Abstract
Pulmonary embolism (PE) is a life-threatening condition characterised by occlusion of the pulmonary vasculature, with a global incidence of approximately 1 in 1,000 patients. While pharmacological therapy remains the cornerstone of PE management, selected patients may benefit from catheter-directed treatments due to their potential for rapid symptom relief and swift haemodynamic stabilisation. Catheter-directed thrombolysis and catheter-directed mechanical thrombectomy are current treatment options, though their availability is still limited. This review provides a practical outlook on catheter-directed therapies for PE, outlining optimal procedural planning, device selection, technical execution, evaluation of results, and management of complications.
Pulmonary embolism (PE) is a life-threatening condition with a heterogeneous clinical presentation, ranging from asymptomatic cases to cardiac arrest, with up to 50% mortality at 90 days1. While most patients are managed with anticoagulation alone, high-risk cases require reperfusion therapy2. Systemic thrombolysis, despite limited evidence from randomised controlled trials34, remains the guideline-recommended strategy for high-risk patients567 but is underutilised because of bleeding concerns89. Surgical embolectomy, in contrast, is less widely available and carries high postprocedural mortality rates59101112. Consequently, less invasive catheter-directed treatments are emerging, divided into two main approaches: catheter-directed thrombolysis (CDT), using low-dose thrombolytics through a small-bore catheter, and catheter-directed mechanical thrombectomy (CDMT), using a medium- to large-bore catheter to mechanically remove the thrombus. Due to limited evidence supporting the safety and efficacy of these techniques13, an individualised approach is essential to maximise the benefit and minimise the risks of these interventions. This review offers a practical guide to transcatheter PE treatment, focusing on procedural planning, device selection, technical execution, and outcome evaluation with the devices presently in use.
Procedural planning
Risk assessment and thrombus localisation
Prior to the procedure, it is essential to assess the clinical presentation14 and haemodynamic status, and perform imaging to evaluate patient risk, detect early signs of deterioration, and optimise the strategy and timing of the intervention (Figure 1).
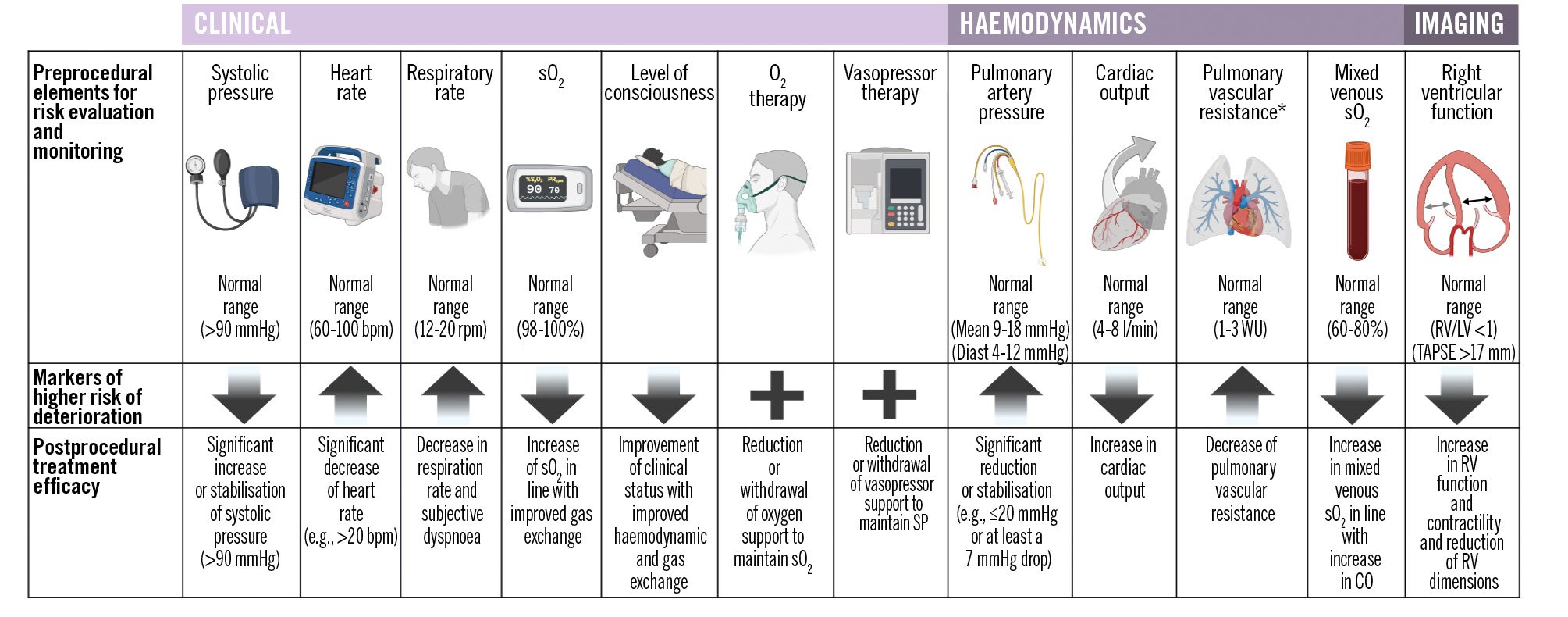
Figure 1. Clinical, haemodynamic, and imaging features for pre- and postprocedural monitoring. In the preprocedural phase, assessing these elements helps to evaluate patients’ clinical risk, identify signs of potential clinical deterioration, and inform decision-making regarding appropriate interventions. In the postprocedural phase, monitoring improvements in these same parameters provides information regarding treatment efficacy and guides further therapeutic strategies. *In the setting of right heart catheterisation during pulmonary embolism, the evaluation of total pulmonary resistance, as the ratio of mean pulmonary artery pressure (mPAP) to cardiac output (CO), may be preferred over pulmonary vascular resistance, which is calculated as the difference between mPAP and pulmonary capillary wedge pressure divided by CO, as pulmonary artery wedging can be challenging due to extensive thrombus distribution obstructing the pulmonary vasculature. Down arrows represent that a reduction in this element is a marker of deterioration; up arrows represent that an increase in the element is a marker of deterioration; the plus symbol represents that if this specific therapy is necessary, it is a marker of deterioration. bpm: beats per minute; Diast: diastolic; LV: left ventricular; O2: oxygen; rpm: respirations per minute; RV: right ventricular; sO2: oxygen saturation; SP: systolic pressure; TAPSE: tricuspid annular plane systolic excursion; WU: Wood units
Ultrasound
A preprocedural transthoracic echocardiogram (TTE) provides critical insights into right ventricular (RV) dysfunction, a major determinant of outcomes. TTE can also detect a clot in transit, raising the risk of paradoxical embolism through a patent foramen ovale (PFO) – a key piece of information for guiding treatment decisions. It can also reveal significant right-to-left shunting through a PFO, potentially worsening oxygenation. Furthermore, Doppler ultrasound of the lower extremities aids in assessing deep vein thrombosis and access site selection.
Computed tomography
Computed tomography (CT) scanning is essential for PE diagnosis and procedural planning5, providing detailed information on thrombus location and anatomical challenges for catheterisation (e.g., a wide main pulmonary artery [MPA] bifurcation angle, a subacute or chronic thrombus adhering to the vessel walls). Understanding vascular anatomy is crucial for correlating preprocedural and angiographic images and ensuring safe distal navigation during the procedure (Supplementary Figure 1). Extending the scan to abdominal and proximal lower limb segments helps exclude peripheral vein thrombosis to ensure safe vascular access. CT may also distinguish between acute and chronic PE, which is useful as chronic forms significantly influence the procedural approach and can impact outcomes (Supplementary Table 1).
Right heart catheterisation
Information from right heart catheterisation is instrumental in assessing the pre- and postprocedural haemodynamic status (Figure 1).
Pulmonary angiography
A preprocedural CT scan alone, especially with coronal reconstructions, is sufficient to plan and successfully guide the interventional procedure in most cases, thus avoiding multiple selective angiograms that could potentially destabilise the patient. However, selective pulmonary angiography may be necessary to re-evaluate the thrombus distribution before intervention if changes caused by medical therapy are expected (e.g., thrombolysis after CT or a long delay between CT and intervention). During pulmonary angiography, 10-20 ml of contrast is usually administered at 3-8 ml/s in the anteroposterior position for MPA and 30-40° homolateral oblique views for the right pulmonary artery and the left pulmonary artery. In complex anatomies, CT reconstruction can predict optimal projections during transcatheter interventions.
Device selection
A primary goal of transcatheter treatment is to rapidly and effectively decompress the right ventricle by reducing thrombus burden, decreasing RV afterload and increasing left ventricular (LV) preload, thereby improving systemic perfusion15. The decision on treatment selection between CDT or CDMT often relies on the clinical presentation, the location and burden of the thrombus, the predicted risk of bleeding, institutional availability and operator expertise (Central illustration)2. The proposed approach is based on currently available data and institutional experience. In haemodynamically unstable PE patients in whom systemic thrombolysis is contraindicated or has failed, CDMT should be preferred because of its faster improvement of haemodynamic status compared to CDT, where thrombolytic infusion occurs over 6-24 hours161718. In addition, in centres with appropriate expertise, extracorporeal membrane oxygenation (ECMO) support could be anticipated in order to rapidly stabilise a patient before mechanical reperfusion. In haemodynamically stable patients, treatment selection could be individualised based on the anatomical distribution of the thrombus and bleeding risk. In case of large volume clots located proximal to the origin of the segmental pulmonary arteries or clots in transit (located in the right atrium, RV, or PFO), large-bore CDMT may be preferred. Whereas, in case of a smaller volume, uniformly dispersed clot, or a clot that extends distal to the origin of the segmental pulmonary arteries, CDT may be preferred. In case of high bleeding risk patients and relative or absolute contraindications to thrombolysis (Table 1), CDMT should be preferred over CDT. Finally, the choice of a specific CDMT system should be based on operator experience and institutional availability, considering the trade-off between larger-bore devices (e.g., 20-24 Fr), which offer higher aspiration performance but increased stiffness, and medium-bore devices (e.g., 12-16 Fr), which provide better trackability and access to more distal segments at the expense of potentially lower aspiration force.
Recently, the PEERLESS trial provided the first direct comparison between large-bore CDMT and CDT in patients with intermediate-risk PE. Large-bore CDMT was performed using the FlowTriever system (Inari Medical), while CDT was conducted according to local standard practices, with 59.8% utilising ultrasound-accelerated CDT19. The composite primary endpoint occurred significantly less frequently with large-bore CDMT. This was driven by a lower rate of clinical deterioration, reduced need for escalation to bailout procedures, and decreased postprocedural intensive care unit (ICU) utilisation. There were no significant differences between the two groups in all-cause mortality, intracranial haemorrhage, or major bleeding events. Comparisons of CDMT and CDT with the standard care of systemic anticoagulation are currently under investigation in dedicated randomised controlled trials (ClinicalTrials.gov: NCT06055920, NCT04790370, NCT05591118, NCT05684796).
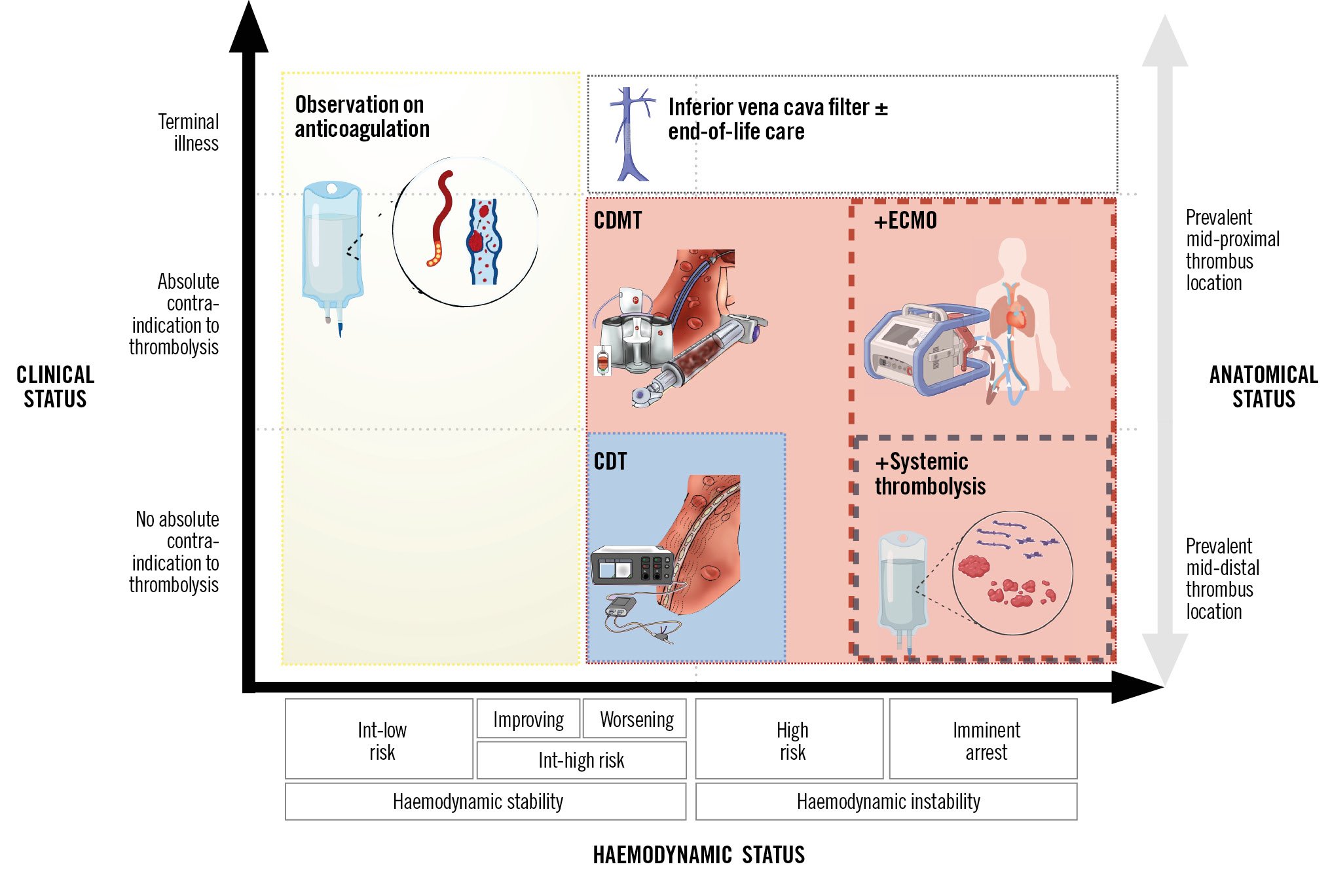
Central illustration. Catheter-directed therapy selection based on clinical presentation, bleeding risk and thrombus location. Treatment strategies based on clinical status with or without absolute contraindications to thrombolysis, haemodynamic stability and thrombus location. The red dashed line represents the option of having ECMO in addition to catheter-directed mechanical thrombectomy. The grey dashed line represents the option of applying concomitant systemic thrombolysis to catheter-directed mechanical thrombectomy. The blue box represents the area of possible application of CDT as an alternative to CDMT. CDMT: catheter-directed mechanical thrombectomy; CDT: catheter-directed thrombolysis; ECMO: extracorporeal membrane oxygenation; int: intermediate
Table 1. Risk features for high bleeding risk, and relative and absolute contraindications to thrombolysis.
| High bleeding risk features during antithrombotic therapy | Relative contraindications to thrombolysis | Absolute contraindications to thrombolysis |
|---|---|---|
| Advanced age (>75 years)Advanced renal diseaseActive cancerSevere anaemia (<11 g/dl)Recent spontaneous bleeding (<6 months)Bleeding diathesis | Recent transient ischaemic attack (<6 months)Ongoing oral anticoagulationPregnancy or first postpartum weekNon-compressible puncture sitesTraumatic resuscitationUse of ECMOAdvanced liver diseaseInfective endocarditisActive peptic ulcerRefractory hypertension (systolic BP >180 mmHg) |
Prior haemorrhagic strokeRecent ischaemic stroke (<6 months)Central nervous system neoplasmRecent major trauma, surgery, or head injury (<3 weeks)Current active bleeding |
| BP: blood pressure; ECMO: extracorporeal membrane oxygenation | ||
Catheter-directed thrombolysis
CDT allows the local infusion of a low-dose thrombolytic agent into the pulmonary artery or directly into the thrombus. Currently, two main techniques are available: conventional local thrombolysis, which can be performed via a common pigtail catheter or via dedicated catheters (Uni-Fuse [AngioDynamics]; Cragg-McNamara [Medtronic]; Pulse*Spray infusion system [AngioDynamics]; BASHIR [Thrombolex]), and ultrasound-accelerated catheter-directed thrombolysis, in which a dedicated catheter emits 2 MHz ultrasound waves during thrombolytic infusion to allow the disaggregation of fibrin fibres and drug penetration into the thrombus. The EKOS system (Boston Scientific) is currently the most commonly used dedicated CDT system. According to a European survey, it is implemented in up to 20.2% of catheter-directed cases20 and has been investigated in multiple randomised trials2122. Nevertheless, there is still a lack of definitive evidence to support the superiority of dedicated CDT devices over standard catheters for local thrombolysis, which should be considered based on their lower cost and ease of use22.
Procedural steps, tips and tricks
The EKOS system is composed of an infusion catheter with an ultrasonic core and a control unit with connector interface cables (Figure 2). The device is available in various lengths – 6, 12, 18, 24, 30, 40, and 50 cm – allowing selection based on the measured length of the thrombus. For pulmonary clots, shorter lengths, such as 12 and 18 cm, are generally preferred. First, central venous access is established, typically via the femoral vein, or alternatively through the jugular or basilic vein, with echo guidance recommended to detect the thrombus and reduce complications. Two packages are used: one for the infusion catheter and one for the ultrasonic core. System priming is obtained by flushing the catheter drug lumen with heparin and the coolant lumen with heparinised saline. Based on thrombus distribution, single-sided or two-sided therapy could be selected. Once the target pulmonary vessel is reached with standard catheters, a 0.035’’ exchange length guidewire is used to place the distal marker of the infusion catheter 1 cm beyond the target clot (Figure 2B). Catheter placement in the basal segments should be preferred to increase system stability and reduce complications. Once the infusion catheter is in place, the 0.035” wire is removed and the ultrasonic core is advanced into the guidewire lumen, paying attention not to kink or bend it (Figure 2C, Figure 3). When the ultrasonic core is locked into the manifold, the infusion of heparinised saline through the coolant port is started at 35 ml/h, and the infusion of the lytic through the drug port is started at 5-35 ml/h at the desired dosage (e.g., 4-10 mg recombinant tissue plasminogen activator per lung for 2-12 hours depending on the chosen protocol) (Table 2, Figure 2D). The interface cable is connected to the control unit and then the device cables to the single interface unit (Figure 2E). When the device is properly connected, the instructions on the screen should be followed to begin ultrasound emission (Figure 2F). In summary:
• Use ultrasound guidance for safe venous access and detecting peripheral vein thrombi.
• Choose an infusion catheter length that matches the thrombus size to ensure effective thrombolysis.
• Decide on single or bilateral catheter placement based on thrombus distribution. Position the infusion catheter’s distal marker 1 cm beyond the target clot, preferentially in basal segments to enhance stability and reduce complications.
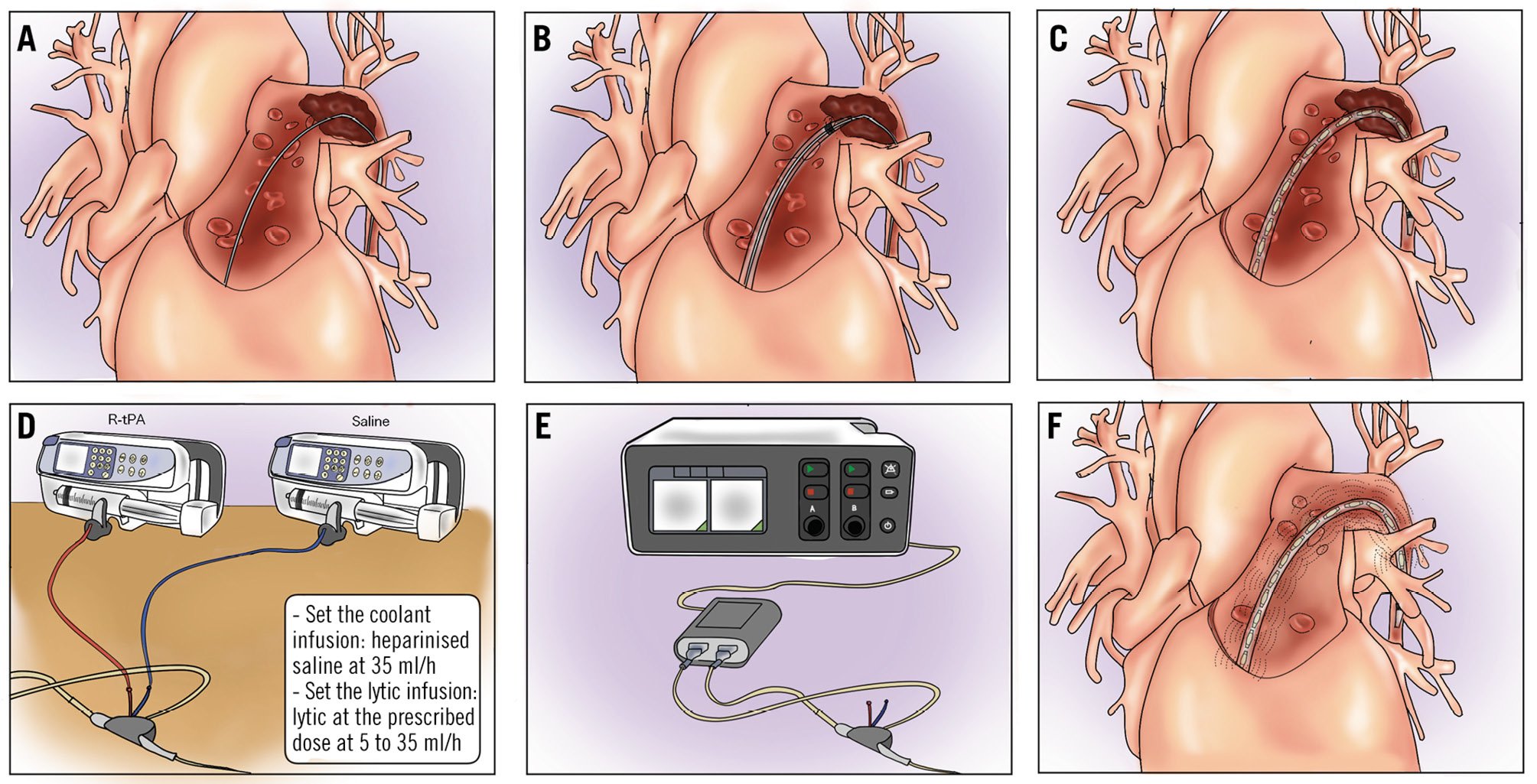
Figure 2. Ultrasound-accelerated catheter-directed thrombolysis with the EKOS system. A) Guidewire positioning (basal segments are preferred). Positioning of the EKOS infusion catheter over the wire and guidewire removal (B). Positioning of the ultrasound probe in the infusion catheter (C). Setting the infusion of the thrombolytic agent and coolant solution; 2 infusion systems are needed for each EKOS catheter (D). Once the infusion of the coolant and thrombolytic agent has been started, ultrasound emission may be initiated (E). Leave the infusion catheter in place during treatment for the prescribed duration (F). Check the result at bedside by temporarily interrupting the emission of ultrasound from the device console. EKOS system by Boston Scientific.
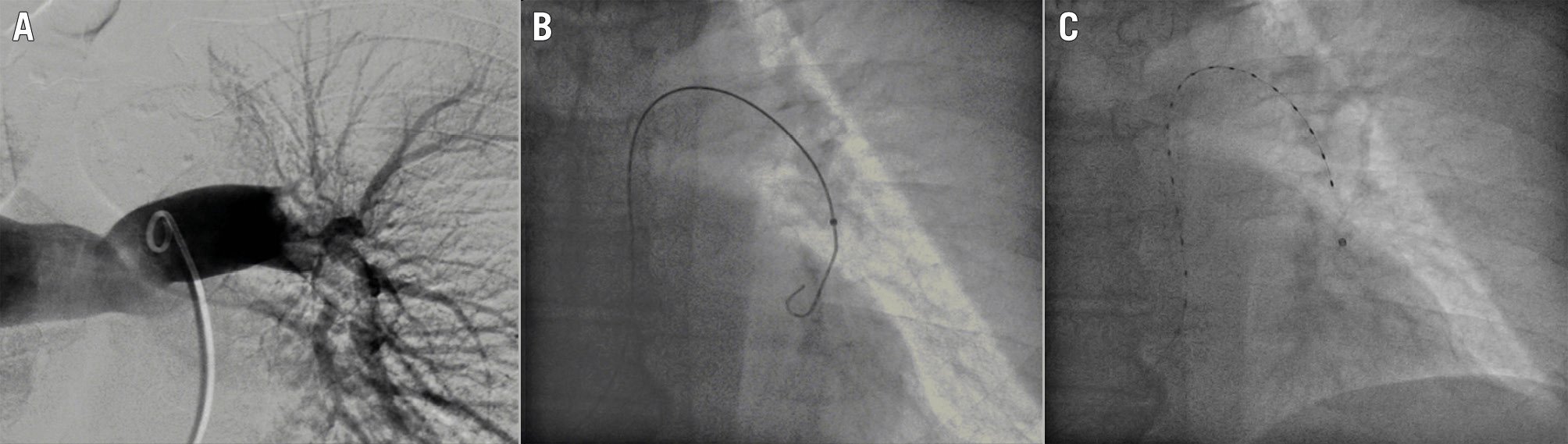
Figure 3. Ultrasound-accelerated catheter-directed thrombolysis with the EKOS system: clinical case. Pulmonary embolism with nearly complete occlusion of the left pulmonary artery (A). Positioning of the guidewire in the basal segment of the left pulmonary artery (B). EKOS infusion and ultrasonic catheter in place (C). EKOS system by Boston Scientific.
Table 2. Dose regimens of tissue plasminogen activator tested in clinical trials of catheter-directed thrombolysis.
| Dose regimens of tPA | |
|---|---|
| Dose regimen of tPA implemented in the OPTALYSE PE trial35 | |
| Arm 1 | 4 mg per lung over 2 h |
| Arm 2 | 4 mg per lung over 4 h |
| Arm 3 | 6 mg per lung over 6 h |
| Arm 4 | 12 mg per lung over 6 h |
| Dose regimen of tPA implemented in the SEATTLE II trial36 | |
| Unilateral PE (1 catheter) | 1 mg/h over 24 h |
| Bilateral PE (2 catheters) | 1 mg/h per lung over 12 h |
| Dose regimen of tPA implemented in the HI-PEITHO trial8 | |
| Unilateral PE (1 catheter) | 9 mg, 2 mg bolus followed by1 mg/h infusion over 7 h |
| Bilateral PE (2 catheters) | 18 mg, 2 mg bolus followed by1 mg/h infusion over 7 h per lung |
| PE: pulmonary embolism; tPA: tissue plasminogen activator | |
Catheter-directed mechanical thrombectomy
Contemporary CDMT involves aspirating thrombi from the pulmonary artery using percutaneous catheters via femoral or jugular venous access. There are currently two devices that are approved for percutaneous treatment of PE and do not require the support of a perfusionist: the FlowTriever system and the Lightning Flash thrombectomy system (Penumbra)2324. The additional benefit of these systems is that they can be used in patients who have an absolute or relative contraindication to thrombolytics and, off-label, can even be used without systemic anticoagulation. The FlowTriever is a large-bore catheter system comprising a 24 Fr catheter connected to a high-pressure syringe to create a vacuum (with other accessories such as a longer 16 Fr catheter, a curved 20 Fr catheter, and a set of nitinol disks to fragment the thrombus). This system is effective for rapid clot debulking but requires a large vascular access and may be more challenging if needed for reaching a distal clot (Figure 4, Figure 5). Contrarily, the Lightning system uses a 12 Fr catheter connected to an automatic negative pressure pump that allows selective aspiration when engagement with the clot is sensed and allows a separator to be used when the clot obstructs the catheter lumen (Figure 6, Figure 7). The reduced profile allows for a smaller access site and can address a clot that is located more distally in the pulmonary tree but also limits its aspiration capability with larger clots25. Lately, a new 16 Fr system with computer-assisted vacuum thrombectomy (CAVT; Penumbra) technology has been introduced to increase aspiration power and reduce blood loss. The 16 Fr catheter, available with a single or a double curve, allows trackable and atraumatic advancement even without a guidewire in place.
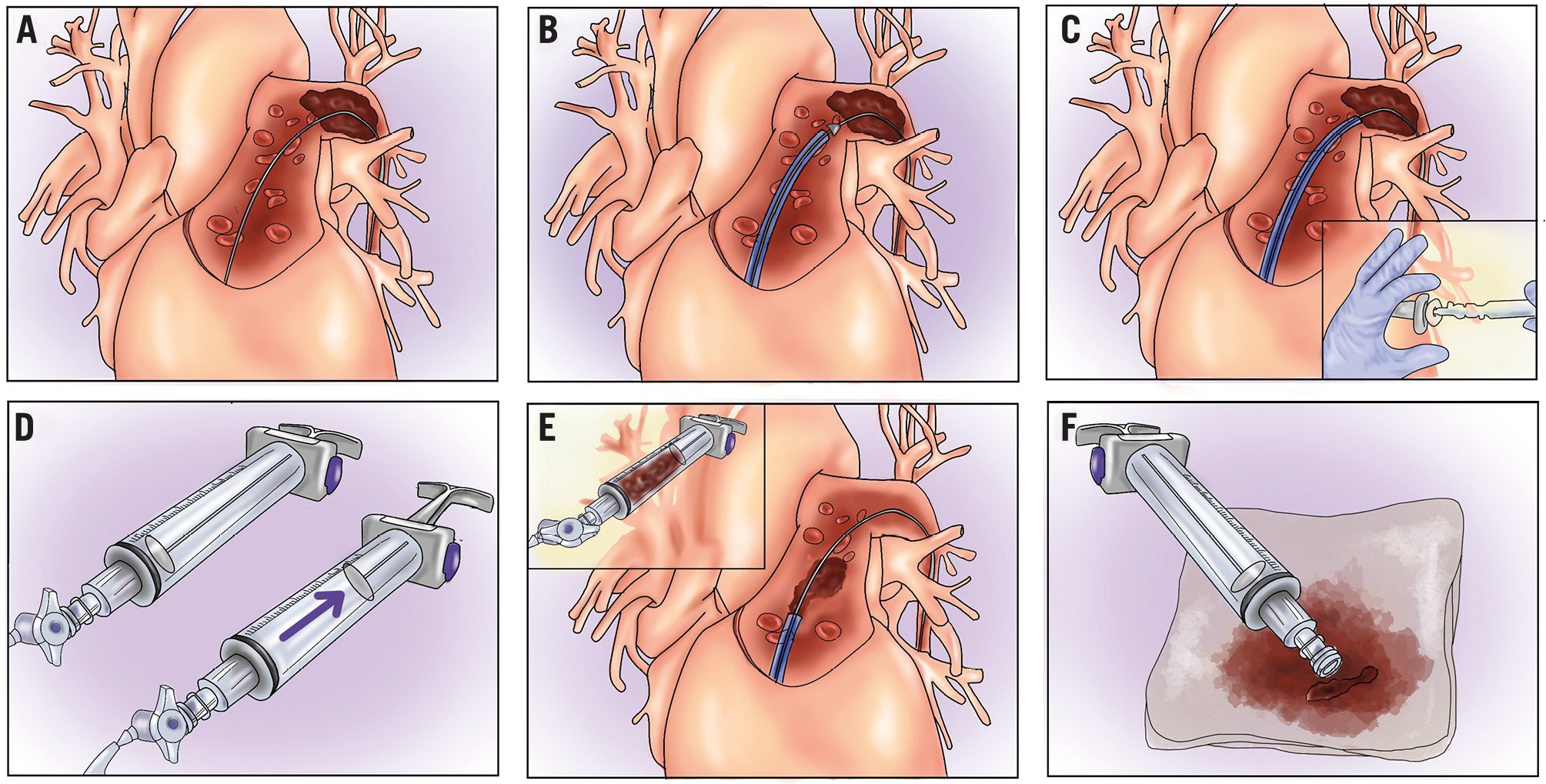
Figure 4. Catheter-directed mechanical thrombectomy with the FlowTriever system. A) Guidewire positioning (generally basal segments are preferred with a high support wire). Advance the aspiration catheter with the inner dilator to avoid vascular damage and approach the thrombus (B). Remove the inner dilator, leaving the guidewire in place to avoid vascular damage (C). Create a vacuum with the syringe aspirator (D) and open the pressure valve to aspirate the thrombus; aspirating in proximity to the thrombus with the “whoosh” technique is advised (E). Remove thrombi or use the dedicated FlowSaver filter (Inari Medical) for blood return (F). FlowTriever system by Inari Medical.
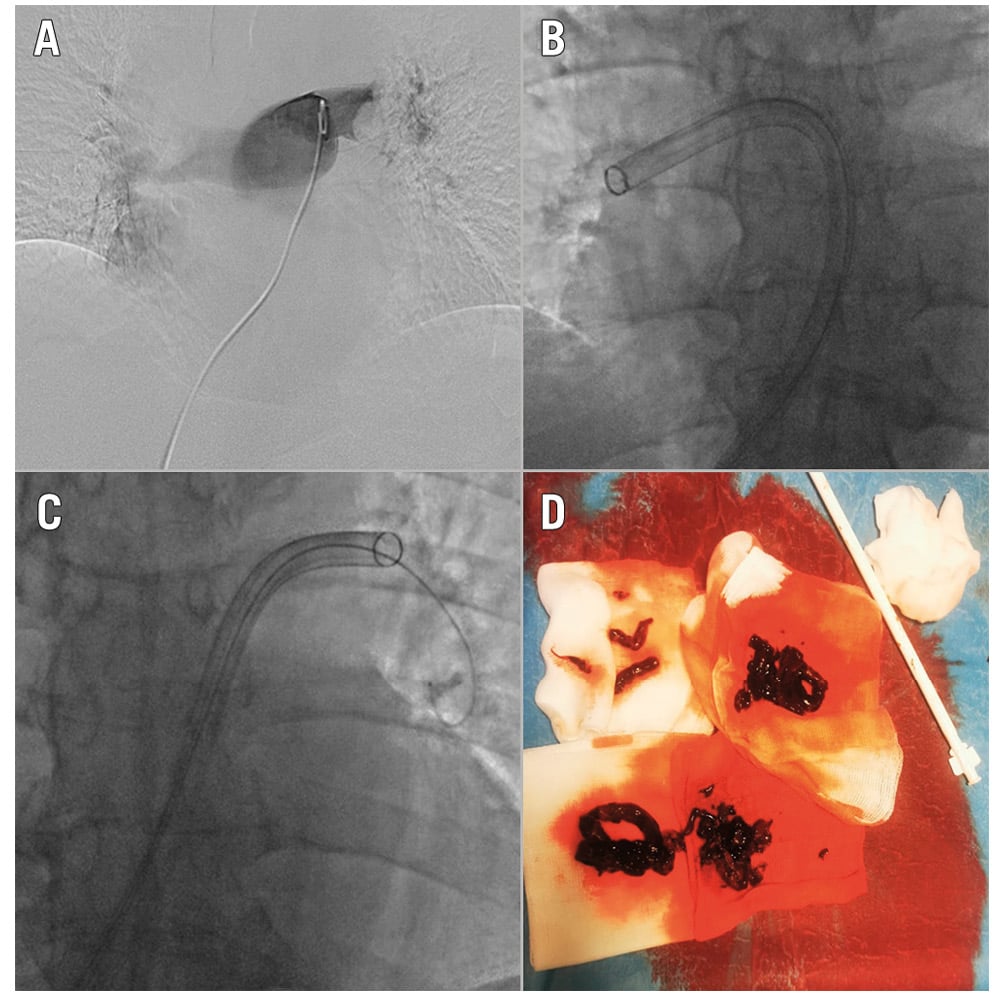
Figure 5. Catheter-directed mechanical thrombectomy with the FlowTriever system: clinical case. Pulmonary angiography with evidence of bilateral pulmonary embolism (A). FlowTriever system (Inari Medical) in the right and left pulmonary arteries (B-C). Large clot burden removed after thrombus aspiration (D).
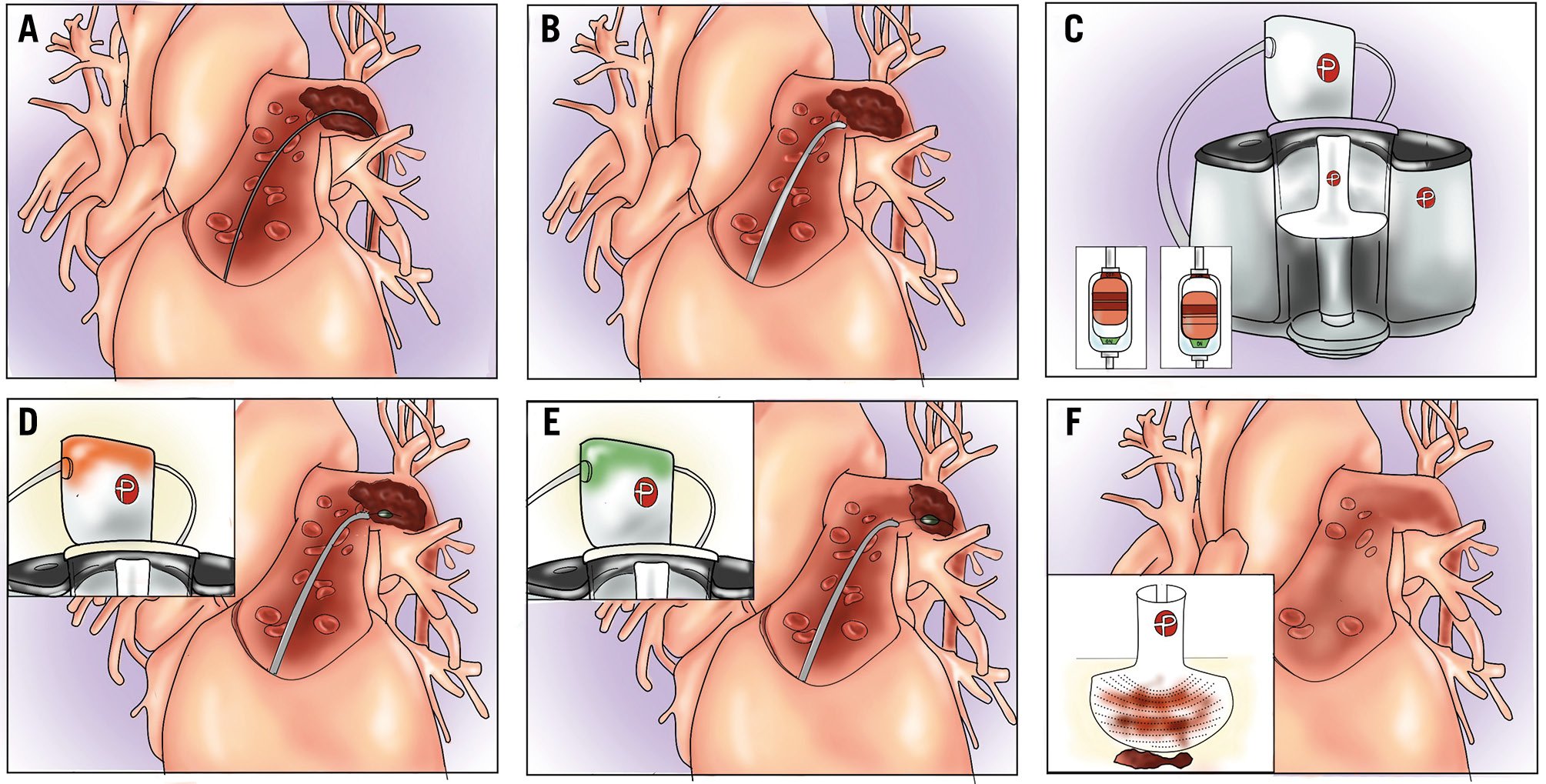
Figure 6. Catheter-directed mechanical thrombectomy with the Lightning Flash system. A) Guidewire positioning (generally basal segments with high support wire are preferred). Advance the aspiration catheter over the wire and approach the thrombus (B). Activate the aspiration switch (C). Discontinuous aspiration will start removing thrombotic material (flashing green light). If the thrombus totally obstructs the catheter lumen, a flashing yellow light will appear (D). Reposition the catheter or use the separator to re-establish aspiration. A flashing green light will confirm successful aspiration (E). Check the amount of thrombus and blood aspirated in the console filter (F). Lightning Flash system by Penumbra.
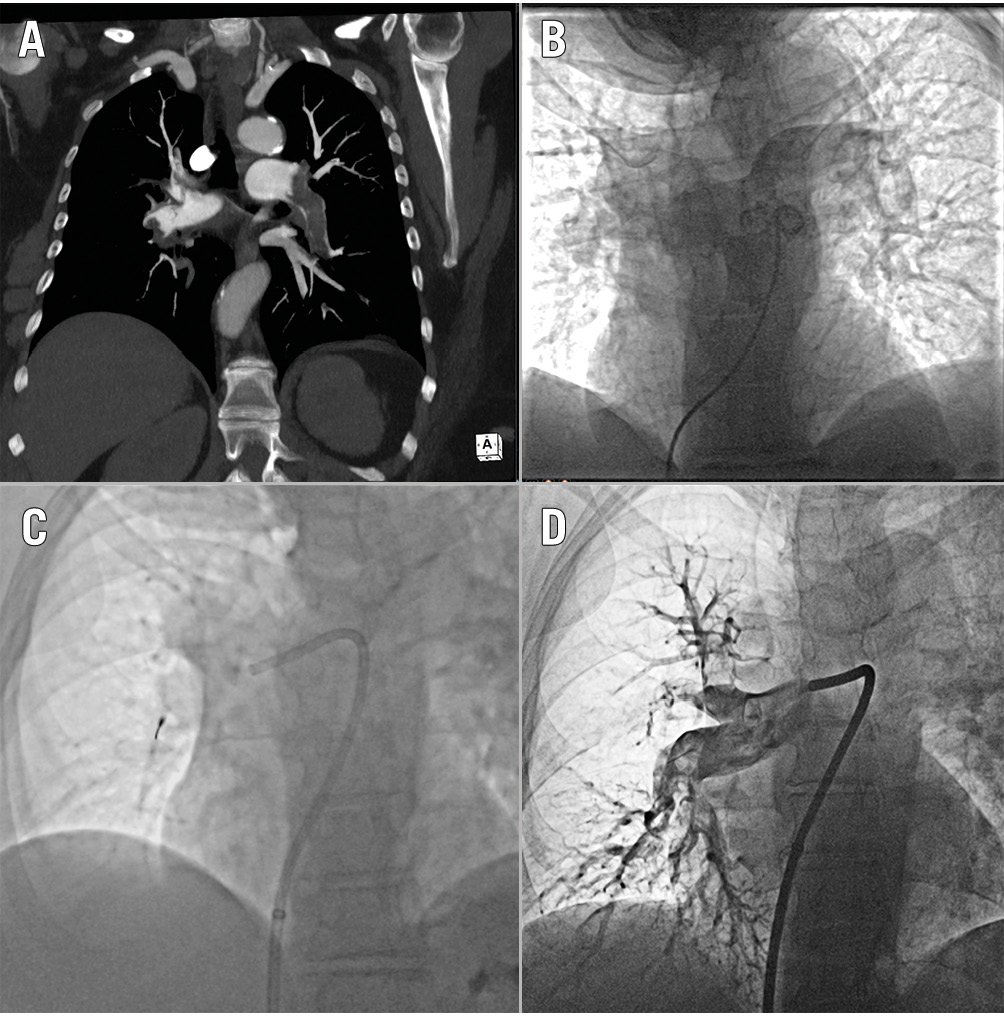
Figure 7. Catheter-directed mechanical thrombectomy with the Lightning Flash system: clinical case. CT angiogram showing bilateral pulmonary embolism (A). Baseline pulmonary artery angiogram (B). Selective cannulation of the right pulmonary artery with the Lightning Flash catheter and separator advanced in the right interlobar artery (C). The postprocedural selective angiogram of the right pulmonary artery shows revascularisation of the interlobar artery with moderate residual thrombus. Lightning Flash system by Penumbra. CT: computed tomography
Procedural steps, tips and tricks
After obtaining venous access, a balloon catheter (or pigtail, alternatively) should be used to advance through the tricuspid valve into the pulmonary artery, preventing entanglement or damage to the subvalvular apparatus by larger catheters. Right heart catheterisation and pulmonary angiography should be performed as previously mentioned. Vessel selection for interventional treatment should prioritise segments with total occlusion without distal visualisation. In cases of subtotal occlusion, a lower success rate and the risk of possible distal embolisation with clinical destabilisation should be considered. A multipurpose catheter with a 0.035” wire is commonly used to cross the thrombus and gain distal position. Hydrophilic guidewires are effective in challenging situations, but an increased risk of distal perforation should be considered. A torqueable, soft-tip wire (e.g., Wholey wire [Medtronic]) may also be useful in challenging cases. Proper distal positioning of the catheter is essential for advancing a high support guidewire with a short, floppy tip (1 cm). This positioning ensures stability when introducing large-bore catheters and minimises the risk of distal perforation. The lower branches of the right or left pulmonary artery (e.g., segments A8-A10) (Supplementary Figure 1) should be preferred, and it is important to confirm that the target vessel is large enough to accommodate the procedure. Lingula segments should be avoided because of the increased risk of distal perforation. After proper wire positioning, the CDMT system can be introduced. The FlowTriever navigates directly from a short femoral sheath, while the Lightning system usually is used in combination with a long sheath (e.g., 60 cm or 80 cm) that reaches the main pulmonary artery. During advancement, special care should be taken in patients with previous left bundle branch block, as instrumentation with large-bore catheters of the right ventricular outflow tract might induce complete heart block.
CDMT with Inari Medical’s FlowTriever
If the FlowTriever catheter is selected, it is safely advanced using dedicated catheter dilators that cover the sharp edge of the catheter, avoiding vascular wall damage (razor effect) (Figure 4B). Navigation is usually simple; however, in case of challenging catheter tracking, an “inverted” mother-and-child technique, advancing the larger catheter through a narrower and longer one could be useful (Figure 8). CDMT may be initiated in the right pulmonary artery as it provides greater stability, simpler navigability, and a higher aspiration-to-time ratio. Once the target thrombus is reached, aspiration can be initiated, maintaining the guidewire in place, which reduces the risk of potential vascular damage. Aspiration attempts should be initiated from proximal to distal, with the catheter tip proximal to the thrombus to avoid catheter obstruction (Figure 4C-Figure 4D-Figure 4E). If free-flowing blood with no clot is aspirated, the catheter should be repositioned closer to the thrombus. If the aspirated material is a mix of blood and clot, additional aspirations can be performed at the discretion of the operator. Considering the significant amount of blood loss with these manoeuvres, careful evaluation of the total amount of blood removed and the baseline haemoglobin values should be considered during the procedure to avoid severe anaemia. To limit this issue, blood reinfusion systems (e.g., FlowSaver [Inari Medical]) have recently become available. In case the aspirated thrombus is occluding the catheter tip during aspiration, it is recommended to perform a second additional aspiration (“double whoosh”) to retrieve the clot. If this is not effective and the catheter remains obstructed during aspiration, removing the catheter over the wire while maintaining suction (“lollipop” technique) should be considered. This manoeuvre should be performed with care to avoid embolisation and should not be performed in case of a PFO due to a possible risk of paradoxical embolisation26. After performing thrombectomy in the first artery or branch, the catheter may be safely redirected to different vessels using a parallel wire technique (Figure 8). The previous steps are repeated until the procedure is concluded. In case of more distal thrombus retrieval from smaller pulmonary branches, distal aspiration with the 16 Fr catheter can be considered using a child-in-mother technique (Figure 8). Thrombi that are adherent to the vessel wall can be difficult to reach and remove, particularly in the inner curve at the level of the interlobar artery, as the guidewire tends to follow the outer curve, preventing the catheter from making contact with the thrombus. In such cases, the Triever20 Curve catheter (Inari Medical), a 20 Fr steerable catheter, is advanced within the 24 Fr system with the distal tip curving up to 225° to approach wall-adherent thrombi. This is particularly useful in the left pulmonary artery interlobar segment, where the curved catheter has a higher chance of being coaxial with the distal vessel and making contact with the thrombus. In cases of a subacute/chronic thrombus that is highly adherent to the vessel wall, mechanical thrombectomy using nitinol disks can be performed. The disks, which are sized according to the target vessel diameter, are advanced inside the thrombus and left in place for 2 minutes to allow slight thrombus compression, then removed. Following this procedure, it is advisable to attempt thrombus aspiration again, as its effectiveness tends to be higher after disk use. In summary:
• Cross the tricuspid valve with a balloon-tipped catheter to avoid chordae entanglement.
• Use a catheter dilator when advancing it to avoid vessel wall injury.
• Start aspiration proximal to the thrombus to prevent catheter blockage.
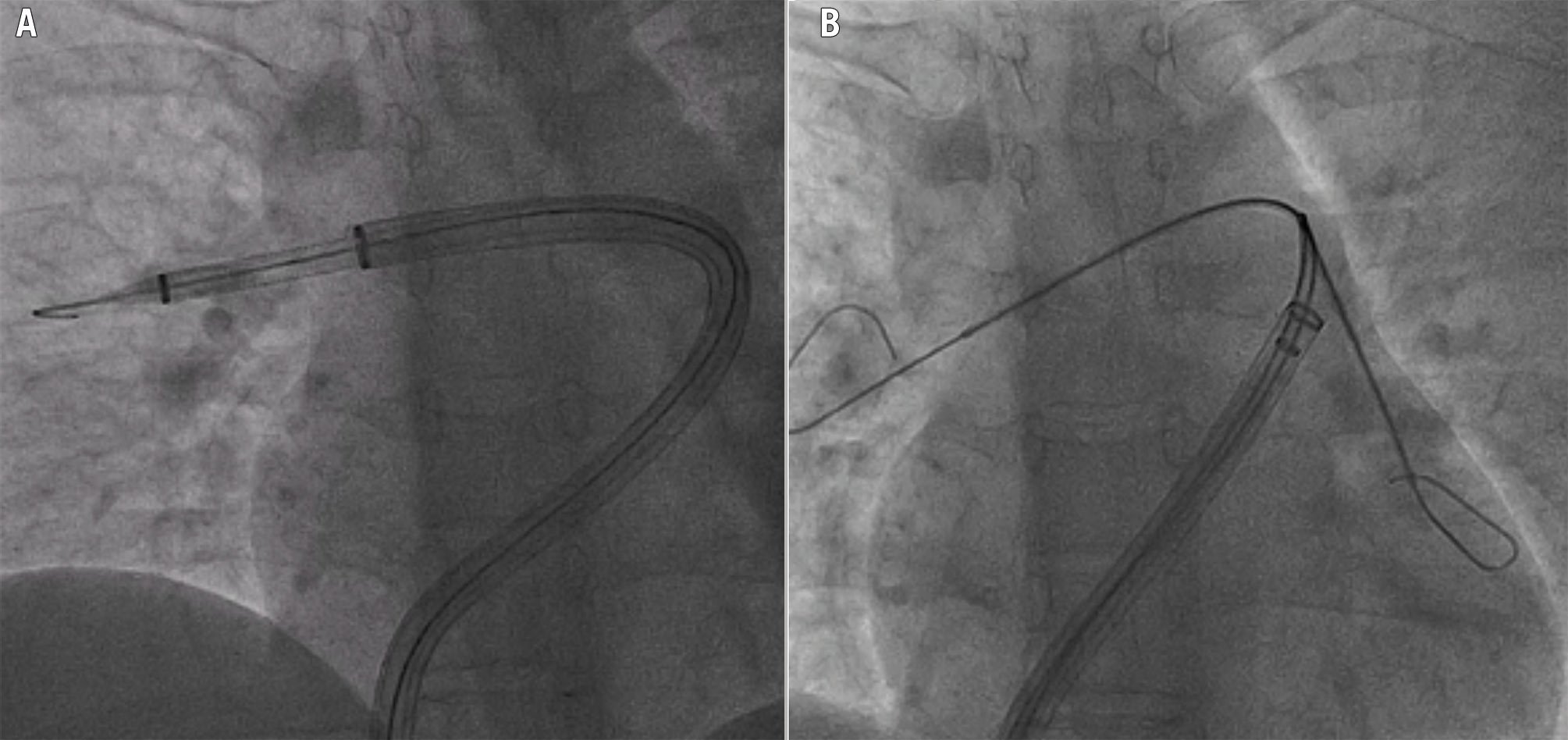
Figure 8. Techniques of catheter-directed mechanical thrombectomy in challenging anatomies. Inverted mother-and-child technique to facilitate FlowTriever 24 Fr catheter (Inari Medical) advancement over a FlowTriever 16 Fr distal catheter (A). Parallel wire technique to switch selective cannulation of a FlowTriever 24 Fr catheter from the right to the left pulmonary artery in a challenging anatomy (B). The extra support wire is maintained in the first branch to stabilise the catheter and prevent withdrawal into the right ventricle; the catheter is retrieved enough to be able to insert and advance a multipurpose long catheter (parallel to the first wire) into the next branch. After positioning the long catheter distally in that branch, a high support wire is advanced through it, the first wire can be retrieved, and the catheter is advanced over the new high support wire towards the second branch.
CDMT with Penumbra Lightning
If the Penumbra system is used, the same technique could be used to cross the tricuspid valve and track the device in proximity to the clot (Figure 6, Figure 7). A 16 Fr long sheath could be useful to improve device tracking with the 12 Fr catheter, while such a sheath is not necessary with the new 16 Fr catheter. Once the catheter has been advanced and the clot has been reached, aspiration can be initiated by turning on the flow switch and advancing the catheter through the thrombus (Figure 6B, Figure 6C). The 12 Fr Lightning system provides intermittent intelligent aspiration to reduce blood loss, accompanied by a light and sound indication of the aspiration status: a flashing green light indicates intermittent aspiration of the clot from the catheter. In case of catheter obstruction, a flashing yellow light will indicate the need to redirect the catheter or use the separator to break the clot and continue aspiration (Figure 6D, Figure 6E). The amount of clot/blood aspirated can be checked through the console. The new CAVT technology allows for more precise aspiration, signalling with a solid green light when the catheter has not reached the thrombus, and a solid yellow light when the thrombus has been reached, at this stage activating faster suction (which is also associated with a higher frequency sound from the aspiration device) that forces clot extraction and automatically reduces the aspiration force once the resistance reduces. In case the catheter is totally obstructed by the thrombus, as prevously mentioned, a flashing yellow light will appear, indicating the need to change catheter position or proceed to catheter removal (lollipop technique). In summary:
• Cross the tricuspid valve with a balloon-tipped catheter to avoid chordae entanglement.
• Pay close attention to the system’s visual and auditory cues to optimise aspiration and minimise blood loss.
• In case of obstruction with the 12 Fr system, use the separator to re-establish aspiration.
Termination of the CDMT procedure
After CDMT, follow-up angiograms can be performed either directly via the thrombectomy catheter or through a pigtail catheter, ensuring that the catheters are free of thrombi (Figure 5, Figure 7). There is no consensus on the definition of reperfusion success with CDMT. As a general rule, removal of all the thrombotic material is not necessary, as clinical improvement is generally obtained even with modest clot extraction. Procedural elements that inform the conclusion of a CDMT are summarised in Table 327. After catheter removal, haemostasis is achieved with different techniques according to operator preferences – figure-of-eight stitch, purse-string suture, Perclose device (Abbott), or manual compression.
Table 3. Procedural elements for informing treatment cessation during catheter-directed mechanical thrombectomy.
| Procedural elements |
|---|
| 1. Improvement of haemodynamic status, achieving clinical stability |
| 2. Improvement in mean pulmonary artery pressure (e.g., ≤20 mmHg or at least a 7 mmHg drop) |
| 3. No more thrombi can be reasonably removed (e.g., all proximal thrombi have been removed; only a chronic or highly organised thrombus remains, or anatomical impediments to reaching the thrombus) |
| 4. Unfavourable blood loss trade-off (e.g., blood loss over 400 cc) |
Control of postprocedural treatment efficacy and monitoring
Postprocedural treatment efficacy should be evaluated based on haemodynamic, imaging and laboratory parameters, with signs of clinical improvement generally observed immediately after CDMT, and 2-6 hours after CDT (Figure 1). From a clinical standpoint, systemic blood pressure and heart rate are simple clinical markers of early improvement. A European consensus paper suggests the use of the National Early Warning Score (NEWS) as a monitoring tool to detect clinical deterioration28. Although there is a lack of evidence supporting this recommendation, the same tool could also be used to monitor clinical improvement. Echocardiography is useful to evaluate treatment efficacy at bedside, yet in case of CDT with EKOS, ultrasound emission should be interrupted to avoid artefacts. Non-invasive imaging markers of treatment response are a change in RV/LV diameter ratio from baseline, pulmonary artery systolic pressures, change in right atrial dimension, tricuspid annular plane systolic excursion (TAPSE) and other indices of RV contractility. In a meta-analysis evaluating the clinical and haemodynamic impact of ultrasound-assisted CDT in patients with intermediate- or high-risk pulmonary embolism, after treatment the RV/LV ratio was reduced by 35% (95% confidence interval [CI]: 30-40%), pulmonary artery systolic pressure by 16.69 mmHg (95% CI: 13.65-19.73 mmHg), while TAPSE improved by 3.68 mm (95% CI: 2.43-4.93 mm) and cardiac index by 0.68 l/m2 (95% CI: 0.49-0.87 l/m2)29. Similar results have been observed with echocardiography 48 hours after CDMT: in the FLASH registry, the echocardiographic RV/LV ratio at 48 hours decreased from 1.23±0.36 to 0.98±0.31, while RV systolic pressure was reduced by 13.4 mmHg30; in the interim analysis of the STRIKE-PE registry, the RV/LV ratio at 48 hours decreased from 1.39±0.35 to 1.01±0.2131. Laboratory testing could also be useful to guide postprocedural treatment strategy. Reduction of lactates is a key element to determine circulatory improvement. Serial monitoring of troponin32 or N-terminal pro-brain natriuretic peptide33 has been evaluated in small series. It is important that haemoglobin values are evaluated after CDMT, as significant blood loss can occur. After CDT, on the other hand, fibrinogen levels can be monitored (e.g., every 2 hours) during thrombolytic infusion to limit the risk of bleeding complications. Postprocedural close monitoring of the patient in an intermediate or critical care unit is recommended after either CDT or CDMT.
Complications
While catheter-directed therapies have generally demonstrated low complication rates in expert centres34, the potential risk for life-threatening complications should be acknowledged and anticipated (Table 4). In recent international registries, major bleeding has been reported in up to 5% and 9% of CDT- and CDMT-treated patients, respectively, while intracranial haemorrhage has remained rarely observed, in less than 1% of cases. In-hospital cardiac arrest has been observed in 4% and 8% of patients, while in-hospital mortality has been observed in 4% and 10% of patients with CDT and CDMT, respectively12.
Specific complications relative to transcatheter interventions are presented in Table 4 and extensively discussed in Supplementary Appendix 1.
Table 4. Potential complications of catheter-directed therapies for pulmonary embolism.
| Complication | Frequency | Cause | Solution |
|---|---|---|---|
| Sudden haemodynamic collapse | Uncommon | Fragmentation of the thrombus during mechanical thrombectomy increases pulmonary resistance | Increase inotropesVA-ECMOIntraprocedural thrombolysis |
| Increased right ventricular strain due to excessive catheter manipulation | Catheter removalIntraprocedural thrombolysisVA-ECMO | ||
| Incorrect tricuspid valve crossing determining acute tricuspid insufficiency or rupture | Catheter removalCardiac surgery | ||
| Free wall perforation and cardiac tamponade | PericardiocentesisCardiac surgery | ||
| Haemoptysis | Uncommon | Guidewire tip perforation | Ventilatory support, selective intubation if neededReverse heparinBalloon inflationSelective coiling |
| Large branch perforation | Ventilatory support, selective intubation if neededReverse heparinBalloon inflationCardiac surgery | ||
| Reperfusion injury | Ventilatory support, selective intubation if needed | ||
| Large branch dissection | Rare | Excessive catheter manipulation | Generally self-limitingCTA during FUBalloon inflation |
| Paradoxical embolism | Rare | Systemic embolism through a patent foramen ovale or other congenital defects | Specific management according to the embolism site |
| Non-pulmonary bleedings | Uncommon | Excessive antithrombotic treatment or side effect of thrombolysis | Stop thrombolysisReverse heparinSpecific management according to the bleeding site |
| CTA: computed tomography angiography; FU: follow-up; VA-ECMO: venoarterial extracorporeal membrane oxygenation | |||
Future technologies
Several devices will expand the armamentarium for PE treatment. These are presented in Table 5 and extensively discussed in Supplementary Appendix 2.
Table 5. Novel devices and future technologies for catheter-directed treatment of pulmonary embolism.
| Device | Description | FDA approval status | CE approval status | Study/trial | NCT number | Population number (n) |
|---|---|---|---|---|---|---|
| AlphaVac F18a | Thrombectomy device for mechanical thrombus aspiration | FDA-approved | CE-approved | APEX-AV | NCT05318092 | 122 |
| Hēlo PEb | Thrombectomy device combining mechanical clot disruption with aspiration | Currently not approved | Currently not approved | ENGULF | NCT05597891 | 25 |
| WOLFc | Thrombectomy device using finger-like prongs for controlled emboli removal | Approved for peripheral arteriesCurrently not approved for PE | Currently not approved | N/A | N/A | N/A |
| JETid | Thrombectomy device with a high-pressure jet of saline to break up thrombus to facilitate aspiration | Approved for peripheral arteriesCurrently not approved for PE | Currently not approved | N/A | N/A | N/A |
| Lagunae | Thrombectomy device combining mechanical clot disruption with aspiration | Approved for peripheral arteriesCurrently not approved for PE | Currently not approved | TRUST | NCT06041594 | 107 estimated patients |
| Cleaner Prof | Thrombectomy device combining thrombectomy and infusion of thrombolytics | Approved for peripheral arteriesCurrently not approved for PE | Currently not approved | CLEAN-PE | NCT06189313 | 125 estimated patients |
| Katanag | Thrombectomy device combining rheolytic-powered aspiration and directional features | Currently not approved | Currently not approved | QUADRA-PE | NCT06672510 | 118 estimated patients |
| Magneto eTrieveh | Thrombectomy device combining large-bore aspiration with electromechanical thrombus extraction | Currently not approved | Currently not approved | Evaluation of the Safety and Performance of Magneto PE Kit | NCT04949048 | 10 |
| aBy AngioDynamics; bby Endovascular Engineering; cby Boston Scientific; dby Abbott; eby Innova Vascular; fby Argon Medical Devices; gby Akura Medical; hby Magneto Thrombectomy Solutions. CE: European Conformity; FDA: U.S. Food and Drug Administration; N/A: not applicable; PE: pulmonary embolism | ||||||
Conclusions
While anticoagulation remains the foundation of PE treatment and the main treatment option in most PE patients, the advent of catheter-directed therapies offers promising alternatives for selected patients. A comprehensive, patient-centred approach, integrating advanced catheter technologies with meticulous procedural planning, remains paramount in the evolving landscape of PE treatment. Future randomised controlled trials, together with new devices and techniques, hold the potential to significantly refine our understanding and application of these interventions, aiming to improve patient outcomes and minimise risks.
Acknowledgements
We thank Rita Lauro for her contribution in the original design and production of the manuscript illustrations.
Conflict of interest statement
F. Götzinger is supported by the German Foundation for Heart Research (Deutsche Herzstiftung); and has received speaker honoraria from AstraZeneca. E.A. Secemsky has received grants to his institution from Abbott/CSI, BD, Boston Scientific, Cook, Medtronic, and Philips; additionally, he has received speaker honoraria and consulting fees from Abbott/CSI, BD, BMS, Boston Scientific, Cagent, Conavi, Cook, Cordis, Endovascular Engineering, Gore, InfraRedx, Medtronic, Philips, RapidAI, Rampart, Shockwave Medical, Siemens, Terumo, Thrombolex, VentureMed, and Zoll. F. Mahfoud is supported by the Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung; his institution, Saarland University, has received scientific support from Ablative Solutions, Medtronic, and Recor Medical; he has also received speaker honoraria and consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari Medical, Medtronic, Merck, Recor Medical, Servier, and Terumo. A. Jurado-Román is a proctor for Abbott, Boston Scientific, World Medica, and Philips; has received consulting fees from Boston Scientific and Philips; and has received speaker fees from Abbott, Boston Scientific, Shockwave Medical, Philips, and World Medica. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

