Abstract
BACKGROUND: Transcatheter mitral valve replacement (TMVR) is a therapeutic option for patients with severe mitral regurgitation (MR) who are ineligible for conventional surgery. There are limited data on the outcomes of large patient cohorts treated with TMVR.
AIMS: This study aimed to investigate the outcomes and predictors of mortality for patients treated with transapical TMVR.
METHODS: This analysis represents the clinical experience of all patients enrolled in the Tendyne Expanded Clinical Study. Patients with symptomatic MR underwent transapical TMVR with the Tendyne system between November 2014 and June 2020. Outcomes and adverse events up to 2 years, as well as predictors of short-term mortality, were assessed.
RESULTS: A total of 191 patients were treated (74.1±8.0 years, 62.8% male, Society of Thoracic Surgeons Predicted Risk of Mortality 7.7±6.6%). Technical success was achieved in 96.9% (185/191), and there were no intraprocedural deaths. At 30-day, 1- and 2-year follow-up, the rates of all-cause mortality were 7.9%, 30.8% and 40.5%, respectively. Complete MR elimination (MR <1+) was observed in 99.3%, 99.1% and 96.3% of patients, respectively. TMVR treatment resulted in consistent improvement of New York Heart Association Functional Class and quality of life up to 2 years (both p<0.001). Independent predictors of early mortality were age (odds ratio [OR] 1.11; p=0.003), pulmonary hypertension (OR 3.83; p=0.007), and institutional experience (OR 0.40; p=0.047).
CONCLUSIONS: This study investigated clinical outcomes in the full cohort of patients included in the Tendyne Expanded Clinical Study. The Tendyne TMVR system successfully eliminated MR with no intraprocedural deaths, resulting in an improvement in symptoms and quality of life. Continued refinement of clinical and echocardiographic risks will be important to optimise longitudinal outcomes.
Current guidelines recommend mitral valve surgery or transcatheter edge-to-edge repair (TEER) as therapeutic options for eligible patients with severe mitral regurgitation (MR)12. In recent years, a steep increase in the annual procedure volumes of TEER has been observed3. Although the majority of patients with MR are amenable to either surgery or TEER, there remains a considerable portion of patients at high or prohibitive surgical risk who are ineligible for these established therapies4567. Moreover, residual or recurrent MR after TEER is associated with adverse outcomes compared to patients with sustained MR reduction89.
Transcatheter mitral valve replacement (TMVR) with dedicated devices is a minimally invasive technology that allows for predictable MR elimination. Several TMVR devices with different designs and anchoring mechanisms have been introduced in recent years101112131415. The largest experience published so far is of the first 100 patients treated with the apically tethered Tendyne Mitral Valve System (Abbott), which demonstrated promising 2-year results1116. While patient selection for TMVR is considered key to ensure beneficial outcomes, reliable predictors of outcome after TMVR have not been described in a large cohort.
The present study comprises the full study cohort of patients enrolled in the Tendyne Expanded Clinical Study and aims to outline detailed results up to 2 years and define the predictors of mortality.
Methods
STUDY DESIGN AND PATIENTS
The Expanded Clinical Study of the Tendyne Mitral Valve System (ClinicalTrials.gov: NCT02321514) is a single-arm, prospective, multicentre investigational study. Details of the study design and methodology have been reported previously111617. Patients participating in this study underwent transapical TMVR with a tethered device between November 2014 and June 2020 at 36 investigation sites worldwide. The cumulative enrolment rate per year is depicted in Supplementary Figure 1. In brief, patients enrolled in the study were diagnosed with MR 3+ or 4+ with functional impairment equal to or greater than New York Heart Association (NYHA) Functional Class II while on guideline-directed medical therapy (GDMT), including cardiac resynchronisation therapy if indicated. All study patients were evaluated by a local Heart Team at baseline and were deemed unsuitable for conventional cardiac surgery. The study was conducted in compliance with ethical principles based on the Declaration of Helsinki, with individual institutional review board approval at each site. Detailed study inclusion and exclusion criteria as well as a list of participating centres and investigators are available in Supplementary Appendix 1 and Supplementary Appendix 2. The study was sponsored by Abbott.
ECHOCARDIOGRAPHY AND CARDIAC COMPUTED TOMOGRAPHY
For eligibility assessment, transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) were performed for a comprehensive anatomical and functional evaluation of the mitral apparatus and ventricle. TTE was performed at both baseline and follow-up visits. Contrast-enhanced cardiac computed tomography (CT) imaging was performed to assess mitral valve anatomy, determine prosthesis type and size, plan the transapical access site, and predict post-deployment neo-left ventricular outflow tract (LVOT) area. All cardiac imaging studies were assessed by independent core laboratories: Beth Israel Deaconess Medical Center (Boston, MA, USA) for echocardiography, and St Paul’s Hospital (Vancouver, BC, Canada) for cardiac CT.
TRANSCATHETER MITRAL VALVE REPLACEMENT
Details of the TMVR procedure with this tethered TMVR device have been previously published17. In brief, the procedure was performed under general anaesthesia via a left-sided mini-thoracotomy. The valve prosthesis was delivered using a 34 Fr or 36 Fr sheath through or near the left ventricular apex and attached to an epicardial pad using a braided, high-molecular-weight polyethylene tether. The valve was delivered and deployed without the need of rapid pacing or cardiopulmonary bypass. The length of the tether was adjusted to optimise the seating and securement of the prosthesis, in order to minimise the risk of device displacement and paravalvular leak (PVL). Patients were anticoagulated for a minimum of 3 months after the procedure using warfarin with a target international normalised ratio of 2.5 to 3.5. More information on the peri- and postprocedural anticoagulation regimens is given in Supplementary Appendix 3.
EVALUATION AT FOLLOW-UP
Per protocol, patients were followed up at discharge, after 1, 3, 6, and 12 months, and annually thereafter up to 5 years. This study reports clinical outcomes up to 2 years. Symptom evaluation and quality of life assessments, including NYHA Functional Class, Kansas City Cardiomyopathy Questionnaire (KCCQ) score, and 6-minute walk distance (6MWD), were performed and compared with those from baseline. CT was studied at the 1-month follow-up, and TTE was studied at each follow-up visit to assess residual MR and prosthesis performance. Structural valve dysfunction was defined as haemodynamic dysfunction (e.g., mean transvalvular gradient ≥6 mmHg or residual MR ≥2+) in the presence of morphological deterioration (e.g., torn or flail leaflet, calcification, frame fracture, tether rupture, or apical pad deterioration)1819. Clinical events including death were adjudicated by an independent clinical events committee (CEC).
STUDY ENDPOINTS
The primary performance endpoint was the reduction of MR to ≤2+ at 30-day follow-up. The primary safety endpoint was a composite of device success and freedom from device-related or procedure-related serious adverse events (SAE) evaluated at 30 days post-index procedure.
Technical success was assessed at exit from the procedure room and was defined as the absence of death along with the following: successful access, delivery of the transcatheter valve delivery system, deployment and correct positioning of the correctly sized valve, and no need for additional emergency surgery or reintervention related to the device or procedure. Procedural outcomes included procedure time, defined as skin-to-skin time, and device time, defined as the time from the start of the apical penetration to final securement of the tether to the epicardial pad.
All-cause mortality was assessed at 30 days, 1 year and 2 years after the procedure. Death was adjudicated by the CEC when cardiovascular in nature or if prosthesis related. Short-term mortality was defined as all-cause mortality occurring within 90 days after the procedure. Bleeding was categorised using the Mitral Valve Academic Research Consortium (MVARC) primary bleeding scale20.
Institutional experience was defined as an overall centre experience of more than two (>2) procedures with the Tendyne TMVR system enrolled into the Expanded Clinical Study.
STATISTICAL ANALYSIS
Continuous variables are summarised as mean±standard deviation (SD). Changes from baseline, if applicable, are included using the same descriptive statistics. Differences between subgroups are summarised with differences of the two means and 95% confidence intervals (CIs). For categorical variables, the results are summarised with subject counts and proportions including exact 95% CIs. The Kaplan-Meier method was used to generate survival estimates for freedom from all-cause mortality. Comparisons between baseline and follow-up parameters were made using a paired Student’s t-test for continuous variables or McNemar’s test for categorical variables. Kaplan-Meier curves were constructed for all-cause mortality up to 2 years. A 3-month landmark analysis was performed to assess the midterm impact of TMVR by excluding events potentially attributable to the procedure. Univariable analysis for short-term (90-day) and midterm (2-year) mortality was conducted for 60 baseline parameters. To distinguish between predictors for short- and midterm mortality, only 90-day survivors were included in the midterm mortality model. In addition, uni- and multivariable Cox regression was performed for the full study cohort. Factors were included in the multivariable model using stepwise regression; if the p-value was ≤0.2 for univariable analysis, the parameter was available for 90% of the subjects and had higher significance if highly correlated (correlation coefficient>0.5) with another variable. A p-value<0.05 was considered statistically significant. Analyses were performed using the Power Analysis & Sample Size software (PASS), version 14 (NCSS Statistical Software). All statistical analyses were performed using Statistical Analysis System (SAS) for Windows, version 9.4 (SAS Institute).
Results
The study cohort included a total of 191 patients (74.1±8.0 years, 62.8% male) treated with a tethered TMVR device in the USA (n=74), Australia (n=24), Germany (n=22), Italy (n=20), France (n=18), United Kingdom (n=18), Norway (n=10), the Netherlands (n=3), Sweden (n=1) and Switzerland (n=1) between November 2014 and June 2020. The last implant was conducted in June 2020. Figure 1 illustrates the completeness of study follow-up up to 2 years.
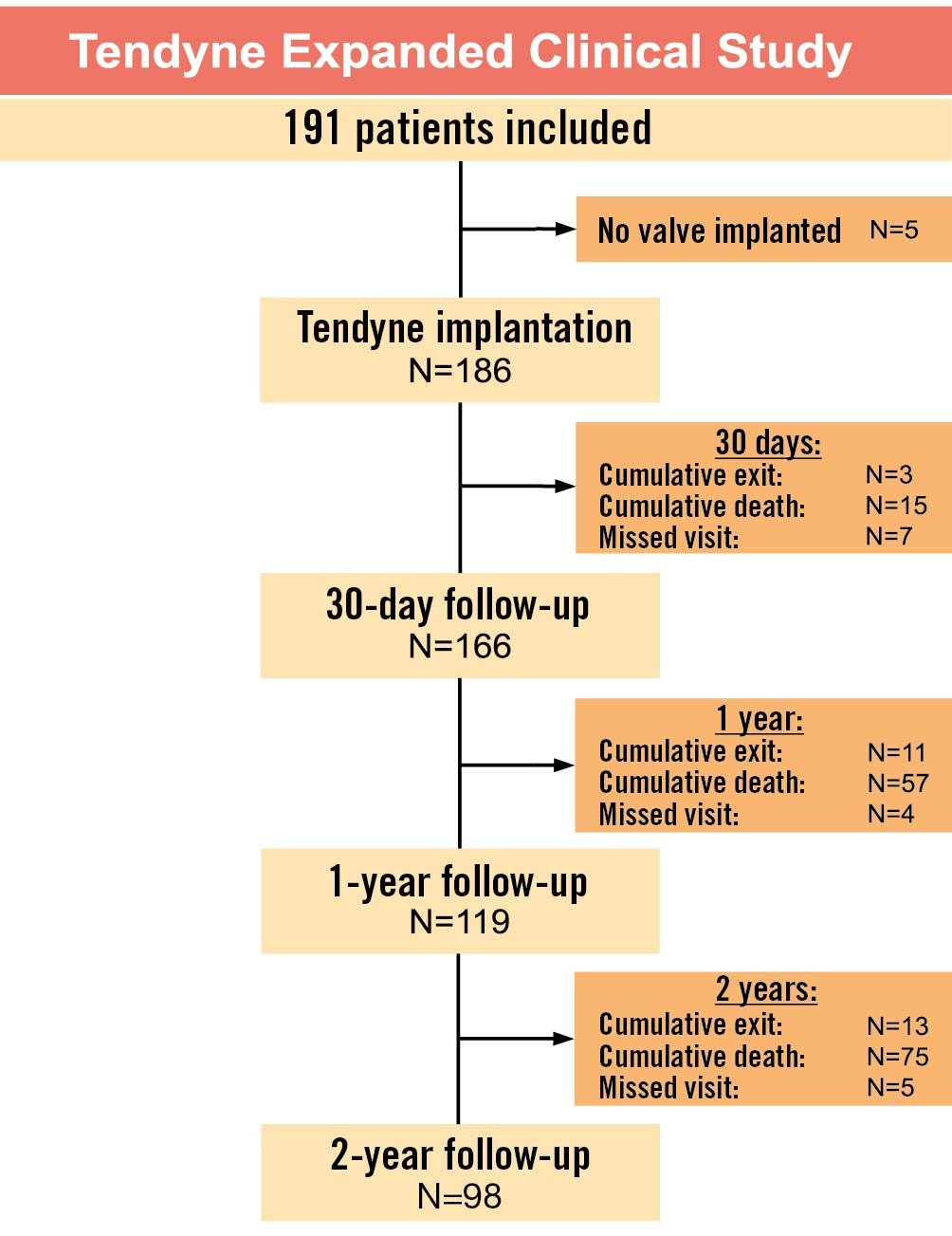
Figure 1. Study flowchart.
BASELINE CHARACTERISTICS
Demographics, baseline characteristics and medical history are presented in Table 1. The predominant mechanism of MR was secondary or mixed aetiology (169/191, 88.5%), and the majority of patients were in chronic heart failure with NYHA Class ≥III at baseline (134/191, 70.2%). The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score (calculated for surgical mitral valve replacement) and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II were 7.7±6.6% and 6.6±5.3%, respectively. The prevalence of risk factors in the study population are presented in Table 1. A comparison between the total study cohort and the first 100 patients included in the study is given in Supplementary Table 1, showing no differences regarding age, STS-PROM score, or MR aetiology.
Table 1. Baseline patient characteristics.
| Baseline patient characteristics | Total (N=191) |
|---|---|
| Age, years | 74.1±8.0 (191) |
| Male sex | 62.8 (120/191) |
| BSA, m2 | 1.88±0.25 (191) |
| BMI, kg/m2 | 27.0±5.9 (191) |
| LVEF, % | 44.7±8.8 (161) |
| NYHA Class III/IV | 70.2 (134/191) |
| II | 29.8 (57/191) |
| III | 64.4 (123/191) |
| IV | 5.8 (11/191) |
| MR aetiology: secondary MR | 88.5 (169/191) |
| MR grade 3+/4+ | 99.5 (190/191) |
| MR 2+ | 0.5 (1/191) |
| MR 3+ | 4.2 (8/191) |
| MR 4+ | 95.3 (182/191) |
| EROA, cm2 | 0.26±0.10 (110) |
| Regurgitant vol, ml | 40.1±11.6 (114) |
| STS-PROM for MV replacement, % | 7.7±6.6 (191) |
| EuroSCORE II, % | 6.6±5.3 (175) |
| Heart failure hospitalisation within 6 months prior to enrolment | 44.5 (85/191) |
| Current or prior smoker | 60.7 (116/191) |
| Diabetes | 27.7 (53/191) |
| Coronary artery disease | 68.1 (130/191) |
| Prior CABG | 38.2 (73/191) |
| Renal failure, GFR <60 mL/min/1.73 m2 | 58.1 (111/191) |
| Atrial fibrillation | 34.0 (65/191) |
| Hypertension | 78.5 (150/191) |
| Prior percutaneous coronary intervention | 48.2 (92/191) |
| Prior myocardial infarction | 45.5 (87/191) |
| Valvular heart disease other than mitral | 35.1 (67/191) |
| COPD | 34.6 (66/191) |
| Pulmonary hypertension | 51.1 (90/176) |
| Prior stroke or TIA | 14.1 (27/191) |
| ICD or pacemaker | 40.3 (77/191) |
| Baseline medications | |
| ACE inhibitor or ARB | 58.1 (111/191) |
| Beta-receptor antagonist | 84.3 (161/191) |
| Vasodilator | 12.6 (24/191) |
| Diuretic | 85.9 (164/191) |
| Digitalis | 7.3 (14/191) |
| Anticoagulant | 53.4 (102/191) |
| Aspirin or antiplatelet | 60.2 (115/191) |
| Continuous values are presented as mean±SD (N) and proportions are presented as % (n/N); n is the number of affected patients, and N is the total number of assessed patients. ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; EROA: effective regurgitant orifice area; GFR: glomerular filtration rate; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MV: mitral valve; NYHA: New York Heart Association; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TIA: transient ischaemic attack; vol: volume | |
PERIPROCEDURAL OUTCOMES
Periprocedural outcomes are summarised in Supplementary Table 2. Successful implantation of the tethered TMVR device occurred in 97.4% (186/191) of patients. Among these, 76.3% (142/186) received a standard profile (SP) valve, while a low profile (LP) valve was implanted in 23.7% (44/186). In all cases with successful valve implantation, the original intended valve remained in place up to 30 days or before study exit (186/186, 100%).
In 5 subjects, implantation of the TMVR device was unsuccessful: device retrieval was performed because of concerns about an elevated LVOT gradient and obstruction (n=1), systolic anterior motion of the anterior mitral valve leaflet (AML; n=1), aortic regurgitation after valve deployment (n=1), or suboptimal positioning of the valve due to suboptimal apical access (n=1). In 1 patient, the procedure was aborted because of unstable haemodynamic conditions before valve deployment.
During the procedure, no patient required cardiopulmonary bypass or extracorporeal circulatory support. An intra-aortic balloon pump (IABP) was used in 4.2% (8/191) of patients. There were no conversions to open-heart surgery nor intraprocedural deaths. Technical success, assessed at exit from the procedure room, was reported in 96.9% (185/191) of patients. There was 1 case of incorrect positioning of the valve (incorrect radial orientation). However, a core lab review of the predischarge echocardiogram identified only trace lateral PVL and no residual central MR in this patient.
Supplementary Table 3 summarises the temporal trends of selected procedural parameters showing a decrease of device and procedure time and an increase of technical success over time.
MORTALITY
The rate of all-cause mortality after 2 years was 38.7% (74/191), mainly due to cardiovascular causes (65/191, 34.0%). Advanced heart failure was the main cause of death among patients at follow-up (24/191, 12.6%), followed by cardiac arrest (13/191, 6.8%), multiple organ failure (6/191, 3.1%), shock (5/191, 2.6%), and endocarditis (3/191, 1.6%). Patients with attempted but unsuccessful valve implantation were followed up to 30 days and exited the study afterwards, unless they withdrew their consent to participate prior to 30-day follow-up; there were no deaths in such patients prior to study exit. After adjudication by the independent CEC, the rates of death attributable to device and/or procedure-related adverse events (AE) were 4.2% (procedure related, 8/191), 4.2% (device related, 8/191) and 7.9% (both device and procedure related, 15/191). Mortality and causes of death are presented in Supplementary Table 4. Supplementary Table 5 shows the baseline characteristics of survivors versus non-survivors at 2-year follow-up.
Figure 2 shows Kaplan-Meier analysis for all-cause mortality up to 2 years. The Kaplan-Meier calculated rate of 2-year all-cause mortality was 40.5% (Figure 2A). When excluding early mortality in a 3-month landmark analysis, all-cause mortality after 2 years was reduced to 29.2% (Figure 2B). The Kaplan-Meier estimated event rates for cardiovascular and non-cardiovascular mortality after 2 years were 36.5% and 6.3%, respectively (Supplementary Figure 2, Supplementary Figure 3).
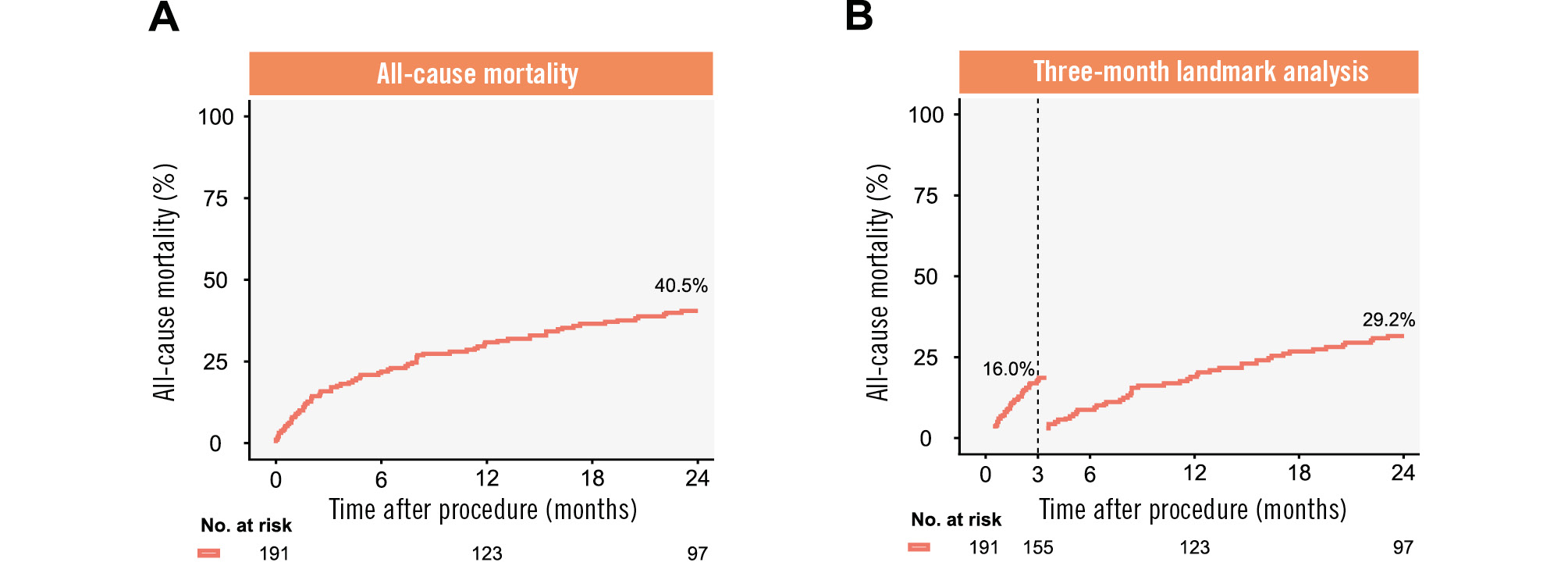
Figure 2. Kaplan-Meier analysis for all-cause mortality up to 2 years. A) Kaplan-Meier analysis for 2-year all-cause mortality. B) Three-month landmark analysis for 2-year all-cause mortality.
SERIOUS ADVERSE EVENTS
Table 2 summarises detailed SAE up to 90 days and 2 years after the index procedure. The rate of readmission for congestive heart failure was 38.7% (74/191) at 90 days and 59.2% (113/191) at 2 years. There were 7 cases of disabling stroke within 2 years (7/191, 3.7%) following the procedure, 4 of which occurred within 90 days (4/191, 2.1%). Major, life-threatening, or fatal bleeding events (assessed by the MVARC scale) were reported in 33.0% of patients (63/191) at 2 years, the majority of which (52/191, 27.2%) occurred within 90 days and were classified as procedure related. Fatal bleeding occurred in 2 patients (1.0%) during the first 90 days of follow-up and in 4 patients (2.1%) up to 2 years. Apical access site complications and infections occurred in 2 patients (1.0%) and 1 patient (0.5%), respectively, by 90-day follow-up. The rates of device-specific AE were 15.7% (30/191) at 90 days and 21.5% (41/191) at 2 years. Detailed device-specific SAE are summarised in Supplementary Table 6.
Nine subjects experienced 10 total events of device thrombus, all with onset within 6 months after the procedure. In most cases (7/10), patients were asymptomatic at presentation, and the device thrombus was discovered as part of the study imaging follow-up. Device thrombus was observed in 6 patients with subtherapeutic international normalised ratios (INRs; 2.0 or lower), 2 patients with an INR between 2.1 and 2.4, and in 2 cases with an INR within the recommended therapeutic window (INR 2.5-3.5). Patients experiencing device thrombus were monitored and medically managed (intravenous [IV] heparin, anticoagulation adjustment). In all cases, follow-up imaging demonstrated thrombus resolution along with optimised oral anticoagulation therapy maintaining a therapeutic INR range (2.5-3.5). Imaging data (TOE, TTE, CT) were used to evaluate the patients’ response to medical management. The independent CEC adjudicated that none of these AE led to death. No new onset of device thrombus was reported after 6 months following the procedure. There was no evidence of device fracture, device embolisation, or structural valve dysfunction up to 2 years of follow-up.
Table 2. Serious adverse events up to 90 days and 2 years.
| Adverse event | % (n/N) | |
|---|---|---|
| 90 days | 2 years | |
| Hospital readmission | 38.7 (74/191) | 59.2 (113/191) |
| HF hospitalisation | 16.2 (31/191) | 29.3 (56/191) |
| Bleeding, all (MVARC) | 27.2 (52/191) | 33.0 (63/191) |
| Major, extensive, life-threatening, or fatal | 19.9 (38/191) | 24.1 (46/191) |
| Life-threatening | 6.8 (13/191) | 9.4 (18/191) |
| Fatal | 1.0 (2/191) | 2.1 (4/191) |
| Acute kidney injury | 13.6 (26/191) | 22.0 (42/191) |
| Not requiring dialysis | 5.8 (11/191) | 10.5 (20/191) |
| Requiring dialysis | 8.4 (16/191) | 13.6 (26/191) |
| New-onset atrial fibrillation | 7.9 (15/191) | 12.0 (23/191) |
| New permanent pacemaker | 3.7 (7/191) | 7.9 (15/191) |
| Myocardial infarction | 1.0 (2/191) | 5.8 (11/191) |
| Stroke, disabling | 2.1 (4/191) | 3.7 (7/191) |
| Stroke, non-disabling | 1.0 (2/191) | 2.1 (4/191) |
| Transient ischaemic attack | 0.5 (1/191) | 2.6 (5/191) |
| Apical access site complications | 1.0 (2/191) | 1.0 (2/191) |
| Infection – surgical site | 0.5 (1/191) | 0.5 (1/191) |
| Mitral valve stenosis | 0 (0/191) | 0 (0/191) |
| Values are presented as % (n/N); n is the number of affected patients, and N is the total number of assessed patients. HF: heart failure; MVARC: Mitral Valve Academic Research Consortium | ||
ECHOCARDIOGRAPHIC EVALUATION
As shown in Figure 3A, at baseline, the vast majority of patients (99.5%, 190/191) had an MR grade of 3+ or 4+. Among all patients treated with the tethered TMVR device, in whom MR was assessed at 30 days, 99.3% (150/151) had MR eliminated to none/trace, and all patients were free from MR greater than mild in severity (>1+). The degree of MR elimination was sustained at 1 year and 2 years, with 99.1% and 96.3% of followed-up patients having no or trace residual MR, respectively, without any cases of more than mild (>1+) residual MR.
TTE findings at 2-year follow-up, compared to baseline, are given in Table 3. Significant changes were observed for left ventricular ejection fraction (LVEF) and right ventricular systolic pressure (RVSP). While LVEF significantly decreased from 44.4±9.1% at baseline to 41.3±11.1% at 2-year follow-up (−3.1±12.4%; p=0.034), a significant reduction of RVSP was also reported from 48.8±10.5 mmHg to 35.4±10.3 mmHg (−13.4±14.2 mmHg; p<0.001). No significant changes were observed for left ventricular end-diastolic or -systolic dimensions at 2-year follow-up.
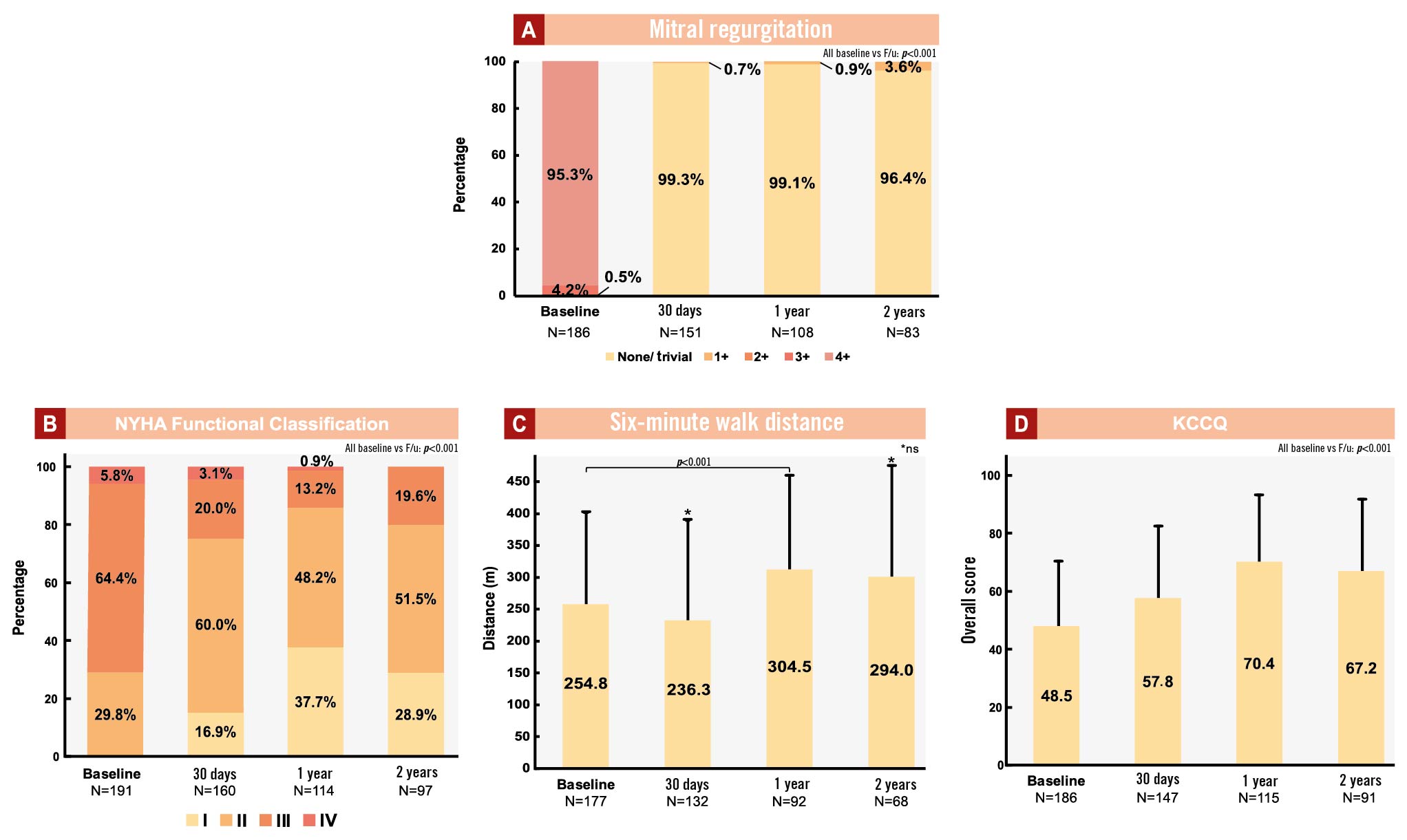
Figure 3. Baseline and follow-up symptom evaluation and quality of life assessments. A) Mitral regurgitation; B) NYHA Functional Classification; C) 6MWD; D) KCCQ. MR severity was assessed by TTE at baseline, 30-day, 1- and 2-year follow-up and adjudicated by an independent echocardiography core lab. Heart failure symptoms and functional capacity were assessed at baseline, 30-day, 1- and 2-year follow-up. 6MWD: six-minute walk distance; F/u: follow-up; KCCQ: Kansas City Cardiomyopathy Questionnaire; MR: mitral regurgitation; NYHA: New York Heart Association; TTE: transthoracic echocardiography
Table 3. Echocardiographic parameters up to 2 years and paired comparisons to baseline.
| Echo parameters | Baseline vs 2-year follow-up | |||
|---|---|---|---|---|
| Baseline | 2 years | Change | p-value | |
| LVEF, % | 44.4±9.1 (75) | 41.3±11.1 (75) | −3.1±12.4 (75) | 0.034 |
| LVEDV, ml | 175.5±57.4 (51) | 166.1±49.0 (51) | −9.4±46.1 (51) | 0.151 |
| LVESV, ml | 98.5±38.5 (51) | 102.6±40.6 (51) | 4.1±36.6 (51) | 0.428 |
| LVEDVi, ml/m2 | 91.4±25.6 (51) | 87.5±26.5 (51) | −3.9±23.8 (51) | 0.249 |
| LVESVi, ml/m2 | 51.5±18.9 (51) | 54.2±22.4 (51) | 2.8±19.6 (51) | 0.321 |
| LVEDD, cm | 6.0±0.7 (77) | 6.1±0.7 (77) | 0.1±0.6 (77) | 0.087 |
| LVESD, cm | 4.9±0.8 (73) | 5.1±1.0 (73) | 0.2±1.0 (73) | 0.057 |
| Forward SV, ml | 50.6±17.3 (55) | 53.4±16.3 (55) | 2.8±16.5 (55) | 0.209 |
| CO, l/min | 3.6±1.0 (52) | 3.7±1.1 (52) | 0.1±1.1 (52) | 0.578 |
| RVSP, mmHg | 48.8±10.5 (22) | 35.4±10.3 (22) | −13.4±14.2 (22) | <0.001 |
| LVOT gradient, mmHg | 1.4±0.7 (63) | 1.6±0.9 (63) | 0.2±0.8 (63) | 0.061 |
| Values are presented as mean±SD (N). CO: cardiac output; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEDVi: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; LVESVi: left ventricular end-systolic volume index; LVOT: left ventricular outflow tract; RVSP: right ventricular systolic pressure; SD: standard deviation; SV: stroke volume | ||||
IMPROVEMENT OF FUNCTIONAL CAPACITY AND QUALITY OF LIFE
Heart failure symptoms were assessed according to NYHA Functional Class. At baseline, 70.2% of patients were at NYHA Class III/IV. Significant symptomatic improvement was observed following TMVR, with 76.9%, 86.0% and 80.4% of surviving patients at NYHA Class I/II at 30-day, 1- and 2-year follow-up, respectively (p<0.001, paired comparison to baseline, McNemar’s test) (Figure 3B).
The functional capacity of study participants was assessed using the 6-minute walk test. At 30-day follow-up, a non-significant 23.4 metre reduction from baseline was observed. At 1-year follow-up, a significant increase of 6MWD (40.1 metres) compared to baseline was reported (p<0.001) (Figure 3C). At 2-year follow-up, no significant change compared to baseline was observed.
Patient-reported quality of life following treatment with the tethered TMVR device, assessed by the KCCQ score, improved at follow-up. Compared to baseline, significant improvements of 9.3 points (at 30 days), 21.9 (at 1 year) and 18.7 points (at 2 years; all p<0.001 compared to baseline) were reported (Figure 3D).
PREDICTORS OF SHORT- AND MIDTERM OUTCOMES
Univariable analysis for the prediction of short-term and midterm mortality was performed for a total of 60 clinical and echocardiographic characteristics (Supplementary Table 7, Supplementary Table 8). Multivariable logistic regression identified 3 independent predictors of short-term all-cause mortality at 90 days: pulmonary hypertension (odds ratio [OR] 3.83, 95% CI: 1.44-10.16; p=0.007), age (OR 1.11, 95% CI: 1.04-1.19; p=0.003), and institutional experience (>2 implants; OR 0.40, 95% CI: 0.17-0.99; p=0.047).
Regarding midterm mortality at 2 years (in 90-day survivors), multivariable Cox regression identified 5 indeÂpendent predictors: arterial hypertension (OR 3.32, 95% CI: 1.16-9.54; p=0.026), pulmonary hypertension (OR 2.15, 95% CI: 1.08-4.29; p=0.03), baseline quality of life (OR 1.08, 95% CI: 1.00-1.17; p=0.042), age (OR 1.06, 95% CI: 1.01-1.11; p=0.021), and baseline creatinine (OR 1.02, 95% CI: 1.00-1.03; p=0.005) (Supplementary Table 9).
After uni- and multivariable Cox regression among the full study cohort, pulmonary hypertension (OR 2.05, 95% CI: 1.22-3.43; p=0.006), coronary artery disease (OR 2.03, 95% CI: 1.10-3.74; p=0.023), and age (OR 1.06, 95% CI: 1.02-1.10; p=0.002) were independent predictors of 2-year all-cause mortality, while prior coronary artery bypass graft (OR 0.37, 95% CI: 0.20-0.68; p=0.001) and left ventricular end-systolic diameter (OR 0.73, 95% CI: 0.54-1.00; p=0.001) were protective against mortality (Supplementary Table 10).
Discussion
The present study comprises the full study cohort of patients enrolled in the prospective Tendyne Expanded Clinical Study, extending previously published data on the outcomes of patients treated with the same device1116.
The main findings of this study are as follows:
I) For patients with MR at high or prohibitive surgical risk with a high burden of comorbidities, transapical TMVR proved to be safe with no intraprocedural deaths and a high technical success rate.
II) Overall rates of SAE were low, particularly regarding access site complications, but not without notable bleeding events.
III) In the vast majority of successfully treated patients, MR was completely eliminated with sustained echocardiographic findings up to 2 years. Consequently, TMVR treatment led to significant improvement of symptomatic burden, functional capacity and quality of life.
IV) Despite favourable outcomes concerning MR elimination and functional improvement, midterm mortality was elevated, with heart failure as the leading cause of death in a study population with predominantly secondary MR and left ventricular dysfunction at baseline.
V) Pulmonary hypertension and age were identified as independent predictors of acute mortality, while institutional experience with transapical TMVR was protective.
In recent years, 1- and 2-year outcomes of the first 100 patients treated with the transapical Tendyne TMVR system have been published, and these have supported the European Conformity (CE) mark approval of this TMVR device1116. The present study expands on these results to the full cohort of 191 treated patients, substantially extending the body of evidence on this transcatheter TMVR device. Compared to the aforementioned studies, this analysis confirms procedural safety with continuously high rates of technical success (96.9%) and the absence of intraprocedural death and surgical conversion in all treated patients. Recently, 1-year data from the the Tendyne European Experience Registry (TENDER) were published demonstrating similar procedural and clinical outcomes in 195 patients treated with the device. Of note, this retrospective study also included 60 (31%) patients treated under off-label conditions, characterised by higher rates of mitral annular calcification and elevated mitral valve gradient21.
Overall postprocedural complication rates were low in the present study. Access site complications are a known and serious threat specific to transapical procedures. The particularly low rate of access site complications within 30 days after the procedure, despite device delivery through a 34 Fr or 36 Fr transapical sheath, supports the safety of the procedure and suggests that the apical pad of this tethered TMVR device may serve as an additional haemostatic feature. However, elevated rates of bleeding events and acute kidney injury were reported. Aside from the potential trauma of a transapical TMVR procedure, the most likely explanation for elevated bleeding rates, especially within the first month after transapical TMVR, may be the need for oral anticoagulation for at least 3 months after the procedure. The majority of patients were already on oral anticoagulants (53.4%) and antiplatelet therapy (60.2%) at baseline, which may have contributed to the elevated bleeding risk in this elderly and comorbid patient cohort. The impact of anticoagulation and comorbidities on postprocedural bleeding, rather than the choice of access, is further supported by the markedly elevated bleeding rates observed in a recently published series with a transeptally delivered TMVR device22.
Patient selection remains key to reproducibly achieve beneficial outcomes and reduce the postprocedural complication rates of patients treated with transapical TMVR. With most deaths classified as cardiovascular and almost half of all deaths deemed definitively not attributable to the device, the prevalence of cardiovascular comorbidities appears to have an important impact on clinical outcomes. Elevated rates of deaths from refractory heart failure up to 90 days suggest the need for improved patient selection and postoperative management of patients receiving transapical TMVR. In particular, postoperative management after TMVR is complex and may be more demanding compared to other transcatheter procedures. The present study identified both pulmonary hypertension and age as independent factors associated with both short- and midterm mortality. The adverse impact of pulmonary hypertension on outcomes after mitral valve interventions is well known and strengthens the hypothesis that patients with long-existing MR and potentially refractory pulmonary hypertension may not tolerate a transapical TMVR procedure well, whereas age represents the patient’s natural risk2324. The fact that institutional experience proved to be independently protective regarding short-term survival supports the use of transapical TMVR preferably at experienced tertiary centres. The learning curve appears to be short, with improved outcomes achieved after the first 2 procedures only. However, since no intraprocedural deaths were observed in the present study, learning curve effects might not only be attributable to improved procedural skill but may include improved patient selection resulting in better short-term outcomes with increasing institutional experience. In summary, age, comorbidities and quality of life have an important impact on midterm mortality, highlighting the importance of patient selection and clinical follow-up.
In accordance with published data on TMVR, effective and predictable MR elimination in the vast majority of patients after transapical TMVR was confirmed in this series as well. Several studies have demonstrated the negative impact of residual or recurrent MR on outcomes after TEER9252627. In particular, MR elimination and a lasting reduction in RVSP may result in a consistent reduction of symptomatic burden as well as an improvement of functional capacity and quality of life up to 2 years.
While data on the long-term impact of TMVR-mediated MR elimination in comparison to TEER or medical therapy are scarce, the present study supports the use of transapical TMVR as a valid complementary treatment option for patients ineligible for both surgery and TEER2829. The ongoing randomised controlled SUMMIT trial (ClinicalTrials.gov: NCT03433274) comparing outcomes of transapical TMVR versus TEER will provide important data that will help define future roles of TMVR within the armamentarium of available MR treatment options.
Limitations
This study is a single-arm interventional study with its inherent limitations and might be subject to provider bias. The lack of a control group limits conclusions with regard to the comparison to other MR therapies. Clinical and echocardiographic follow-up in surviving patients was comprehensive but not complete, thereby limiting paired echocardiographic comparisons to those with available data. Moreover, data on tricuspid regurgitation and right ventricular function were not available.
Conclusions
This study investigated 2-year outcomes following transapical TMVR in the full study cohort of the Tendyne Expanded Clinical Study. TMVR yielded predictable elimination of MR and sustained reduction of pulmonary artery pressure accompanied by improvements in symptoms, functional capacity, and quality of life. Individual clinical and echocardiographic risk assessments remain important within the selection process of potential TMVR candidates.
Impact on daily practice
Transapical transcatheter mitral valve replacement (TMVR) with the Tendyne Mitral Valve System is associated with a predictable elimination of mitral regurgitation and a sustained reduction of pulmonary artery pressure, resulting in an improvement of symptoms, functional capacity and quality of life. Patient survival largely depends on age and cardiovascular comorbidities, highlighting the need for optimised patient selection. While TMVR already represents a complementary treatment option for patients at high or prohibitive surgical risk who are ineligible for transcatheter edge-to-edge repair (TEER), studies comparing TMVR to TEER in patients amenable to both transcatheter therapies are warranted.
Funding
This study was sponsored by Abbott, Santa Clara, CA, USA.
Conflict of interest statement
L. Conradi has been an advisory board member for Abbott, Medtronic and JenaValve; and has received personal fees from Boston Scientific, Edwards Lifesciences, Micro Interventional Devices, MicroPort, Neovasc, Pi-Cardia, and Venus Medtech. S. Ludwig has received a grant from the German Heart Foundation (DHS); speaker honoraria from Abbott; travel compensation from Edwards Lifesciences; advisory fees from Bayer; and is a consultant to NVT. D.W. Muller has served as a consultant for Medtronic, Abbott, and Edwards Lifesciences; and has received research grant support from Abbott and Medtronic. P. Sorajja has served as a consultant for 4C Medical, Abbott, Adona, Boston Scientific, Cultiv8, Edwards Lifesciences, Foldax, GE HealthCare, GLG, Laza Medical, Medtronic, Philips, W.L. Gore & Associates, VDyne, and xDot. A. Duncan has served as a proctor and consultant for Abbott, Edwards Lifesciences, Medtronic, and Neochord. B. Bethea has served as a consultant for Abbott. G. Dahle has been proctor/speaker for Abbott; and speaker for Edwards Lifesciences. V. Babaliaros has served as a consultant for Edwards Lifesciences and Abbott; and has equity in Transmural Systems. M. Guerrero has served as a consultant for Edwards Lifesciences and Abbott; and has received research grant support from Edwards Lifesciences. V. Thourani has served as a consultant for and received research grant support from Abbott. N. Dumonteil has been a consultant for Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. T. Modine has served as a proctor and consultant for Abbott, Edwards Lifesciences, and Medtronic. A. Garatti has served as a proctor for Abbott. P. Denti has received speaker honoraria from Abbott and Edwards Lifesciences; and has been a consultant for Approxima, HVR, InnovHeart, and Pi-Cardia. J. Leipsic has provided institutional core laboratory services to Edwards Lifesciences, Abbott, Medtronic, Boston Scientific, Pi-Cardia, and Neovasc, without direct personal compensation. P. Blanke has provided institutional core laboratory services to Edwards Lifesciences, Abbott, Medtronic, Boston Scientific, Pi-Cardia, and Neovasc, without direct personal compensation; and has been a consultant to Edwards Lifesciences. V. Badhwar has served as an uncompensated consultant for Abbott. M.L. Chuang has no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

