Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Transcatheter mitral valve replacement (TMVR) offers a potential treatment option for select patients with mitral regurgitation (MR) deemed unsuitable for surgery or transcatheter repair, but data are limited on its long-term durability and performance.
Aims: We evaluated 5-year outcomes from the global Pilot Study with the Intrepid transapical (TA) TMVR system.
Methods: This multicentre, single-arm study evaluated the early-generation Intrepid TA system in patients with symptomatic ≥moderate-severe MR at high risk for mitral valve (MV) surgery. Echocardiograms and clinical events were independently adjudicated, and patients were followed for up to 5 years.
Results: Ninety-five patients were enrolled at 21 sites between 2015 and 2019. The mean age was 74.0±9.2 years, 43.2% of patients were female, the mean Society of Thoracic Surgeons Predicted Risk of Mortality score was 6.5±4.8%, 57.9% had prior heart failure hospitalisation (HFH), and 88.4% were in New York Heart Association (NYHA) Functional Class III/IV. Secondary MR was present in 78.7%, and 76.6% had a left ventricular ejection fraction ≤50%. Up to 5 years, all-cause mortality was 66.7% and HFH was 55.4%, with one 30-day MV reintervention (1.1%). Haemodynamic valve deterioration occurred in 1.4%, the median MV mean gradient remained stable at 3.6 mmHg (first and third quartiles: 3.0, 4.8 mmHg), ≤mild MR was present in 100% of patients, and no patient experienced paravalvular leak. NYHA Functional Class I/II was maintained in 84.6%.
Conclusions: In this 5-year follow-up of the early-generation Intrepid TA TMVR system, we observed sustained MR reduction, durable haemodynamic valve performance, and improved functional status among survivors. The APOLLO (ClinicalTrials.gov: NCT03242642) and APOLLO-EU (NCT05496998) trials using the transfemoral Intrepid system will further determine the role of TMVR in managing this high-risk patient population. ClinicalTrials.gov: NCT02322840
Conventional surgical mitral valve (MV) repair or replacement improves longevity and quality of life for patients with MV disease. However, fewer than one-half of patients with ≥moderate-severe mitral regurgitation (MR) are referred for MV surgery, primarily due to high surgical risk12. The self-expanding Intrepid transcatheter mitral valve replacement (TMVR) system (Medtronic) is a less invasive investigational technology to treat MR. Data from the pooled analysis of the Pilot Study (ClinicalTrials.gov: NCT02322840) and the initial phase of the APOLLO trial (NCT03242642) using the early-generation transapical (TA) Intrepid system showed excellent device haemodynamics with the ability to eliminate MR up to 2 years3. The device performance data were further confirmed in the next-generation transfemoral system, which demonstrated improved safety outcomes up to 2 years in patients treated under an early feasibility study456.
In order to treat severe MR in patients who are ineligible for conventional MV surgery or transcatheter MV repair, two TMVR devices are currently approved for commercial use in Europe (Tendyne [Abbott], SAPIEN M3 [Edwards Lifesciences]). Additionally, the Tendyne system recently received U.S. Food and Drug Administration approval for treating patients with symptomatic severe MV disease associated with severe mitral annular calcification. However, long-term data on device durability and clinical outcomes after TMVR beyond 3 years have not been reported7. The present Pilot Study aimed to evaluate the 5-year clinical and echocardiographic outcomes focused on device performance after TMVR with the Intrepid TA TMVR system.
Methods
Study design and patient population
The Intrepid TMVR global Pilot Study is a multicentre, prospective, non-randomised study evaluating the safety and performance of the Intrepid TA TMVR system in patients at high risk for conventional MV surgery. Patients were recruited from 21 hospitals in Australia, Europe, and the US (Supplementary Table 1). Key eligibility criteria, study device, procedure-related details, and endpoints of the Pilot Study have been reported previously38. Briefly, inclusion criteria were age >18 years, symptomatic ≥moderate-severe MR (3-4+), no or minimal MV calcification, and a left ventricular ejection fraction (LVEF) ≥20%. Key exclusion criteria were severe pulmonary hypertension, need for coronary revascularisation, haemodynamic instability, need for other surgical valvular therapy, severe renal insufficiency, and prior MV surgery or intervention. The complete inclusion/exclusion criteria are listed in Supplementary Table 2. Institutional review board approval was obtained in all centres, and patients provided informed consent for study participation.
The early-generation Intrepid TMVR system comprised a self-expanding, nitinol dual-stent valve and a TA delivery system. A circular inner stent frame houses a 27 mm trileaflet bovine pericardial valve, and a conformable outer stent anchors to the native anatomy without leaflet capture. The valve is delivered transapically via a 35 Fr catheter access sheath. The early-generation system included valves with outer fixation ring diameters of 43, 46, and 50 mm, whereas 42 and 48 mm valves are used in current clinical trials38.
Anatomical suitability for TA TMVR was determined using transoesophageal echocardiography and multidetector computed tomography (MDCT). Study eligibility was determined by local Heart Teams at the study sites (including, at the minimum, a cardiac surgeon, an interventional cardiologist, and an echocardiologist) and approved by an independent physician committee. An independent clinical events committee, which also served as the data and safety monitoring board (Stanford University, Stanford, CA, USA), adjudicated endpoint-related adverse events and reviewed the safety results. Echocardiographic endpoints were assessed by an independent echocardiographic core laboratory (Mayo Clinic, Rochester, MN, USA).
Study endpoints and definitions
Clinical and transthoracic echocardiography assessments were performed at discharge, 1 month, 3 months, 6 months, 12 months, and biannually thereafter for up to 5 years. Unscheduled echocardiograms were performed by sites if clinically indicated and reviewed by the echocardiographic core laboratory. The severity of MR was assessed according to American Society of Echocardiography criteria9. Moderate haemodynamic valve deterioration was defined according to the Heart Valve Collaboratory 2022 and Mitral Valve Academic Research Consortium (MVARC) 2015 criteria as an increase in the mean transmitral gradient of ≥5 mmHg from 30 days/discharge to the last available echocardiogram or transvalvular MR ≥moderate, while severe haemodynamic valve deterioration was defined as a mean transmitral gradient of ≥10 mmHg or MR ≥moderate-severe1011.
MDCT was collected per protocol at discharge and 1 year for patients enrolled at US sites. Quality of life was evaluated using the Minnesota Living with Heart Failure Questionnaire at baseline and 1 year, as previously reported3. New York Heart Association (NYHA) Functional Class was assessed from baseline to 5 years. Standard definitions for clinical events were used in accordance with the MVARC 2015 criteria11, except for device thrombosis, as described in Supplementary Appendix 1. Post-procedure anticoagulation was prescribed per physician discretion but was recommended for at least 3-6 months post-implant, or longer unless there was a clinical indication to discontinue it.
Statistical analysis
Continuous variables are summarised as mean±standard deviation, or median and first (Q1) and third quartiles (Q3), as appropriate. Categorical variables are reported as frequencies and percentages. Adverse event rates were estimated as Kaplan-Meier estimates and reported at 30 days, 1 year, and 5 years. Thrombosis and endocarditis events were also reported as linearised rates with 95% confidence intervals (CIs), expressed per 100 patient-years. All-cause, cardiovascular, and non-cardiovascular mortality were landmarked at 1 year post-procedure to assess the later impact of TMVR by excluding events potentially attributable to the TA approach. Paired echocardiographic analysis was performed using the Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables. Change in NYHA Class from baseline was assessed using the Wilcoxon signed-rank test. A two-sided p-value<0.05 was considered statistically significant. Statistical analyses were performed by the sponsor using SAS software, version 9.4 (SAS Institute).
Results
Baseline characteristics
The study cohort included 95 patients who had undergone TA TMVR between 2015 and 2019 and completed 5-year follow-up. Demographics, baseline characteristics, and medical history are presented in Table 1. The mean age was 74.0±9.2 years, 43.2% of patients were female, the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score for MV replacement was 6.5±4.8%, 57.9% had experienced a heart failure hospitalisation (HFH) within the year preceding enrolment, and 88.4% were in NYHA Class III/IV. The predominant mechanism of MR was secondary (78.7%), 70.2% had an LVEF ≤50%, and nearly all had ≥moderate-severe MR (95.8%). Four patients were initially treated for ≥moderate-severe MR based on the site echocardiogram reading but were later found to have lower MR severity after formal core lab review.
Table 1. Baseline patient characteristics.
| (n=95) | |
|---|---|
| Age, years | 74.0±9.2 |
| Sex | |
| Male | 56.8 (54) |
| Female | 43.2 (41) |
| STS-PROM score, % | 6.5±4.8 |
| NYHA III/IV | 88.4 (84) |
| Diabetes | 37.9 (36) |
| Hypertension | 78.9 (75) |
| Prior MI | 42.1 (40) |
| HFH within the past year | 57.9 (55) |
| ≥Moderate chronic lung disease | 25.3 (24) |
| Peripheral artery disease | 15.8 (15) |
| Prior stroke | 13.7 (13) |
| Prior PCI | 42.1 (40) |
| Prior cardiac surgery | 47.4 (45) |
| Prior valve surgery | 10.5 (10) |
| CABG | 40.0 (38) |
| GFR <60 mL/min/1.73 m2 | 57.4 (54/94) |
| Atrial fibrillation/atrial flutter | 60.0 (57) |
| Prior ICD | 28.4 (27) |
| Prior CRT | 15.8 (15) |
| Aetiology of MR | |
| Primary MR | 21.3 (20/94) |
| Secondary MR | 78.7 (74/94) |
| ≥Moderate-severe MR | 95.8 (91) |
| LVEF, % | 45.2±10.6 |
| LVEF ≤30% | 6.4 (6/94) |
| LVEF 30-50% | 63.8 (60/94) |
| LVEF >50% | 29.8 (28/94) |
| Valve size deployed | |
| 43, 46, or 50 mm | 94.7 (89/94) |
| 42 or 48 mm | 5.3 (5/94) |
| Data are presented as mean±standard deviation, % (no. of patients), or % (n/N). CABG: coronary artery bypass graft; CRT: cardiac resynchronisation therapy; GFR: glomerular filtration rate; HFH: heart failure hospitalisation; ICD: implantable cardioverter defibrillator; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MR: mitral regurgitation; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | |
Intraprocedural and 30-day clinical outcomes
A summary of the patient flow is provided in Figure 1. The Intrepid valve was successfully implanted in 92 (96.8%) of 95 patients. In one patient, the procedure was aborted prior to valve deployment because of uncontrolled bleeding around the sutures at the apical incision site. The other two patients underwent conversion to surgical mitral valve replacement during the index procedure due to device malposition/migration. Clinical outcomes for the attempted implant cohort, reported as Kaplan-Meier estimates, are shown in Table 2. A total of 18 deaths (18.9%) occurred within 30 days post-procedure; the majority were attributed to cardiovascular causes (n=15, 15.8%).
Eight HFH events occurred within 30 days (9.6%), and 3 patients experienced a disabling ischaemic stroke (3.6%); one was procedure related, while two were both device and procedure related. A total of 20 patients experienced life-threatening (n=16) or fatal bleeding events (n=4) due to access-related apical or intrathoracic bleedings. There was 1 MV (device-related) reintervention (1.1%) due to device malposition within 30 days, with successful percutaneous valve-in-valve implantation. No myocardial infarction, clinically significant device thrombosis, clinical haemolysis, or prosthetic MV endocarditis events were reported within the first 30 days.
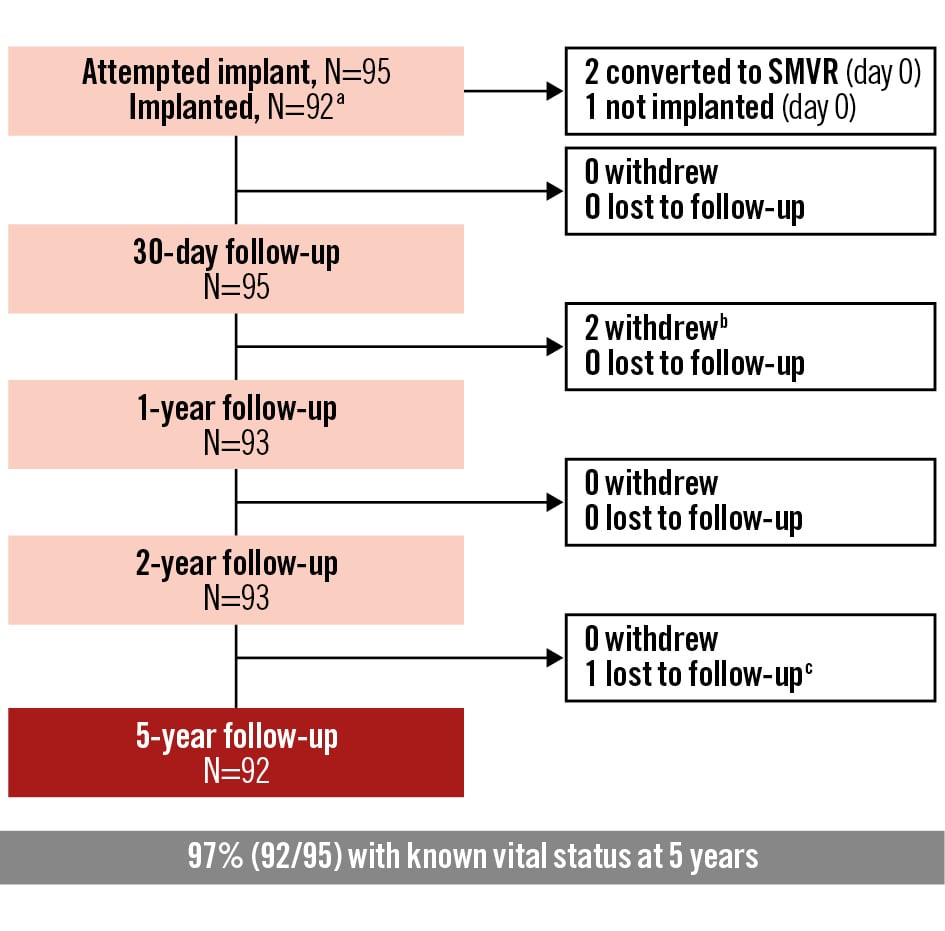
Figure 1. Patient flowchart. Flowchart depicting the number of patients enrolled in the analysis cohort, number of successful implants, and number of patients with known vital status at follow-up. aThe analysis of clinical outcomes is based on the attempted implant cohort, and the analysis of echocardiographic outcomes is based on the implanted cohort. bOne patient who converted to SMVR at day 0 and one patient who converted to SMVR at day 1 were followed for 30 days then withdrew from the study. cOne patient missed the 54- and 60-month visits and was considered lost to follow-up. Each follow-up includes patients who were evaluated, died prior to, or were observed alive at a later timepoint. SMVR: surgical mitral valve replacement
Table 2. Clinical outcomes up to 5 years.
| 30 days | 1 year | 5 years | New patients with events between 1 and 5 years | |
|---|---|---|---|---|
| All-cause mortality | 18.9 (18) | 31.9 (30) | 66.7 (62) | 32 |
| Cardiovascular mortality | 15.8 (15) | 26.1 (24) | 51.6 (43) | 19 |
| Non-cardiovascular mortality | 3.7 (3) | 7.9 (6) | 31.4 (19) | 13 |
| Disabling stroke | 3.6 (3) | 3.6 (3) | 9.1 (6) | 3 |
| Myocardial infarction | 0 (0) | 0 (0) | 22.9 (10) | 10 |
| Cardiovascular hospitalisation | 12.1 (10) | 48.2 (37) | 79.0 (57) | 20 |
| Heart failure hospitalisation | 9.6 (8) | 26.0 (20) | 55.4 (37) | 17 |
| Bleeding event ≥major (MVARC definition) | 24.3 (23) | 24.3 (23) | 32.5 (27) | 4 |
| Fatal | 4.2 (4) | 4.2 (4) | 4.2 (4) | 0 |
| Life-threatening | 17.1 (16) | 17.1 (16) | 21.8 (18) | 2 |
| MV reintervention | 1.1 (1) | 1.1 (1) | 1.1 (1) | 0 |
| Device thrombosis | ||||
| Clinically significant with sequelae | 0 (0) | 3.0 (2) | 10.5 (5) | 3 |
| Clinically significant without sequelae | 0 (0) | 0 (0) | 1.7 (1) | 1 |
| MV endocarditis | 0 (0) | 2.9 (2) | 4.6 (3) | 1 |
| Haemolysis | 0 (0) | 0 (0) | 0 (0) | 0 |
| Data are presented as Kaplan-Meier rates (no. of patients with the event). MV: mitral valve; MVARC: Mitral Valve Academic Research Consortium | ||||
One-year clinical outcomes
All-cause mortality and HFH at 1 year were 31.9% and 26.0%, respectively (Table 2). A total of 12 patients had their first HFH between 31 days and 1 year. No additional cases of disabling stroke occurred between 31 days and 1 year. Two cases of clinically significant device thrombosis with sequelae (3.0%) were diagnosed. At the time of diagnosis, the first patient was on warfarin but had a subtherapeutic international normalised ratio (INR) value, while the second patient was not on anticoagulation after completing the protocol-recommended 6-month period. In both cases, intensification or reinitiation of anticoagulation therapy led to resolution of thrombosis as confirmed by imaging.
There were 2 cases of MV endocarditis between 31 days and 1 year (observed on post-procedure days 84 and 167). The first resolved following antibiotic therapy, while the second case was fatal. Details on all device thrombosis and endocarditis events can be found in Supplementary Table 3 and Supplementary Table 4, respectively. There were no new MV reinterventions or bleeding events between 31 days and 1 year.
Five-year clinical outcomes
At 5 years, 62 patients were deceased, and 2 patients missed their follow-up visit. The remaining 28 patients that were still in contact completed their 5-year follow-up visit (Table 2). The Kaplan-Meier rates for all-cause mortality, cardiovascular mortality, non-cardiovascular mortality, and HFH at 5 years were 66.7%, 51.6%, 31.4%, and 55.4%, respectively (Central illustration A and B, Table 2). The composite rate of all-cause mortality or HFH at 5 years was 78.6%. Per the independent clinical events committee, a total of 5 deaths were attributed to the device. One death was deemed definitely related (endocarditis, as described previously), while four were considered possibly related (2 fatal strokes, 1 intracranial bleeding following a fall due to cardiac arrest, and 1 stroke followed by hospital-acquired pneumonia). One-year landmark analyses for all-cause, cardiovascular, and non-cardiovascular mortality are shown in Supplementary Figure 1. When excluding 1-year mortality, all-cause, cardiovascular, and non-cardiovascular mortality estimates up to 5 years were 51.2%, 34.5%, and 25.5%, respectively.
After 1 year, an additional 19 patients died due to cardiovascular causes (Table 2). Worsening HF was the main cause of death among these patients (n=12), followed by sudden/unwitnessed death (n=3), death due to a neurological event (n=2), due to myocardial infarction (n=1), and of unknown cause (n=1). There were 17 patients that had their first HFH between 1 and 5 years. Among these, there were 4 patients with progression of other non-MV diseases that contributed to the advancement of HF (3 patients with severe aortic valve disease, and 1 patient with severe tricuspid regurgitation).
Between 1 and 5 years, myocardial infarction occurred in a total of 10 patients, all but two of whom had a history of prior myocardial infarction and/or revascularisation with percutaneous coronary intervention or coronary artery bypass grafting. Three additional patients experienced their first disabling stroke, with two of these events being device related. Additionally, no new fatal bleeds occurred between 1 and 5 years, while 2 patients had their first new life-threatening bleeding event. One life-threatening subdural haematoma occurred on day 1,185, associated with overanticoagulation (INR 9.6), and one life-threatening bleeding following postperipheral stenting occurred on day 1,545.
Intrepid valve function up to 5 years
The rate of significant device thrombosis per 100 patient-years with and without sequelae were 1.95 (95% CI: 0.81-4.69) and 0.39 (95% CI: 0.06-2.77), respectively. Three clinically significant device thrombosis events with sequelae and 1 event without sequelae occurred after 1 year (Supplementary Table 3). At the time of the event, 2 patients were receiving warfarin (the INR was 2.1 in one patient and unknown in the other patient), and 2 patients were receiving clopidogrel. Management involved intensifying or adding anticoagulation therapy. Of these 4 cases, two completely resolved per follow-up imaging, one remained of unknown status, and one persisted in the setting of disseminated intravascular coagulation and a COVID-19 infection. Among the total of 6 cases of clinically significant device thrombosis up to 5 years of follow-up, the independent clinical events committee determined that none of the 5 subsequently occurring mortalities was caused by implant thrombosis.
The rate of MV endocarditis per 100 patient-years was 1.17 (95% CI: 0.38-3.63). There was one new case of MV endocarditis between 1 and 5 years (post-procedure day 500), which resolved following antibiotic therapy (Supplementary Table 4). There was no new incidence of MV reinterventions between 1 and 5 years.
Improvement in functional status
At baseline, 88.4% of patients were in NYHA Class III/IV. Significant symptom improvement was observed following Intrepid TMVR, with 77.3%, 89.8%, and 84.6% of surviving patients in Class I/II at the 30-day, 1-, and 5-year follow-ups, respectively (Central illustration C).
Five-year echocardiographic outcomes
Twenty-one of 28 patients (75%) with 5-year follow-up had transthoracic echocardiographic images for core lab evaluation of MR severity. Among survivors at 5 years, all patients were free from residual MR greater than mild in severity (Figure 2A), and no patients had more than trace paravalvular leak (PVL) (Figure 2B). Similar findings were observed in a paired MR analysis (Central illustration D). A review of all available scheduled and clinically driven unscheduled echocardiograms revealed no MR or PVL greater than mild in severity in the study. The rate of moderate haemodynamic valve deterioration was 1.4% (1/69), while there was no evidence of severe haemodynamic deterioration during the 5 years of follow-up.
The median MV mean gradient at 5 years among survivors was 3.6 mmHg (Q1: 3.0 mmHg, Q3: 4.8 mmHg) (Figure 3A), and the median left ventricular (LV) outflow tract peak gradient was 6.6 mmHg (Q1: 3.8 mmHg, Q3: 8.8 mmHg) (Figure 3B). A paired comparison of echocardiographic outcomes at baseline and 5 years is shown in Table 3. There were no significant changes in the LV end-systolic diameter index, LV end-diastolic diameter index, cardiac output, or tricuspid regurgitation severity. The LVEF decreased from baseline to 5-year follow-up. Although not statistically significant, forward stroke volume increased, while pulmonary artery systolic pressure and right ventricular dysfunction decreased.
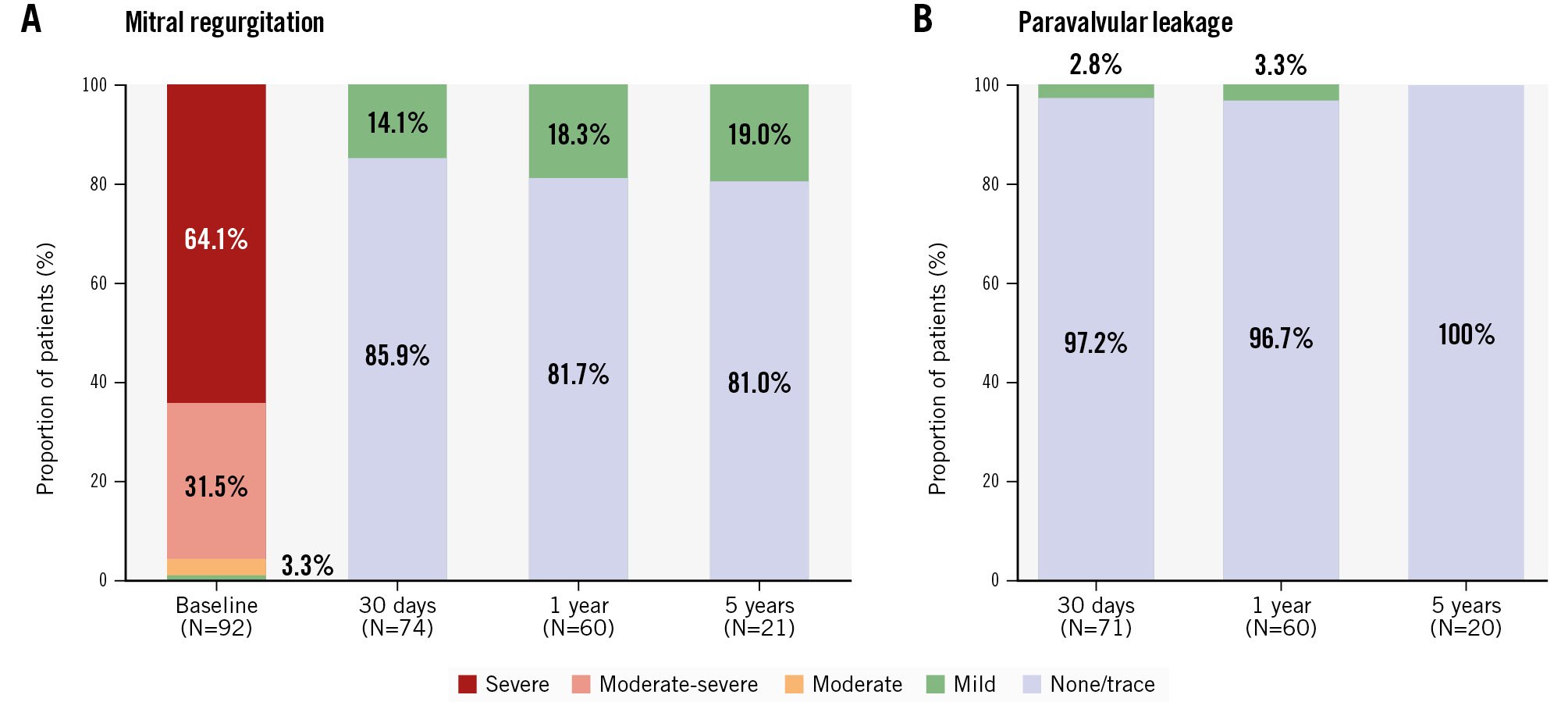
Figure 2. Mitral regurgitation severity over time. A) Total mitral regurgitation from baseline to 5 years; (B) paravalvular leakage from 30 days to 5 years. Data are reported for the implanted cohort (N=92) in patients who were alive with evaluable echocardiograms at protocol-specific visits.
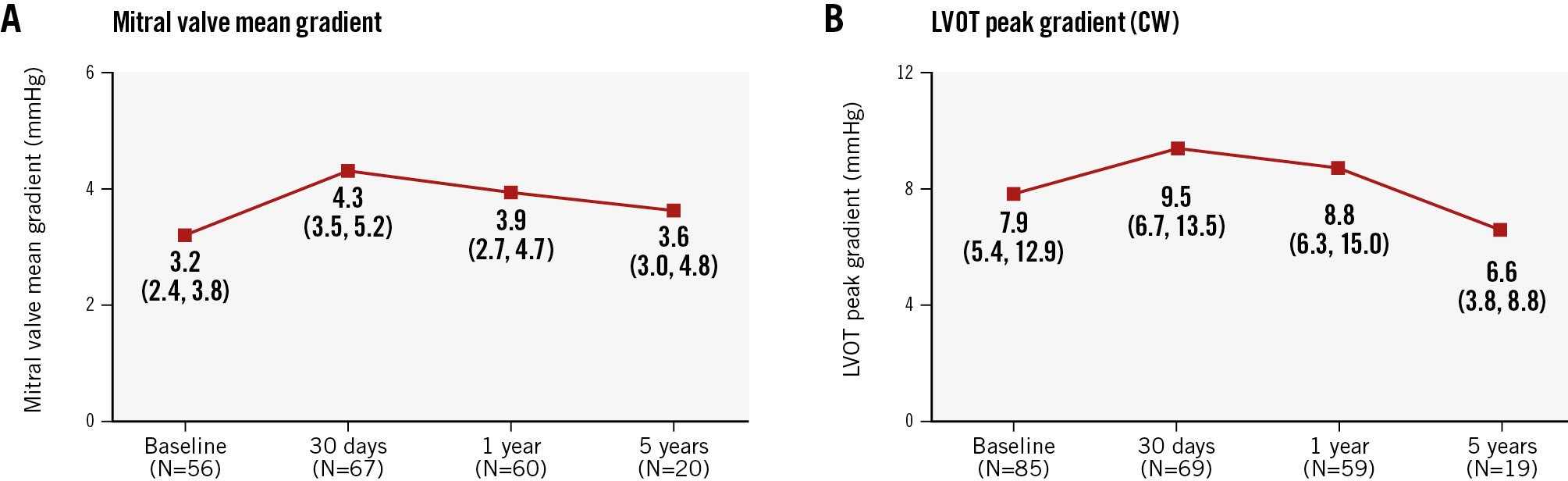
Figure 3. Gradients over time. A) Mitral valve mean gradient over time; (B) left ventricular outflow tract peak gradient over time. Data are reported for the implanted cohort (N=92) in patients who were alive with evaluable echocardiograms at protocol-specific visits. Values are reported as median (Q1, Q3). CW: continuous wave; LVOT: left ventricular outflow tract; Q1: first quartile; Q3: third quartile
Table 3. Paired comparison of echocardiographic outcomes at 5 years.
| n | Baseline | 5 years | p-value | |
|---|---|---|---|---|
| MV mean gradient, mmHg | 14 | 3.2 (2.3, 3.9) | 3.7 (3.0, 4.7) | 0.08 |
| LVOT peak gradient, mmHg | 17 | 6.1 (4.5, 6.6) | 6.0 (3.8, 8.8) | 0.94 |
| LVESD index | 7 | 2.4 (2.1, 2.9) | 2.3 (1.9, 3.1) | 0.84 |
| LVEDD index | 15 | 3.1 (2.9, 3.4) | 3.1 (2.7, 3.5) | 0.46 |
| LVEF, % | 20 | 44.0 (36.0, 55.0) | 39.5 (26.5, 46.5) | 0.008 |
| Forward stroke volume, mL | 14 | 56.1 (47.4, 65.1) | 64.5 (47.4, 69.1) | 0.15 |
| Cardiac output, L/min | 14 | 4.7 (3.2, 4.7) | 4.4 (3.9, 5.1) | 0.33 |
| RV dysfunction ≥mild | 17 | 76.5 (13/17) | 47.1 (8/17) | 0.06 |
| PASP, mmHg | 11 | 46.0 (33.0, 59.0) | 39.0 (32.0, 54.0) | 0.42 |
| TR ≥moderate | 21 | 38.1 (8/21) | 38.1 (8/21) | >0.99 |
| Data are presented as median (Q1, Q3) or % (n/N). Paired comparisons were made using the Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables. LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVOT: left ventricular outflow tract; MV: mitral valve; PASP: pulmonary artery systolic pressure; Q1: first quartile; Q3: third quartile; RV: right ventricular; TR: tricuspid regurgitation | ||||
Discussion
The major findings in this study are as follows (Central illustration): (1) Intrepid TA TMVR resulted in near-elimination of MR during 5-year follow-up among survivors, with durable haemodynamic valve performance and a low rate of haemodynamic valve deterioration; (2) there was one 30-day MV reintervention and none thereafter; (3) device-related complications (thrombosis and endocarditis) were infrequent during 5-year follow-up, with no apparent clustering of events and no cases of haemolysis; and (4) there was sustained improvement in functional status in survivors. In this high-risk patient population treated with the early-generation Intrepid TA TMVR system, 78.6% of the patients either died or were hospitalised for heart failure (HF) within 5 years. These findings highlight the complex comorbid patient population evaluated in this Pilot Study and the need for systematic optimisation of patient selection, guideline-directed medical therapy for HF, and a less invasive transfemoral delivery system.
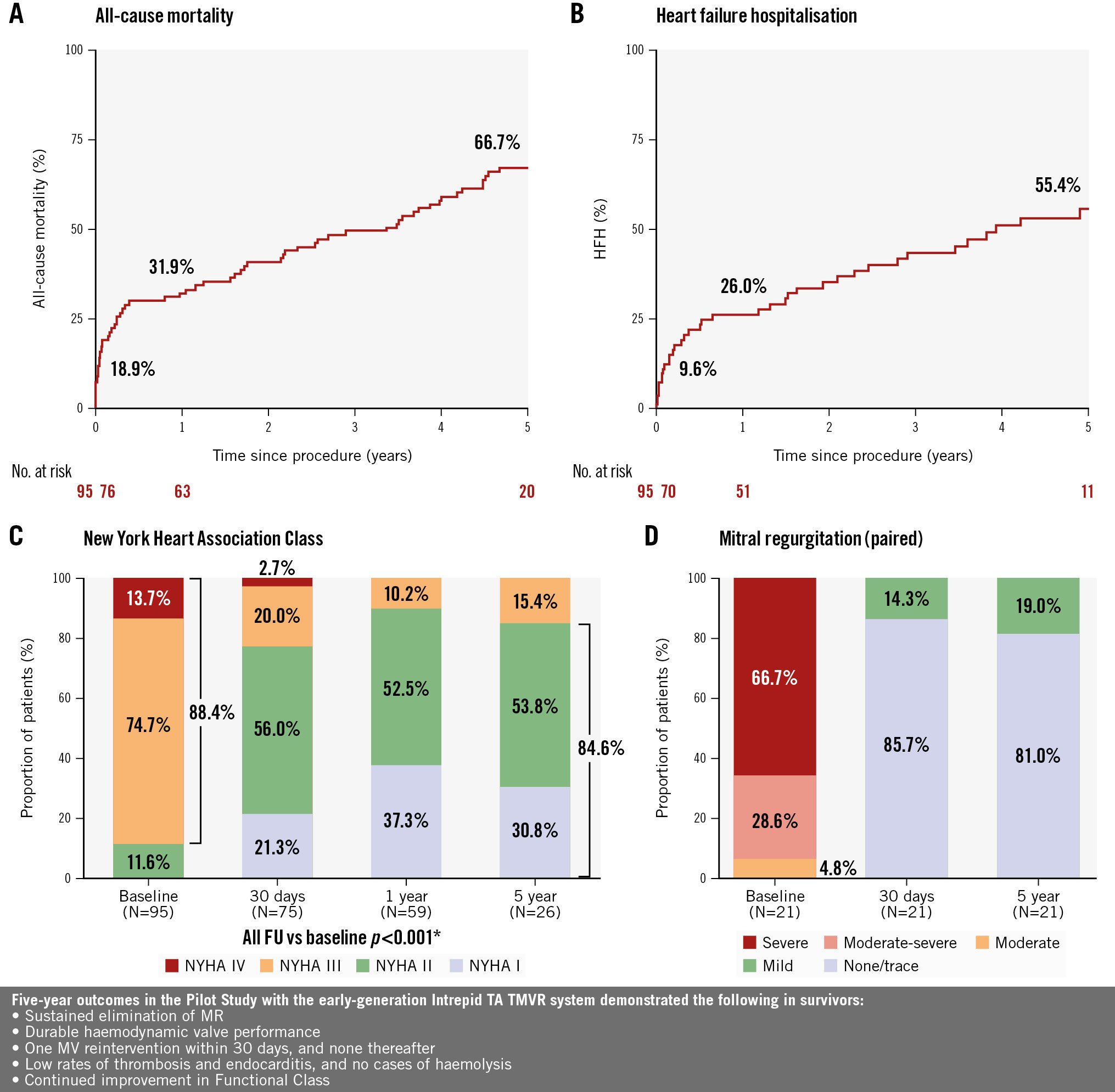
Central illustration. Five-year clinical outcomes with the Intrepid transapical TMVR system. A) Kaplan-Meier estimate of all-cause mortality up to 5 years; (B) Kaplan-Meier estimate of heart failure hospitalisation up to 5 years; (C) symptom status (NYHA Functional Class) at baseline, 30 days, 1 year, and 5 years; *Wilcoxon signed-rank test; (D) mitral regurgitation severity over time (paired, N=21). FU: follow-up; HFH: heart failure hospitalisation; MR: mitral regurgitation; MV: mitral valve; NYHA: New York Heart Association; TA: transapical; TMVR: transcatheter mitral valve replacement
Durable valve performance of the Intrepid TMVR system
Building on previously published 2-year Intrepid TA TMVR data3, the elimination of MR and low transvalvular gradients seen at 5 years are important factors when considering TMVR as an alternative treatment option to surgery or transcatheter repair. Despite an excellent safety profile, the Achilles’ heel of transcatheter edge-to-edge repair (TEER) is residual or recurrent MR, as well as elevated transmitral gradients, both of which have been associated with adverse clinical outcomes121314151617. Similar to other Intrepid studies56, the Pilot Study showed that among survivors, 100% had ≤mild MR and no PVL, with stable transmitral gradients for up to 5 years of follow-up. Clinically significant device thrombosis with sequelae, a concern for TMVR, was observed in this study, with no distinct pattern in the timing of events post-procedure, while MV endocarditis events remained infrequent (1.17 [95% CI: 0.38-3.6] per 100 patient-years). These findings align with other midterm TMVR7 and conventional MV replacement studies1819 and reinforce the importance of valve performance as a key factor, supporting the continued use of the Intrepid TMVR system. Extending anticoagulation beyond 6 months after TMVR should be strongly considered in patients deemed at high risk for thrombosis (e.g., with a history of hypercoagulability, and/or severe left ventricular dysfunction) and at acceptable risk for bleeding. Further studies will be necessary to evaluate this hypothesis, given the balance between valve thrombosis and bleeding in this high-risk population.
Transfemoral favoured over transapical approach in TMVR
TA transcatheter aortic valve implantation has largely been replaced by a transfemoral approach due to increased safety and better patient recovery2021. Similarly, we have seen significant access site-related complications with TA TMVR, both with the Intrepid system and other systems2223. However, there were almost no device-related events beyond the first year in the Pilot Study. The next-generation Intrepid transfemoral TMVR system has demonstrated improved procedural safety compared to the TA system reported in this study, with 0% 30-day and 6.7% 1-year mortality rates5. The most recent ENCIRCLE trial (ClinicalTrials.gov: NCT04153292) data on the SAPIEN M3 system further confirm the safety of transfemoral TMVR over a TA approach24. Transfemoral TMVR is now the only approach with the latest-generation 29 Fr Intrepid system in the APOLLO and APOLLO EU trials, with other TMVR systems also evolving to the transfemoral approach (e.g., Cephea [Abbott], InnoValve [Edwards Lifesciences], AltaValve [4C Medical]).
Impact of patient risk profile on long-term outcomes after TMVR
This long-term study showed that both all-cause and cardiovascular mortality after TA TMVR were relatively high at 5 years, at 66.7% and 51.6%, respectively. The HFH rate was 55.4%. These findings paralleled those reported at 1 year in the TENDER registry with the Tendyne system25, at 2 years with the CHOICE-MI registry with 11 different TMVR devices26, at 3 years with other TA TMVR systems7, and at 5 years with TEER2728. Indeed, the Pilot Study population was a truly high-risk patient cohort: the mean STS-PROM score was 6.5% for MV replacement, nearly 50% had prior cardiac surgery, 28.4% had an implantable cardioverter defibrillator, 15.8% had an implantable cardiac resynchronisation therapy device, almost 80% had secondary MR, 70% had an LVEF ≤50%, and almost 60% had a prior HFH within the year preceding enrolment. Whether the high mortality rates relate to MR aetiology (primary MR vs secondary MR) remains unclear, given the relatively small sample sizes in the above studies and the limited ability to compare outcomes based on MR aetiology. However, TA TMVR with the Tendyne system had lower 1-year mortality in 2 real-world series with fewer secondary MR patients2529. Results from the larger registries (e.g., ENCIRCLE, APOLLO, SUMMIT [ClinicalTrials.gov: NCT03433274]) will provide a more robust comparison in outcomes between primary and secondary MR patients undergoing TMVR.
With the TA TMVR system, the Kaplan-Meier analysis appeared to show an elevated risk of early mortality from day 0 to 6 months, followed by a plateau from 6 months to 1 year. After the first year, landmark analysis did reveal an ongoing mortality risk after TA TMVR, with 5-year cardiovascular and non-cardiovascular mortality rates of 34.5% and 25.5%, respectively. These findings suggest residual MR is not the main factor after TMVR with Intrepid; rather, mortality appears to be more influenced by patient comorbidities and progressive cardiomyopathy.
Interestingly, 5-year outcomes after TEER in the COAPT Trial were also sobering, with all-cause mortality, cardiovascular mortality, and HFH at 57.3%, 49.0%, and 61.0%, respectively27. The 5-year results of the EuroSMR registry showed a similar all-cause mortality of 65% in patients with secondary MR28. These similar findings, regardless of whether MR reduction or elimination was successful, suggest that we are treating a patient population with severe illness and advanced heart disease. This holds true despite the fact that the two study groups come from different patient populations and time periods. Interestingly, two recent propensity-matched studies between TA TMVR with Tendyne and surgical MV replacement showed no significant outcome differences, but TMVR patients had fewer blood transfusions and shorter hospital stays2930. A less invasive strategy to eliminate MR may be beneficial in this high-risk population. Nevertheless, implementing a more precise patient selection strategy and optimising HF medical therapy after a successful procedure will be crucial to better address this high-risk patient group beyond just treating their MR.
Functional improvement over time in TA TMVR survivors
Despite a relatively high early mortality after TA TMVR with the Intrepid system, patients who survived to 5 years did exhibit sustained functional improvement, with 84.6% remaining at NYHA Class I/II. This is consistent with the sustained improvements observed with other TMVR systems7. Although left ventricular dimensions and cardiac output were unchanged over time in this 5-year study, similar to other midterm TMVR series31, forward stroke volume, right ventricular dysfunction, and pulmonary arterial systolic pressure showed improvements following Intrepid TA TMVR, consistent with the improvements observed in the early feasibility study using a transfemoral approach6. The Intrepid APOLLO and APOLLO EU trials will show whether improvements in these cardiac function metrics are observed in a larger patient cohort.
By paired analysis, LVEF numerically declined from 44% at baseline to 40% at 5 years in this study; however, it is unclear whether this decrease is clinically meaningful. Given that approximately 40% of our patients had a history of coronary artery bypass grafting, percutaneous coronary intervention, and myocardial infarction, underlying myocardial dysfunction could be a contributing factor. A similar postprocedural decline in LVEF has been reported with surgery3233, TEER2734, and TA TMVR3135. It is likely that outcomes may continue to improve with the routine use of a transfemoral approach, device iterations, and procedural maturity in TMVR. Seeing durable valve performance at 5 years, even with this early-generation Intrepid system, is important information for discussing treatment options with patients with symptomatic MR at high risk for open surgery.
Limitations
The current work describes the longest follow-up of patients treated to date by TA TMVR. Nonetheless, it remains a relatively small, single-arm study of the early experience with a new TMVR device using a TA approach and may reflect the initial learning curve associated with the procedure and site experience. The lack of a control group limits conclusions with regard to the comparison to other MR therapies. Although clinical follow-up was comprehensive in surviving patients, echocardiograms were not obtained in all patients at all timepoints. Thus, paired comparisons of parameters of cardiac function could only be performed for a subset of patients. Furthermore, results are limited by the competing risk of mortality and reflect outcomes in a minority of surviving patients. Kansas City Cardiomyopathy Questionnaire assessment was not collected in the Pilot Study, which restricts our ability to assess patient-reported quality-of-life outcomes. Anticoagulation therapy was recommended for at least 3-6 months, but the rates of continuation or discontinuation were unknown. Perioperative management of this high-risk population and long-term medical therapy were not captured by the study protocol. Rigorous and intensive medical therapy with input from HF specialists might have led to improved longer-term outcomes.
Conclusions
In the longest follow-up series of TA TMVR using the early-generation Intrepid system in a high-risk patient population, we observed 5 years of sustained MR elimination and durable valve performance, along with sustained functional improvement among survivors, despite predictable mortality and HFH. Ongoing clinical trials using the less invasive transfemoral approach will help define the patient population most likely to benefit from TMVR.
Impact on daily practice
Intrepid transapical (TA) transcatheter mitral valve replacement (TMVR) was associated with long-term mitral regurgitation (MR) elimination, durable haemodynamic valve performance, and improved functional status among survivors up to 5 years in selected patients with symptomatic ≥moderate-severe MR. The 5-year clinical and echocardiographic outcomes will help Heart Teams in the decision-making process for MR treatment and underscore the need for optimal patient selection and heart failure therapies. With 5-year valve performance of the Intrepid TA TMVR system now available, future studies on transfemoral TMVR and comparison studies with transcatheter edge-to-edge repair will better define the role of TMVR in the management of high surgical risk patients with ≥moderate-severe MR.
Acknowledgements
The authors thank Gan Dunnington, MD; David Lee, MD; David Liang, MD; Jack Boyd, MD; Neil Schwartz, MD; and Ronald Witteles, MD, for their expert patient review and study oversight; Evgenia Nikolsky, MD, employee of Medtronic, for providing scientific review; and Andres Caballero, PhD, CMPP, employee of Medtronic, for providing medical writing assistance under the direction of the lead and senior authors. The authors thank all the study sites and patients that participated in the Pilot Study.
Funding
This work was supported by Medtronic.
Conflict of interest statement
G.H.L. Tang has received speaker honoraria from and served as a physician proctor, consultant, advisory board member, TAVR publications committee member, RESTORE study steering and screening committee member, APOLLO trial screening committee member, and IMPACT MR steering committee member for Medtronic; has received speaker honoraria from and served as a physician proctor, consultant, advisory board member, ENVISION trial screening committee member, and TRILUMINATE trial anatomical eligibility and publications committee member for Abbott; has served as an advisory board member for Boston Scientific; a consultant for Shockwave Medical, Anteris, Philips, Edwards Lifesciences, Peija Medical, and Shenqi Medical Technology; and has received speaker honoraria from Siemens Healthineers. V. Rajagopal has received personal fees for speaking from Medtronic, Boston Scientific, and Abbott; is on the screening committee for the APOLLO trial sponsored by Medtronic; and has equity stake in and is Chief Medical Officer of Opus Medical Therapies. P. Sorajja has served as a consultant for Medtronic, 4C Medical, Abbott, Adona, Arcos, Boston Scientific, ConKay, Coramaze, CroiValve, Cultiv8, Edwards Lifesciences, Egg Medical, Evolution-Med, Foldax, GE HealthCare, Haemonetics, inQB8, Laguna Tech, Laza Medical, Mirus, Philips, Polares, Tricares, W.L. Gore & Associates, VDyne, Unorthodox Ventures, Valcare, and xDot. T. Bajwa has received personal and institutional consulting fees from Medtronic. R. Gooley has received consulting fees from Medtronic, Boston Scientific, Abbott, and Teleflex. A. Walton has served as a physician proctor and an advisory board member for and has received institutional research grant support from Medtronic, Edwards Lifesciences, and Abbott. T. Modine is a consultant for Abbott, Boston Scientific, Cephea, Edwards Lifesciences, GE HealthCare, Medtronic, and MicroPort; and is a proctor for and receives speaker fees from Medtronic. M.K. Ng has received institutional grant support from and is a proctor for Edwards Lifesciences and Abbott. A. Zajarias is a consultant for Medtronic, Edwards Lifesciences, and Anteris. D. Hildick-Smith has received speaker honoraria from and served as a physician proctor for Medtronic, Edwards Lifesciences, Terumo, Abbott, and Boston Scientific. D. Tchétché has served as a consultant for Medtronic, Abbott, Boston Scientific, and Edwards Lifesciences. K. Spargias has served as a physician proctor and consultant for Medtronic, Edwards Lifesciences, and Abbott. V.N. Bapat is a consultant for Medtronic, Edwards Lifesciences, Abbott, and Reniva. O. De Backer has received institutional research grant support and consulting fees from Medtronic, Abbott, and Boston Scientific. D. Blackman is a consultant and proctor for Medtronic and JenaValve Technology; is a consultant and speaker for Abbott; and has received institutional research grant support from Medtronic. P. McCarthy has received speaker fees and royalties from Edwards Lifesciences; speaker fees from Atricure; served on the advisory board for Arthrex; received royalties from Genesee; served as the surgical primary investigator for the REPAIR-MR trial (unpaid); and served on the advisory board for Abbott. R. Jain is a consultant for Medtronic, Edwards Lifesciences, Philips Healthcare, and GE HealthCare; and is an advisory board member for Medtronic. R. Martin is on the executive steering committee for the APOLLO trial sponsored by Medtronic; and on the steering committee of REPAIR MR sponsored by Abbott. J.J. Thaden reports research support for the echocardiographic core laboratory from Medtronic. N.A. Marka is an employee and shareholder of Medtronic. M. Mack is on the executive board for the APOLLO trial sponsored by Medtronic; and is co-principal investigator for the PARTNER 3 and COAPT trials sponsored by Edwards Lifesciences and Abbott. D.H. Adams has served as the national co-principal investigator of the Medtronic APOLLO Food and Drug Administration pivotal trial, the NeoChord ReChord Food and Drug Administration pivotal trial, the Medtronic CoreValve US pivotal trial, and the Abbott TRILUMINATE pivotal trial. M.B. Leon has received personal and institutional grant support from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. M.J. Reardon reports receiving personal consulting fees from Abbott, Boston Scientific, W.L. Gore & Associates, and Medtronic, outside of the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

