Abstract
Robotics entered the cardiovascular field in the late 1990s with a robot-assisted coronary artery bypass graft. Since then, the use of robots has become a common part of cardiovascular surgery in several types of interventions. The experience in transcatheter interventions has been slower, and the application of robotics to percutaneous coronary interventions has shown some encouraging results but also some technical limitations. Following the growth of structural heart interventions, attention has recently switched to the potential application of robotics in this field. So far, several cases have been performed in animal models and only a few cases in humans. The opportunity to perform a procedure (almost) without any X-ray exposure or lead garments is extremely attractive, especially for operators. Alongside these, there are several further potential advantages, but there are also many challenges to overcome. The integration of artificial intelligence and machine learning in the near future might further contribute to improve the performance of future generations of robots. In this review, the current and future applications of robotics in structural heart interventions and transoesophageal echocardiography will be discussed, together with the potential advantages, challenges and future perspectives.
Robotics has entered several fields of daily life over the last half century, making it possible to increase precision and to simplify several tasks. Its introduction into the healthcare field was initially in the laboratory setting, while its integration into clinical medicine has been slow. The first surgical application of robotic technology was represented by an industrial robotic arm modified to perform a stereotactic brain biopsy with 0.05 mm accuracy, in 19851. Since then, several robotic technologies have been developed and applied in different surgical areas. The first use in the cardiovascular field was a coronary artery bypass graft in 1998 with the da Vinci system (Intuitive Surgical)23. Following the positive experience in the surgical field, robotics has been increasingly used in the field of percutaneous coronary intervention (PCI) since its first description in 200545. Compared to surgery, robotics in transcatheter interventions has two additional advantages for the operators: (1) reduced/zero radiation exposure and (2) fewer orthopaedic injuries. Despite this, robotic PCI did not take off because of high costs and limited application in complex coronary anatomies.
Following the growth of structural heart interventions in the last decade, there has been a renewed interest in robotics, and initial experiences have been described for transcatheter aortic valve implantation (TAVI), transcatheter edge-to-edge repair (TEER) for mitral and tricuspid valves, and for transoesophageal echocardiography (TOE).
This review provides an overview of the current experience of robotics in the field of percutaneous structural heart interventions (Table 1) with a glimpse towards possible future applications and integration with artificial intelligence and machine learning.
Table 1. Current experience with robotics in structural heart disease interventions and TOE.
| Procedure | Procedure details | Level of autonomy | Experience | Company |
|---|---|---|---|---|
| TAVI | MRI-guided robotic TAVI | 0 | Eight cases in porcine models | Johnson & Johnson |
| Transapical | ||||
| Robot-AI-assisted TAVI | 1 | Single case in a porcine model | Caranx Medical | |
| Transfemoral | ||||
| M-TEER | Robotic-guided M-TEER | 1 | First-in-human | SurgiPulse Robotics |
| Manual TSP | ||||
| Telerobotic-guided M-TEER | 1 | Eight cases in humans | SurgiPulse Robotics | |
| Manual TSP | ||||
| TMVI | Robotic-guided TMVI | 2 | Animal model | Capstan Medical |
| TOE | Robotic-assisted TOE | 0 | Five cases in humans | ROB’E GmbH |
| AI: artificial intelligence; MRI: magnetic resonance imaging; M-TEER: mitral transcatheter edge-to-edge repair; TAVI: transcatheter aortic valve implantation; TMVI: transcatheter mitral valve implantation; TOE: transoesophageal echocardiography; TSP: transseptal puncture | ||||
Transcatheter aortic valve implantation
Currently, while several cases have been described for robotic-assisted surgical aortic valve replacement, the experience with robotic TAVI is limited67.
Initial studies in which robotics was applied to a TAVI procedure were conducted on in vitro models, using the Magellan system (Hansen Medical) as the robotic platform8. The results showed that the use of robotics was particularly effective at reducing contact with the aortic arch wall and thereby reducing the possible consequent embolic risk, as compared to a manual technique.
Further experiences have been described on a swine model with robotic-assisted, real-time magnetic resonance-guided TAVI using a transapical access site9. The platform used in these cases was a dedicated magnetic resonance imaging (MRI)-compatible robotic surgical assistant system (Johnson & Johnson) with a positioning module and a valve delivery module, allowing 5 degrees and 3 degrees of freedom, respectively. The results showed feasibility and multiple potential advantages such as more predictable valve positioning and reduced use of contrast, in addition to avoiding X-rays and reducing orthopaedic injuries. On the other hand, the use of MRI might be a limitation for those with non-MRI-compatible devices, and a transapical approach is a second-choice alternative access for TAVI.
Recently, the first case of robot-assisted TAVI in a porcine model was performed using a completely dedicated robotic system supported by artificial intelligence10. The system is based on the TAVIPILOT technology developed by Caranx Medical and provides assistance throughout the entire procedure, from preprocedural planning using a three-dimensional (3D) digital twin reconstruction to access site management (femoral access) and to navigation and valve placement (Figure 1). Once mature, TAVIPILOT aims to improve the accuracy of transcatheter heart valve positioning, with the ultimate goal of full robotic deployment under physician supervision. The technology is promising, but clinical data in humans are needed in order to understand its true potentialities compared to manual TAVI, especially in terms of lifetime management11. In light of the continuously growing number of transcatheter procedures compared to surgery, such technologies are expected to draw particular attention in the near future.

Figure 1. TAVIPILOT technology developed by Caranx Medical for performing robot-assisted transcatheter aortic valve implantation. A) The TAVIPILOT arm with the transcatheter heart valve system, and (B) with an ultrasound probe; (C) the TAVIPILOT control system. Modified with permission from10.
Mitral and tricuspid transcatheter edge-to-edge repair
TEER is the most used technique for the percutaneous treatment of both mitral and tricuspid regurgitation, although recently, transcatheter replacement has emerged as a potential alternative therapy1213.
After in vitro validation of robotic TEER, the first-in-human experience of robotic-assisted mitral TEER with pure echo guidance has recently been described for the treatment of mitral regurgitation (MR) with the Kyrin TEER system (Shenqi Medical)14. The first part of the procedure, including the transseptal puncture (TSP), was performed manually by the operator, while all the subsequent steps leading to clip delivery were performed with a newly designed robotic system (SurgiPulse Robotics) compatible with third- and fourth-generation (G3/G4) MitraClip devices (Abbott) or with other similar devices15. The system consists of the following components (Figure 2):
• a robotic articulating arm with single-use sterile functional modules. It has 3 components allowing the manipulation of the steerable guiding catheter, clip delivery sheath and clip control catheter16;
• a control computer for calibration and response rate control of the whole system;
• a cockpit, with three joysticks and several buttons for complete control of the articulating arm.
The final echocardiographic result was optimal (0+ MR), the intervention time (after TSP) was 38 minutes, and no intra-/postprocedural complications occurred; thus, the initial feasibility and safety of robotic-assisted mitral TEER were demonstrated.
Following this, a second case was performed using the commercially available MitraClip G4 confirming the feasibility and safety of the system17. Finally, telerobotic mitral TEER procedures have been successfully performed using a MitraClip G4: the operator was in Beijing and the patients in Kunming and Shenzhen, a distance of over 2,500 km, and 13 channels of control signals were transmitted by a 5G internet connection with a maximum response delay <150 ms. So far, a total of 8 patients (including 2 telerobotic procedures) have undergone a robotic-assisted mitral TEER procedure with 100% procedural success. Notably, remote interventions can be performed by downloading a dedicated app from any tablet, and no dedicated hardware is needed. Large-scale randomised trials will be required to validate these new technologies.
Thanks to the “modular” structure of this technology, it might also be adapted to tricuspid TEER using dedicated modules. In the meantime, several companies are working on robotic tricuspid TEER, although no cases have been described or performed, so far.
The robotic technologies described above are the closest to translation into real-world practice, and, from a technological standpoint, all share two key features, with each one providing a significant improvement to TEER procedures: (1) dedicated cockpits that allow teleoperation and the avoidance of X-ray exposure for operators; (2) robotic actuators that are designed to be coupled with a standard, i.e., non-robotic, delivery system in order to manipulate it with higher precision as compared to a human operator.
At the same time, these technologies do not improve two other relevant aspects of TEER procedures, which are instead tackled by solutions that are still in their embryonic stage. The first aspect is the navigation of TOE imaging and the correct interpretation of mitral valve (MV) lesions and catheter position. These are non-trivial tasks despite the existence of established procedures in the use of TOE for intraprocedural guidance18. To make these tasks intuitive, deep convolutional neural networks are useful to automatically trace the MV in real time, including its leaflets, from 3D TOE. In particular, a new method has been proposed to distinguish between the two leaflets and to reconstruct the pattern of leaflet billowing and the detailed profile of the coaptation line, including coaptation gaps, thereby simplifying the detection of the lesion and of the clip’s target19. The second aspect is the identification of the catheter path from the fossa ovalis to the MV target lesion and of the corresponding sequence of manoeuvres to be performed using the delivery system. This step, which normally relies on the experience of operators, could be achieved using sensor catheters, not only from the distal access to the right heart while limiting catheter-vessel wall interactions, but also in the atrial chamber according to an automatically computed safe route20. Such technologies might simplify the operator’s tasks and, at the same time, increase procedural precision and safety.

Figure 2. Robotic system for performing mitral transcatheter edge-to-edge repair compatible with a MitraClip G3/G4. A) Robotic TEER system (SurgiPulse Robotics). B) Control cockpit with ergonomic design. White arrows indicate forward/backward motion of articulating arm. C) The articulating arm consists of three functional modules which manipulate the steerable guiding catheter (C1), clip delivery catheter (C2) and clip control sheath (C3). D) Control cockpit buttons and knob use. First functional module: clockwise/counter-clockwise rotation, forward/backward motion and +/– knob of the guiding catheter (red arrows and box); second functional module: catheter’s forward/backward motion and A/P, L/M buttons (green arrows and box); third functional module: clockwise/counter-clockwise rotation, forward/backward motion (blue arrows and box), gripper/locker lines (yellow box and purple box) and clip open/close (orange arrows and box). MitraClip G3/G4 by Abbott. A: anterior; G3/G4: third-/fourth-generation; L: lateral; M: medial; P: posterior; TEER: transcatheter edge-to-edge repair
Transcatheter mitral valve replacement
Transcatheter mitral valve replacement (TMVR) in the different settings of MV disease (valve-in-native, valve-in-valve, valve-in-ring and valve-in-mitral annular calcification) has expanded the toolbox of MV therapies. Despite the recent awarding of the European Conformity (CE) mark to Tendyne (Abbott) and increasing experience with some devices, the growth of TMVR has been slow because of multiple issues related to the complex MV anatomy1321. Above all, the risk of left ventricular outflow tract obstruction (LVOTO) has accounted for several screening and procedural failures, directly affecting clinical outcomes2223.
Capstan Medical has developed a percutaneous compact MV prosthesis with a compliant outer frame that can be precisely positioned with a robotic platform (Figure 3). The unique valve and delivery system design allows the operator to slowly expand the valve and reposition it to ensure proper placement before the final deployment. The multijointed robotic arm and catheter, together with the user-friendly digital controller, facilitate precise placement independently of the transseptal puncture site. Preprocedural planning and practice with the robot can be achieved with imaging and with a training simulator, providing an optimal driving experience (level 2 autonomy).
The first studies in animal models have shown good valve performance in terms of thrombogenicity, secure anchoring without LVOTO, and a total procedural time <1 hour. First-in-human studies are being planned and will be useful to understand whether robotic TMVR might be superior to “manual” TMVR and how to expand the future use of this technology.
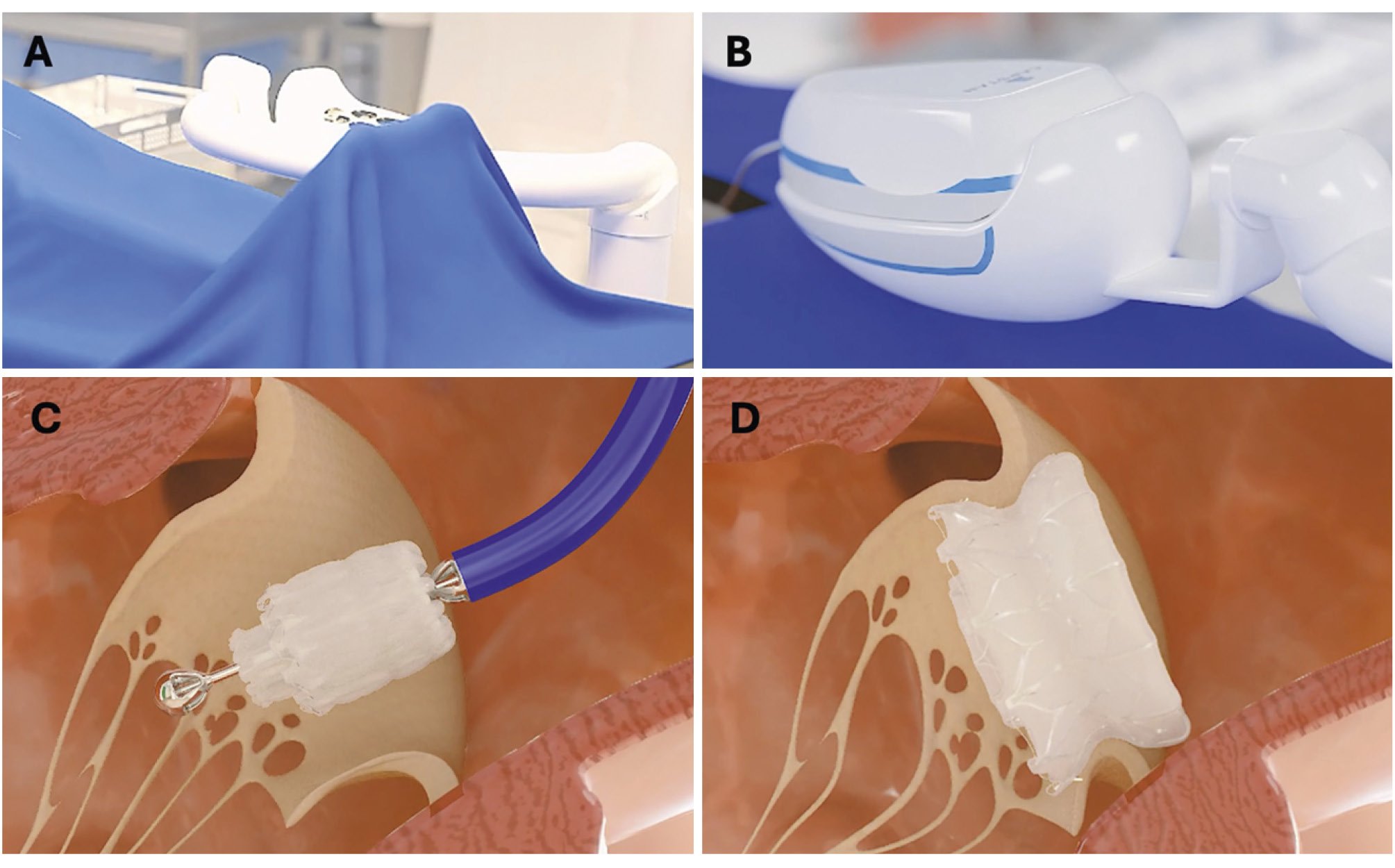
Figure 3. Percutaneous mitral prosthesis and its associated robotic platform. A) Table-mounted adjustable support for the robotic catheter manipulator; (B) delivery system attached to the robotic catheter manipulator; (C) repositionable, partially expanded mitral valve; (D) fully deployed low-profile mitral prosthesis. Robotic platform developed by Capstan Medical.
Transoesophageal echocardiography
Alongside the continuous growth of structural heart interventions over the last years, the role of interventional imaging has become central in several structural procedures requiring imaging guidance2425. Current data clearly indicate that, among the structural Heart Team, interventional echocardiographers are exposed to higher radiation doses. From this perspective, the use of a robotically controlled TOE probe might represent an attractive solution for interventional imagers.
Preclinical testing has recently been described using a new system (ROB’E [ROB’E GmbH])26. It is composed of three parts (Figure 4):
• ROB’E guide: it is placed close to the patient’s mouth and is responsible for probe advancing/retracting, stabilising and for rotation around the longitudinal axis.
• ROB’E base: it contains the motor and allows axial rotations (antegrade/retrograde and left/right bending).
• ROB’E controller: it interacts with and controls the previous two components.
Besides the in vitro testing, which demonstrated its reliability and safety, the system was also tested in five human cases and achieved clinical and technical success27. Such encouraging results should be confirmed in a larger sample size and in different TOE-guided procedures with varying complexity levels.
Another company (Laza Medical) is working on robotic-assisted TOE, although no data have been published/presented so far.
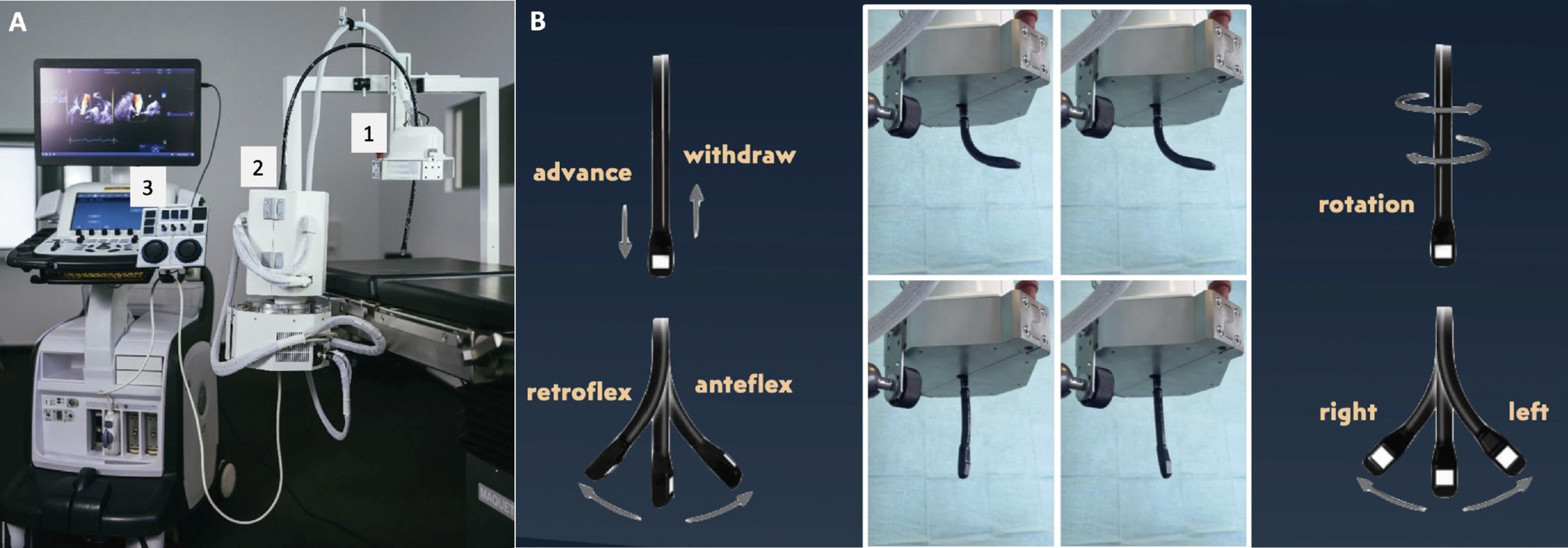
Figure 4. ROB’E system for transoesophageal echocardiography. A) The three components of ROB’E: (A1) ROB’E guide, (A2) ROB’E base with integrated computer, and (A3) ROB’E controller. B) ROB’E guide and associated probe’s possible movements.
Levels of autonomy and social/ethical challenges
While robotics is increasingly becoming part of several activities in everyday life, and robots have reached high levels of autonomy, especially in the vehicles field, the levels of autonomy of each robot in the field of surgical/percutaneous interventions are highly debated. In fact, if, on the one hand, highly autonomous robots might increase accuracy, and reduce the learning curve and human errors, on the other hand, some ethical and legal questions might arise.
Currently, the autonomy grade of available robots includes 6 levels (Figure 5)28:
• Level 0 (no autonomy): the robot is completely controlled by the operator, with no support or constraint provided.
• Level 1 (robot assistance): the robot is capable of interacting with the operator to guide or support the execution of a particular task. The provided assistance consists of either active constraints to guide the surgeon’s motion or virtual fixtures to enhance surgical site visualisation.
• Level 2 (task autonomy): the robot is capable of accomplishing specific surgical tasks based on indications provided by the operator. Control switches from the human operator to the machine for the duration of the task to be executed.
• Level 3 (conditional autonomy): the robot is provided with perceptual capabilities to understand the surgical scenario, plan and execute specific tasks, and update the plan during execution.
• Level 4 (high autonomy): the robot can interpret preoperative and intraoperative information, produce an interventional plan composed of a sequence of tasks, execute this plan autonomously, and replan if necessary. The operator supervises the system under the discrete control paradigm.
• Level 5 (full autonomy): the robot can perform the intervention on its own with no human input.
So far, no systems have reached level 5, and while autonomous driving vehicles have reached level 3 and are approaching level 4, the commercially available platforms for robotic interventions (both surgical and percutaneous) remain mainly at level 0/1. This might be explained by the different complexity between the two fields, involving some technical challenges (e.g., any robot should combine sensing, compensation in case of unexpected events, and motion control) and non-technical challenges. Although it is questionable whether full autonomy (level 5) in surgery will be achievable in the foreseeable future, the consequences of introducing completely autonomous machines are examined herein.
From a social standpoint, the introduction of machines might address the important issue of surgeon/operator shortage, raising the possible fear, on the other hand, of worker replacement. In the medical field, this might not happen, as most activities require adaptation and personal interaction, at least in the surgical/interventional field.
In addition, the social acceptance of being treated by a robot and not by humans will represent a further challenge to overcome, although highly autonomous technology is becoming widely spread in several fields aside from healthcare. The current evidence on robot acceptance suggests that perceiving the robot as similar to a human being might be beneficial, although it should not completely resemble humans (the “uncanny valley phenomenon”), and a robot’s design should be adapted to its task: a robot designed to provide companionship might have a more human-like appearance, while a robot for a surgical task might look and behave more like a machine2930. Recent data on robot trustworthiness indicate that after three trust violations (mistakes) from the robot, human trust is lost with no chance of being restored31.
Another fundamental point will be the legal and ethical issues in consideration of the fact that technology might be “responsible” for critical choices/actions with direct effects on patients’ safety. In 2024, the European Parliament approved the Artificial Intelligence (AI) Act, in which AI systems are classified as unacceptable risk, high risk, and low/minimal risk. All AI systems involved in healthcare are considered high risk, as they are potentially harmful for health and safety. The AI Act outlines the primary role of human beings in the decision-making process: the human being will be responsible for monitoring the functioning of the high-risk AI system, intervening promptly in the event of anomalies, malfunctions, or unexpected performance. Despite this, several gaps are still present as the rapid development of robotics and AI is far exceeding the capacity of the legal framework to fully understand their implications.
Currently, we are in a hybrid phase where the role of the physician is still central, although technology is continuously gaining importance. Keeping in mind all these premises, neither excessive enthusiasm nor pessimism towards the implementation of robotics and AI in healthcare should be deemed the correct approach. The goal of medicine is patients’ health, and whatever may help to achieve this goal should be supported. In particular, robotics may provide several advantages but may also present important ethical and legal challenges. A deep collaboration among healthcare professionals, legal experts, legislators and stakeholders is fundamental to overcome such challenges and to maximise the benefits of robotics in healthcare.

Figure 5. Levels of autonomy in interventional robotics. Strengths, opportunities, weaknesses and threats of robotics in structural heart disease interventions.
Robotics: opportunities and challenges
Alongside some non-technical challenges described above, the introduction of robotics into catheterisation laboratories offers a number of possible opportunities for consideration (Central illustration):
- X-ray-free procedure: occupational effects related to X-ray exposure have been widely demonstrated for interventional cardiologists and, as already discussed above, for interventional imagers32. Such effects involve not only the direct effects of X-rays on human tissues/organs but also orthopaedic issues related to the wearing of protective lead garments. This point might represent a huge change for the health of operators and the whole cath lab team.
- Accuracy: this point is closely connected to safety and, as discussed in the previous paragraph, how to react to any possible error made by a robot might be highly debated. It is reasonable that robot-assisted procedures, together with the implementation of AI, might increase the quality of an operator’s performance and lead to better outcomes.
- Telemedicine: remote interventions have several potential benefits. First, they offer immediate availability, thereby reducing or even eliminating the need to transfer patients between hospitals for urgent care, resulting in consequent benefits in terms of patient outcomes and costs for the healthcare system. Second, telemedicine might allow efficient resource allocation that enables patients in remote or underserved areas to access skilled operators who might not be available locally. Third, experienced operators can manage and supervise multiple procedures simultaneously. Fourth, as learned from the COVID-19 experience, it can minimise infection risks by eliminating the need for a physical presence in the operating room. Fifth, telemedicine might represent an opportunity for training and mentorship under the guidance of experienced but distant operators. Finally, in case of the need for urgent bailout to solve a complication or a challenging situation, an experienced operator has the possibility to assist or to take over remotely.
- Black box: tracing of the procedural steps and, in some cases, learning from errors and possibly creating a more advanced algorithm might enhance procedural safety and efficiency, potentially improving procedural outcomes.
- Reallocation of medical resources: all four previous points have direct or indirect implications on resources with both the potential costs (e.g., the robot itself and its maintenance) and revenues (e.g., resource optimisation with telemedicine, potentially better patient outcomes without human error).
Connected to these possible advantages, there are some challenges:
- Internet speed/stability: this might be an important issue for telemedicine as internet breakdown or delay might significantly affect the intervention. Moreover, underserved areas very often also have poor internet connections and, consequently, internet infrastructure should be improved in order to offer assistance in such areas.
- Safety: as has already been discussed, one challenge is the complexity of some structural heart interventions. System malfunctions, software errors, connection failures, or mismatches in human-robot interaction could lead to adverse outcomes. Continuous monitoring, regular updates, and rigorous testing of these systems are essential to ensure patient safety. Moreover, this point is closely connected to ethical and legal issues and might become more significant as the levels of autonomy of robots/machines increase.
- Costs and sustainability: costs include not only the initial investment but also maintenance, training, and updates. This might also imply an “accessibility gap”, with only a few institutions being able to afford the most advanced robotic technologies. Cost-benefit analyses in other fields, such as robotic coronary artery bypass, showed no differences between robotic-assisted and conventional strategies33. Similar results were also observed in other surgical fields, suggesting that the social/financial perspective, longer-term time horizon and high volumes favour robotic procedures34. Moreover, the implementation of AI in the near future is likely to further change the cost-benefit ratio in favour of robots. A dedicated cost-benefit analysis in the field of structural heart interventions will be necessary to understand the sustainability for healthcare systems as well as to define the true benefits in terms of outcomes.
- Learning curve: the more complex the procedure, the more sophisticated the robot, and consequently, the steeper the learning curve. This has implications both for the cost and time invested by institutions and for the education models for new generations of operators.
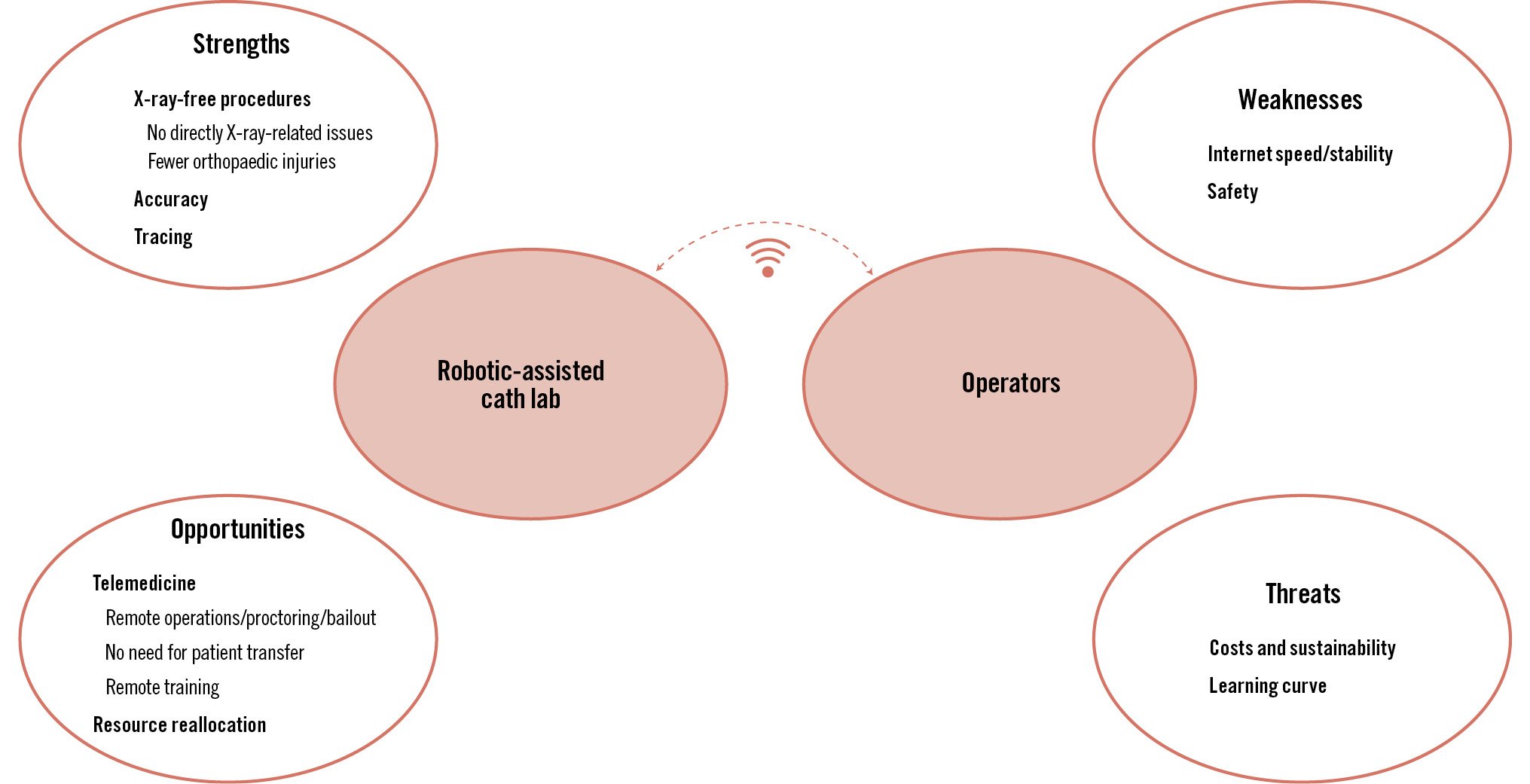
Central illustration. Strengths, opportunities, weaknesses and threats of robotics in structural heart disease interventions.
Future perspectives
Although currently, robotics in the field of cardiovascular intervention is characterised by very low levels of autonomy, integration with AI, machine learning, and augmented reality might significantly change robot performance in the near future35.
Indeed, AI integrates tasks like learning, reasoning, problem solving, perception, and understanding language, allowing computers to thereby derive insight from data, make informed decisions, and solve complex problems. Consequently, any robot supported by AI might collect clinical, laboratory and imaging data; implement risk scores and current guidelines; and finally, provide diagnostic, prognostic, and treatment output accordingly3637.
As regards clinical data collection, current evidence indicates that AI is sufficiently reliable for face recognition, speech analysis, and retinal diagnostics383940. Similarly, AI, thanks to convolutional neural networks and deep learning, has shown promising results when applied to electrocardiogram and imaging analysis4142. Alongside this, the application of augmented reality, such as HoloLens (Microsoft), and fusion imaging might further increase accuracy434445.
All these features will facilitate diagnosis, preprocedural planning and personalised risk-benefit assessment. However, when applied to the interventional context, it is less clear how the output of diagnostic information should guide treatment and, specifically, whether the robot should act autonomously during the procedure (level 5) or whether the operator should use AI information only as a support, keeping full control of the procedure.
Conclusions
Over the last two decades, robotics has become an integrated part of several surgical fields, but its adoption in interventional cardiology has been slower. Nonetheless, initial experiences in PCI have shown encouraging results despite several technical limitations. Currently, the adoption of robotics in structural heart interventions is in the first stage, and so far, only robotic-assisted mitral TEER has been performed in humans. Despite this, transcatheter interventions are growing fast, and it is likely that robotics will grow alongside them and play an important role therein in the near future. Robotics in the field of structural heart interventions might offer several opportunities for operators, patients, and healthcare systems, but at the same time, some challenges, technical and otherwise, will also need to be overcome. Moreover, integration with AI will improve robot/operator performance on the one hand, but might increase the level of robot autonomy on the other hand and, thus, raise some ethical and legal issues.
Considering how quickly technology development progresses, robots might enter catheterisation laboratories very soon, provided that (1) cost-effectiveness analyses demonstrate sustainability, (2) ethical/legal issues are accurately addressed, and most importantly, (3) Asimov’s first law of robotics is satisfied: “A robot may not injure a human being.”
Conflict of interest statement
G. Russo received a fellowship training grant from the EAPCI, sponsored by Edwards Lifesciences. M. Chen has served as a consultant for Jenscare Scientific; and received grants from Edwards Lifesciences and Boston Scientific. E. Ho has served as a consultant and received consulting fees from NeoChord Inc. P. Denti received speaker honoraria from Abbott; and is a consultant for Approxima, HVR, InnovHeart, Pi-Cardia, and Simulands. D. Tchétché is a consultant for Abbott, Edwards Lifesciences, Medtronic, Boston Scientific, T-Heart, and Caranx Medical. A. Latib has served on the advisory board for Medtronic, Abbott, Boston Scientific, Edwards Lifesciences, Shifamed, NeoChord Inc., VDyne, and Philips. M. Taramasso reports consultancy fees from Abbott, Edwards Lifesciences, 4Tech, Boston Scientific, CoreMedic, Mitraltech, and SwissVortex, outside the submitted work. The other authors have no conflicts of interest to declare.

