable-polymer stent followed by 12 months of DAPT after 5 years of follow-up.
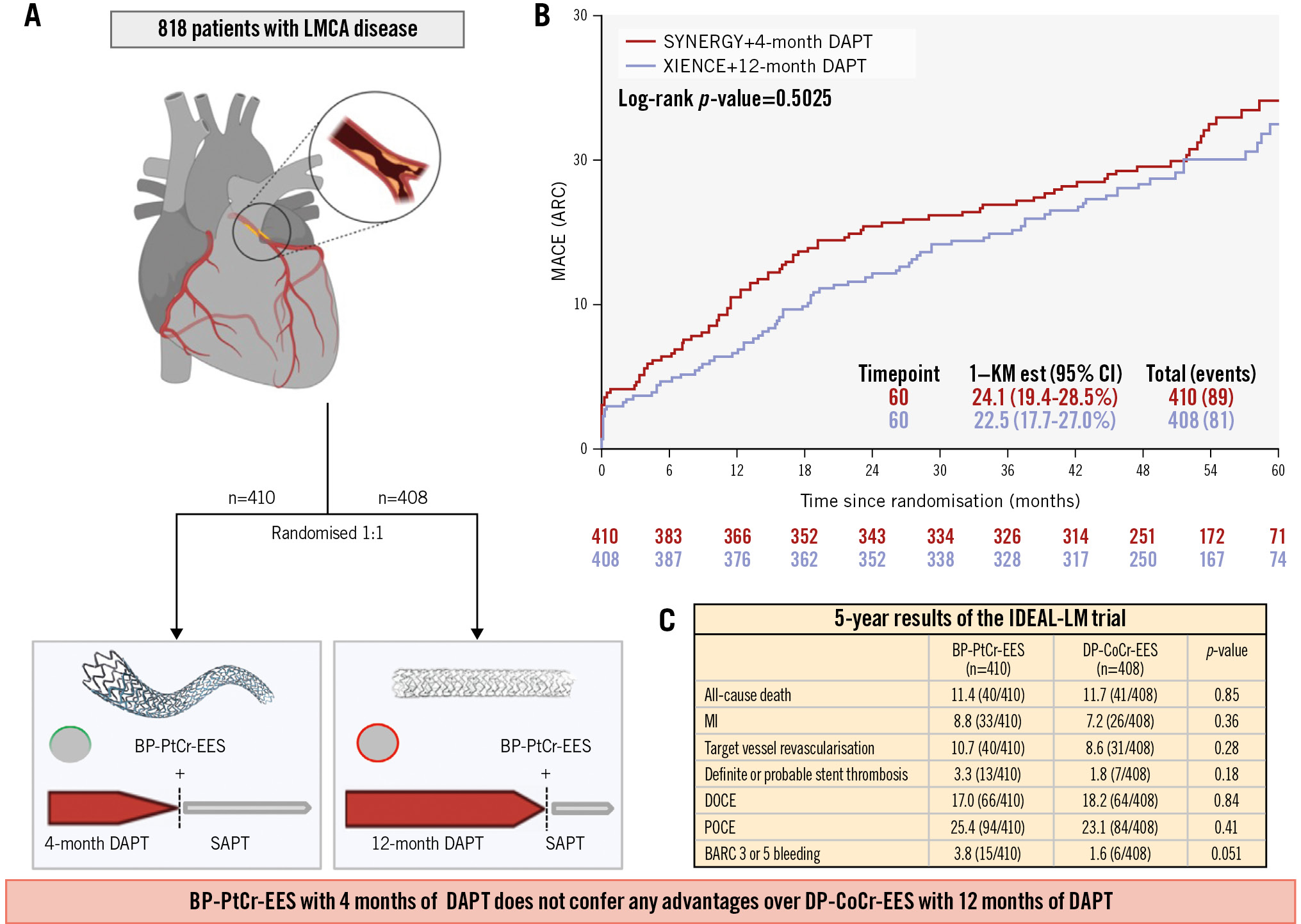
Central illustration. Overview of the IDEAL-LM trial. A) Flowchart of study design; (B) incidence of MACE by stent type; (C) 5-year results of the IDEAL-LM trial. ARC: Academic Research Consortium; BARC: Bleeding Academic Research Consortium; BP-PtCr-EES: biodegradable-polymer platinum-chromium everolimus-eluting stent; CI: confidence interval; DAPT: dual antiplatelet therapy; DOCE: device-oriented composite endpoint; DP-CoCr-EES: durable-polymer cobalt-chromium everolimus-eluting stent; LMCA: left main coronary artery; MACE: major adverse cardiovascular event; MI: myocardial infarction; POCE: patient-oriented composite endpoint; SAPT: single antiplatelet therapy
Funding
Boston Scientific has provided financial support for this trial.
Conflict of interest statement
R.-J.M. van Geuns reported grants and personal fees from Boston Scientific, Abbott, AstraZeneca, and Amgen; and grants from InfraRedx. M. Lesiak reported being on the speaker bureau for Abbott and Boston Scientific. P. O’Kane reported speaker fees from Abbott and Boston Scientific. E. Bressolette reported being a consultant for Boston Scientific. K.G. Oldroyd reported being an employee for Biosensors. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

