Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The COMPLETE trial demonstrated a reduction in cardiovascular (CV) death or new myocardial infarction (MI) after complete, rather than culprit-only, revascularisation in patients with ST-segment elevation myocardial infarction (STEMI) and multivessel disease (MVD). However, it is unknown whether this benefit varies according to baseline left ventricular ejection fraction (LVEF).
Aims: We aimed to determine the effects of complete versus culprit-only revascularisation according to LVEF.
Methods: Baseline LVEF was available for 2,214 of 4,041 randomised patients. The effect of both strategies on the first co-primary outcome of CV death or new MI and the second co-primary outcome of CV death, new MI, or ischaemia-driven revascularisation (IDR) was determined within the prespecified LVEF ranges of <45% (N=660) and ≥45% (N=1,554). An analysis of clinical outcomes by LVEF according to thirds was also conducted.
Results: Patients with LVEF <45% experienced a significantly higher incidence of the first co-primary outcome compared with those with LVEF ≥45% (4.2%/year vs 2.8%/year; hazard ratio [HR] 1.51, 95% confidence interval [CI]: 1.15-1.98; p=0.003). Compared with a culprit-only strategy, complete revascularisation consistently reduced the first co-primary outcome in patients with LVEF <45% (3.0%/year vs 5.5%/year; HR 0.55, 95% CI: 0.36-0.86) and those with LVEF ≥45% (2.4%/year vs 3.2%/year; HR 0.74, 95% CI: 0.52-1.04; interaction p=0.31). Complete revascularisation also consistently reduced the second co-primary outcome in patients with LVEF <45% (3.5%/year vs 7.3%/year; HR 0.49, 95% CI: 0.33-0.74) and those with LVEF ≥45% (2.7%/year vs 6.3%/year; HR 0.44, 95% CI: 0.33-0.60; interaction p=0.67). Consistent results were observed for both co-primary outcomes when LVEF was further stratified into categories of LVEF ≤35%, 36-49% and ≥50%.
Conclusions: Among patients presenting with STEMI and MVD, those with reduced LVEF are at higher risk of ischaemic events than patients with preserved LVEF. There is a consistent benefit of complete revascularisation regardless of baseline LVEF.
Baseline left ventricular ejection fraction (LVEF) is the most powerful independent predictor of short- and long-term mortality after an acute coronary syndrome123. Furthermore, the presence of multivessel disease is associated with significantly increased risks for cardiovascular events and mortality34. The COMPLETE trial demonstrated that, among patients presenting with ST-segment elevation myocardial infarction (STEMI) and multivessel coronary artery disease, complete revascularisation reduced the composite outcome of cardiovascular (CV) death or new myocardial infarction (MI) compared with culprit lesion-only percutaneous coronary intervention (PCI)4. Whether this benefit varies according to baseline left ventricular (LV) function remains unclear. The aim of this prespecified subgroup analysis of the COMPLETE trial is to determine the treatment effects of complete versus culprit lesion-only revascularisation according to baseline LVEF.
Methods
Design overview
The full study design and protocol have been previously published45. Briefly, COMPLETE was a multicentre randomised controlled trial that recruited 4,041 patients internationally and assigned them to either a strategy of complete revascularisation or a strategy of infarct-related (culprit) lesion-only PCI among patients with STEMI and multivessel coronary artery disease4. The main objective of the trial was to determine if a strategy of complete revascularisation is superior to culprit lesion-only revascularisation following successful PCI of the infarct-related artery. Revascularisation of the non-culprit lesion could be performed during the index hospitalisation or after hospital discharge but no later than 45 days after randomisation.
Eligibility criteria
Patients presenting to hospital with STEMI were eligible if they could be randomised within 72 hours of successful culprit lesion PCI. Suitable patients were required to have multivessel coronary artery disease, defined as the presence of at least one angiographically significant non-infarct-related (non-culprit) lesion that was amenable to successful treatment with PCI and was located in a vessel with a diameter of at least 2.5 mm that was not stented as part of the index culprit-lesion PCI. Non-culprit lesions were deemed angiographically significant if they were associated with at least 70% stenosis of the vessel diameter on visual estimation or with 50-69% stenosis accompanied by a fractional flow reserve measurement of 0.80 or less. Patients in whom there was a prerandomisation intent to revascularise a non-culprit lesion or perform surgical revascularisation and those with previous coronary artery bypass graft surgery, non-CV comorbidity associated with life expectancy of less than 5 years, or any other medical, geographical, or social factor making study participation impractical or precluding long-term yearly follow-up were excluded from the study.
Outcomes
The first co-primary outcome was the composite of CV death or new MI. The second co-primary outcome was the composite of CV death, new MI, or ischaemia-driven revascularisation (IDR). Safety outcomes included major bleeding and contrast-associated acute kidney injury. An event-adjudication committee, which consisted of clinicians who were unaware of the treatment assignments, adjudicated the primary, secondary, and safety outcome events. To explore the effects of baseline LV function on outcomes, LVEF <45% versus ≥45% was identified as a prespecified subgroup analysis of the trial5. Further post hoc analyses were conducted with LV function categorised into LVEF ≤35%, 36-49% and ≥50%, which represents one of the most commonly used classifications in clinical practice.
Statistical analysis
Analyses were carried out on the intention-to-treat population, which included all patients with an available evaluation of baseline LVEF who underwent randomisation, regardless of the treatment they actually received. Baseline and procedural characteristics are summarised as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and as frequency/percentage for categorical variables. Differences between LVEF (<45% and ≥45%) subgroups and treatment allocation were tested using the two-sample Student's t-test for normally distributed variables, the Wilcoxon rank-sum test for non-normally distributed variables, and the chi-square test for categorical variables. The treatment effect of complete revascularisation versus culprit lesion-only PCI was estimated using stratified Cox proportional hazard models with an interaction term between treatment allocation and LVEF. Estimates of the hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and the interaction effect was assessed with a likelihood ratio test. The adjusted analysis accounted for age, sex, diabetes, hypertension, dyslipidaemia, smoking, history of heart failure; and an interaction term between treatment allocation and LVEF was included. A similar analysis was performed on the three LVEF groups (≤35%, 36-49% and ≥50%). Cumulative incidences of the primary outcomes were plotted using the Kaplan-Meier method. All tests of significance were 2-sided with an alpha level of 0.05. All analyses were conducted using SAS 9.4 software (SAS Institute), and the figures were created using R software, version 4.4.1 (R Foundation for Statistical Computing) and S-PLUS software (TIBCO Software).
Results
Of the 4,041 randomised patients in COMPLETE, baseline LVEF data, collected during the index event, were available for 2,214 patients. There was a total of 660 patients in the LVEF <45% cohort (331 randomised to complete revascularisation and 329 to culprit lesion-only PCI) and 1,554 patients in the LVEF ≥45% cohort (782 patients randomised to complete revascularisation and 772 to culprit lesion-only PCI). Male patients comprised the majority in both LVEF groups. In patients with LVEF <45%, there were higher proportions of prior MI (10% vs 6.6%), prior history of heart failure (4.1% vs 2.4%), and worse New York Heart Association (NYHA) Class at baseline than in patients with LVEF ≥45%. There were also proportionally more patients who had Killip class ≥2 at presentation in the LVEF <45% group compared with the LVEF ≥45% group (22.5% vs 7.9%). Medications were similar across both groups except for oral diuretics which accounted for 20% in those with LVEF <45% versus 8.3% in the LVEF ≥45% cohort. The baseline mean SYNTAX score was 18 in patients with LVEF <45% compared with 15 in patients with LVEF ≥45%. Culprit lesions were more frequently located in the left anterior descending artery in patients with LVEF <45% than in those with LVEF ≥45% (59.3% vs 24.6%; p<0.001). The success rates of culprit-lesion PCI at index procedure (90.5% vs 92.3%) and of non-culprit lesions (96.3% vs 97.8%) were similar in the two cohorts. Baseline LV function did not appear to impact the operator’s decision on the intended timing of complete revascularisation, with the majority opting to perform the staged PCI during the index hospitalisation (68.3% for LVEF <45% vs 68% for LVEF ≥45%; p=0.24). Baseline and procedural characteristics are shown in Table 1 and Table 2.
Table 1. Baseline characteristics according to LVEF.
| LVEF <45% | LVEF ≥45% | p-value† | |||||
|---|---|---|---|---|---|---|---|
| Total (N=660) | Complete (N=331) | Culprit-only (N=329) | Total (N=1,554) | Complete (N=782) | Culprit-only (N=772) | ||
| Age, years | 62.8±10.8 | 61.9±10.8 | 63.6±10.8 | 61.8±10.8 | 61.3±10.7 | 62.3±10.8 | 0.05 |
| Male sex | 536 (81.2) | 263 (79.5) | 273 (83.0) | 1,227 (79.0) | 622 (79.5) | 605 (78.4) | 0.23 |
| Diabetes | 120 (18.2) | 55 (16.6) | 65 (19.8) | 317 (20.4) | 156 (19.9) | 161 (20.9) | 0.23 |
| Chronic renal insufficiency | 10/595 (1.7) | 3/297 (1.0) | 7/298 (2.3) | 24/1,432 (1.7) | 10/719 (1.4) | 14/713 (2.0) | >0.99 |
| Prior myocardial infarction | 66 (10.0) | 31 (9.4) | 35 (10.6) | 103 (6.6) | 54 (6.9) | 49 (6.3) | 0.006 |
| Current smoker | 260 (39.4) | 134 (40.5) | 126 (38.3) | 628 (40.4) | 333 (42.6) | 295 (38.2) | 0.65 |
| Hypertension | 335 (50.8) | 164 (49.5) | 171 (52.0) | 770 (49.5) | 379 (48.5) | 391 (50.6) | 0.6 |
| Dyslipidaemia | 261 (39.5) | 137 (41.4) | 124 (37.7) | 621 (40.0) | 302 (38.6) | 319 (41.3) | 0.85 |
| Prior PCI | 57 (8.6) | 27 (8.2) | 30 (9.1) | 101 (6.5) | 54 (6.9) | 47 (6.1) | 0.07 |
| Prior stroke | 18 (2.7) | 9 (2.7) | 9 (2.7) | 52 (3.3) | 24 (3.1) | 28 (3.6) | 0.45 |
| History of heart failure | 27 (4.1) | 12 (3.6) | 15 (4.6) | 35 (2.3) | 19 (2.4) | 16 (2.1) | 0.016 |
| Body mass index, kg/m2 | 28.6±5.1 | 29.0±5.1 | 28.3±5.0 | 28.3±6.1 | 28.5±7.2 | 28.0±4.7 | 0.13 |
| Time from symptom onset to first device activation | 0.2 | ||||||
| <6 hours | 433/654 (66.2) | 222/329 (67.5) | 211/325 (64.9) | 1,074/1,539 (69.8) | 543/774 (70.2) | 531/765 (69.4) | |
| 6-12 hours | 113/654 (17.3) | 52/329 (15.8) | 61/325 (18.8) | 251/1,539 (16.3) | 122/774 (15.8) | 129/765 (16.9) | |
| >12 hours | 108/654 (16.5) | 55/329 (16.7) | 53/325 (16.3) | 214/1,539 (13.9) | 109/774 (14.1) | 105/765 (13.7) | |
| Killip class ≥2 | 147/654 (22.5) | 74/329 (22.5) | 73/325 (22.5) | 122/1,546 (7.9) | 59/779 (7.6) | 63/767 (8.2) | <0.001 |
| NYHA Class | 0.038 | ||||||
| I | 152/210 (72.4) | 90/116 (77.6) | 62/94 (66.0) | 319/388 (82.2) | 158/195 (81.0) | 161/193 (83.4) | |
| II | 38/210 (18.1) | 14/116 (12.1) | 24/94 (25.5) | 48/388 (12.4) | 22/195 (11.3) | 26/193 (13.5) | |
| III | 13/210 (6.2) | 9/116 (7.8) | 4/94 (4.3) | 15/388 (3.9) | 11/195 (5.6) | 4/193 (2.1) | |
| IV | 7/210 (3.3) | 3/116 (2.6) | 4/94 (4.3) | 6/388 (1.5) | 4/195 (2.1) | 2/193 (1.0) | |
| Medications at discharge | |||||||
| ASA | 656 (99.4) | 330 (99.7) | 326 (99.1) | 1,550 (99.7) | 779 (99.6) | 771 (99.9) | 0.25 |
| P2Y12 inhibitor (any) | 657 (99.5) | 328 (99.1) | 329 (100) | 1,544 (99.4) | 776 (99.2) | 768 (99.5) | 0.77 |
| Ticagrelor | 415 (62.9) | 217 (65.6) | 198 (60.2) | 968 (62.3) | 489 (62.5) | 479 (62.0) | 0.79 |
| Prasugrel | 42 (6.4) | 20 (6.0) | 22 (6.7) | 175 (11.3) | 98 (12.5) | 77 (10.0) | <0.001 |
| Clopidogrel | 201 (30.5) | 91 (27.5) | 110 (33.4) | 405 (26.1) | 192 (24.6) | 213 (27.6) | 0.034 |
| Beta blocker | 591 (89.5) | 291 (87.9) | 300 (91.2) | 1,364 (87.8) | 677 (86.6) | 687 (89.0) | 0.24 |
| ACEi/ARB | 573 (86.8) | 286 (86.4) | 287 (87.2) | 1,323 (85.1) | 671 (85.8) | 652 (84.5) | 0.3 |
| Statin | 636 (96.4) | 323 (97.6) | 313 (95.1) | 1,524 (98.1) | 766 (98.0) | 758 (98.2) | 0.017 |
| Oral diuretics | 132 (20.0) | 57 (17.2) | 75 (22.8) | 129 (8.3) | 63 (8.1) | 66 (8.5) | <0.001 |
| SGLT2 inhibitors | 42 (6.4) | 20 (6.0) | 22 (6.7) | 172 (11.1) | 87 (11.1) | 85 (11.0) | <0.001 |
| Ivabradine | 8 (1.2) | 4 (1.2) | 4 (1.2) | 4 (0.3) | 2 (0.3) | 2 (0.3) | 0.009 |
| NSAIDs | 17 (2.6) | 6 (1.8) | 11 (3.3) | 68 (4.4) | 40 (5.1) | 28 (3.6) | 0.044 |
| Biological markers | |||||||
| Haemoglobin A1c, % | 6.4±1.6 | 6.4±1.6 | 6.4±1.6 | 6.3±1.5 | 6.2±1.4 | 6.3±1.7 | 0.13 |
| LDL cholesterol, mmol/L | 3.1±1.2 | 3.1±1.1 | 3.0±1.3 | 3.1±1.2 | 3.1±1.2 | 3.1±1.2 | 0.57 |
| Peak creatinine, µmol/L | 86.0±25.3 | 84.0±22.8 | 88.1±27.5 | 85.1±30.2 | 85.1±34.2 | 85.1±25.4 | 0.47 |
| Data are given as mean±SD, n (%), or n/N (%). †p-value for LVEF <45% versus LVEF ≥45%. Baseline clinical and demographic characteristics of patients are stratified by LVEF <45% vs ≥45%. Patients with LVEF <45% had higher rates of prior MI, heart failure, Killip class ≥2 and use of oral diuretics. ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ASA: acetylsalicylic acid; LDL: low-density lipoprotein; LVEF: left ventricular ejection function; MI: myocardial infarction; NSAID: non-steroidal anti-inflammatory drug; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; SGLT2: sodium-glucose cotransporter-2 | |||||||
Table 2. Procedural characteristics according to LVEF.
| LVEF <45% | LVEF ≥45% | p-value† | |||||
|---|---|---|---|---|---|---|---|
| Total (N=660) | Complete (N=331) | Culprit-only (N=329) | Total (N=1,554) | Complete (N=782) | Culprit-only (N=772) | ||
| Index procedure for STEMI | |||||||
| Primary PCI | 603 (91.4) | 298 (90.0) | 305 (92.7) | 1,457 (93.8) | 731 (93.5) | 726 (94.0) | 0.043 |
| Pharmacoinvasive PCI | 21 (3.2) | 12 (3.6) | 9 (2.7) | 37 (2.4) | 18 (2.3) | 19 (2.5) | 0.28 |
| Rescue PCI | 36 (5.5) | 21 (6.3) | 15 (4.6) | 60 (3.9) | 33 (4.2) | 27 (3.5) | 0.09 |
| Radial access | 538 (81.5) | 276 (83.4) | 262 (79.6) | 1,218 (78.4) | 619 (79.2) | 599 (77.6) | 0.1 |
| Thrombus aspiration | 142/586 (24.2) | 70/294 (23.8) | 72/292 (24.7) | 338/1,406 (24.0) | 162/705 (23.0) | 176/701 (25.1) | 0.93 |
| SYNTAX score (core lab) | |||||||
| STEMI culprit lesion-specific score | 9.0 (7.0-15.0) | 9.0 (6.5-14.5) | 10.0 (7.0-15.5) | 7.0 (4.0-10.0) | 7.0 (4.0-10.0) | 7.0 (4.0-9.5) | <0.001 |
| Non-culprit lesion-specific score | 3.5 (2.0-5.0) | 3.7 (2.0-5.5) | 3.0 (2.0-5.0) | 4.3 (2.5-6.0) | 4.0 (2.5-6.0) | 4.5 (2.5-6.0) | <0.001 |
| Baseline (including STEMI culprit) | 18.1±7.4 | 17.9±7.4 | 18.3±7.3 | 15.3±6.4 | 15.6±6.5 | 15.1±6.3 | <0.001 |
| Residual (after index PCI) | 5.0 (3.0-9.0) | 6.0 (3.0-9.0) | 5.0 (3.0-9.0) | 6.0 (3.0-10.0) | 6.0 (3.0-10.0) | 6.0 (3.0-9.0) | 0.004 |
| Culprit lesion location (core lab) | |||||||
| Left main | 3/646 (0.5) | 1/323 (0.3) | 2/323 (0.6) | 2/1,511 (0.1) | 1/756 (0.1) | 1/755 (0.1) | 0.16 |
| LAD | 383/646 (59.3) | 187/323 (57.9) | 196/323 (60.7) | 371/1,511 (24.6) | 189/756 (25.0) | 182/755 (24.1) | <0.001 |
| LCx | 100/646 (15.5) | 56/323 (17.3) | 44/323 (13.6) | 293/1,511 (19.4) | 160/756 (21.2) | 133/755 (17.6) | 0.031 |
| RCA | 160/646 (24.8) | 79/323 (24.5) | 81/323 (25.1) | 845/1,511 (55.9) | 406/756 (53.7) | 439/755 (58.1) | <0.001 |
| Number of residual diseased vessels (core lab) | |||||||
| 1 | 490/645 (76.0) | 240/323 (74.3) | 250/322 (77.6) | 1,155/1,509 (76.5) | 574/756 (75.9) | 581/753 (77.2) | 0.77 |
| ≥2 | 155/645 (24.0) | 83/323 (25.7) | 72/322 (22.4) | 354/1,509 (23.5) | 182/756 (24.1) | 172/753 (22.8) | |
| Non-culprit lesion location (core lab) | |||||||
| Left main | 1/902 (0.1) | 1/460 (0.2) | 0/442 (0) | 4/2,092 (0.2) | 3/1,081 (0.3) | 1/1,011 (0.1) | 0.88 |
| LAD | 221/902 (24.5) | 113/460 (24.6) | 108/442 (24.4) | 923/2,092 (44.1) | 462/1,081 (42.7) | 461/1,011 (45.6) | <0.001 |
| Proximal LAD | 53/902 (5.9) | 33/460 (7.2) | 20/442 (4.5) | 238/2,092 (11.4) | 119/1,081 (11.0) | 119/1,011 (11.8) | <0.001 |
| Mid LAD | 129/902 (14.3) | 60/460 (13.0) | 69/442 (15.6) | 527/2,092 (25.2) | 263/1,081 (24.3) | 264/1,011 (26.1) | <0.001 |
| LCx | 346/902 (38.4) | 172/460 (37.4) | 174/442 (39.4) | 750/2,092 (35.9) | 388/1,081 (35.9) | 362/1,011 (35.8) | 0.19 |
| Proximal LCx or OM/Ramus | 272/902 (30.2) | 133/460 (28.9) | 139/442 (31.4) | 561/2,092 (26.8) | 297/1,081 (27.5) | 264/1,011 (26.1) | 0.06 |
| Distal LCx or PLB | 74/902 (8.2) | 39/460 (8.5) | 35/442 (7.9) | 189/2,092 (9.0) | 91/1,081 (8.4) | 98/1,011 (9.7) | 0.89 |
| RCA | 334/902 (37.0) | 174/460 (37.8) | 160/442 (36.2) | 415/2,092 (19.8) | 228/1,081 (21.1) | 187/1,011 (18.5) | <0.001 |
| Non-culprit lesion reference diameter, mm | 2.8±0.6 | 2.8±0.5 | 2.9±0.6 | 2.8±0.5 | 2.7±0.5 | 2.9±0.5 | 0.036 |
| Non-culprit lesion diameter stenosis (core lab), % | 65.8±19.8 | 64.5±11.1 | 67.1±25.8 | 64.7±10.4 | 64.9±10.7 | 64.5±9.9 | 0.08 |
| Non-culprit lesion diameter stenosis (visual), % | 79.5±8.7 | 79.2±8.5 | 79.7±9.0 | 78.8±7.7 | 79.1±8.1 | 78.5±7.2 | 0.06 |
| Non-culprit lesion diameter stenosis (visual), % | 0.039 | ||||||
| 50-69% | 5/844 (0.6) | 3/426 (0.7) | 2/418 (0.5) | 16/1,987 (0.8) | 8/1,016 (0.8) | 8/971 (0.8) | |
| 70-79% | 340/844 (40.3) | 175/426 (41.1) | 165/418 (39.5) | 849/1,987 (42.7) | 423/1,016 (41.6) | 426/971 (43.9) | |
| 80-89% | 291/844 (34.5) | 145/426 (34.0) | 146/418 (34.9) | 693/1,987 (34.9) | 356/1,016 (35.0) | 337/971 (34.7) | |
| 90-99% | 178/844 (21.1) | 88/426 (20.7) | 90/418 (21.5) | 403/1,987 (20.3) | 210/1,016 (20.7) | 193/971 (19.9) | |
| 100% | 30/844 (3.6) | 15/426 (3.5) | 15/418 (3.6) | 26/1,987 (1.3) | 19/1,016 (1.9) | 7/971 (0.7) | |
| Index PCI | 0.005 | ||||||
| Success | 1,015/1,122 (90.5) | 517/558 (92.7) | 498/564 (88.3) | 2,402/2,602 (92.3) | 1,218/1,324 (92.0) | 1,184/1,278 (92.6) | |
| Partial success | 97/1,122 (8.6) | 41/558 (7.3) | 56/564 (9.9) | 195/2,602 (7.5) | 104/1,324 (7.9) | 91/1,278 (7.1) | |
| Failure | 10/1,122 (0.9) | 0/558 (0) | 10/564 (1.8) | 5/2,602 (0.2) | 2/1,324 (0.2) | 3/1,278 (0.2) | |
| Non-culprit PCI | 0.27 | ||||||
| Success | 386/401 (96.3) | 386/401 (96.3) | - | 933/954 (97.8) | 933/954 (97.8) | - | |
| Partial success | 11/401 (2.7) | 11/401 (2.7) | - | 16/954 (1.7) | 16/954 (1.7) | - | |
| Failure | 4/401 (1.0) | 4/401 (1.0) | - | 5/954 (0.5) | 5/954 (0.5) | - | |
| IABP | 9 (0.8) | 3 (0.5) | 6 (1.1) | 9 (0.3) | 5 (0.4) | 4 (0.3) | 0.06 |
| TIMI flow grade before PCI | 0.004 | ||||||
| 0 | 435/660 (65.9) | 214 (64.7) | 221 (67.2) | 957/1,551 (61.7) | 480/779 (61.6) | 477/772 (61.8) | |
| 1 | 63/660 (9.5) | 28 (8.5) | 35 (10.6) | 115/1,551 (7.4) | 62/779 (8.0) | 53/772 (6.9) | |
| 2 | 88/660 (13.3) | 44 (13.3) | 44 (13.4) | 221/1,551 (14.2) | 98/779 (12.6) | 123/772 (15.9) | |
| 3 | 74/660 (11.2) | 45 (13.6) | 29 (8.8) | 258/1,551 (16.6) | 139/779 (17.8) | 119/772 (15.4) | |
| TIMI flow grade after PCI | 0.09 | ||||||
| 0 | 1 (0.2) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | |
| 1 | 1 (0.2) | 0 (0) | 1 (0.3) | 2 (0.1) | 0 (0) | 2 (0.3) | |
| 2 | 23 (3.5) | 11 (3.3) | 12 (3.6) | 34 (2.2) | 25 (3.2) | 9 (1.2) | |
| 3 | 635 (96.2) | 320 (96.7) | 315 (95.7) | 1,518 (97.7) | 757 (96.8) | 761 (98.6) | |
| Intended timing of complete revascularisation | 0.24 | ||||||
| Index hospitalisation | 434 (65.8) | 226 (68.3) | 208 (63.2) | 1,062 (68.3) | 532 (68.0) | 530 (68.7) | |
| After discharge | 226 (34.2) | 105 (31.7) | 121 (36.8) | 492 (31.7) | 250 (32.0) | 242 (31.3) | |
| Data are given as mean±SD, n (%), n/N (%), or median (IQR).†p-value for LVEF <45% versus LVEF ≥45%. This table summarises the procedural details stratified according to LVEF category (<45% vs ≥45%). IABP: intra-aortic balloon pump; IQR: interquartile range; LAD: left anterior descending artery; LCx: left circumflex artery; LVEF: left ventricular ejection function; OM: obtuse marginal artery; PCI: percutaneous coronary intervention; PLB: posterolateral branch; RCA: right coronary artery; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction | |||||||
Clinical outcomes
The incidence of the first co-primary outcome of CV death or new MI was higher (4.2%/year vs 2.8%/year; p=0.003) in patients with LVEF <45% than in those with LVEF ≥45% (Central illustration). A similar trend was observed for the second co-primary outcome of CV death, new MI or IDR (5.3%/year vs 4.4%/year; p=0.09). There was also a higher incidence of the key secondary outcomes of cardiovascular death, MI, IDR, unstable angina, or NYHA Class IV heart failure in patients with LVEF <45% than in patients with LVEF ≥45% (7.9%/year vs 5.8%/year; p=0.003). The incidences of CV death (1.6%/year vs 0.7%/year; p<0.001), NYHA Class IV (2.1%/year vs 0.4%/year; p<0.001) and rehospitalisation due to heart failure (1.3%/year vs 0.2%/year; p<0.001) were also higher in patients with LVEF <45% (Figure 1). Among patients with LVEF <45%, the incidence of the first co-primary outcome of CV death or new MI was 3%/ year in the complete revascularisation group versus 5.5%/ year in the culprit lesion-only PCI group (HR 0.55, 95% CI: 0.36-0.86). Of those with LVEF ≥45%, the composite of CV death or new MI occurred at a rate of 2.4%/year in the complete revascularisation group versus 3.2%/year in the culprit lesion-only PCI group (HR 0.74, 95% CI: 0.52-1.04; interaction p=0.31). The second co-primary outcome of CV death, new MI, or IDR occurred at 3.5%/year in the complete revascularisation group versus 7.3%/year in the culprit lesion-only PCI group in patients with LVEF <45% (HR 0.49, 95% CI: 0.33-0.74) and at 2.7%/year in the complete revascularisation group versus 6.3%/year in the culprit lesion-only PCI group in patients with LVEF ≥45% (HR 0.44, 95% CI: 0.33-0.60; interaction p=0.67) (Figure 2). Further analysis of baseline LVEF demonstrated a consistent benefit of complete revascularisation for the first co-primary outcome in patients with LVEF ≤35% (HR 0.57, 95% CI: 0.31-1.04), LVEF 36-49% (HR 0.72, 95% CI: 0.47-1.12) and LVEF ≥50% (HR 0.67, 95% CI: 0.44-1.01; interaction p=0.81). A similar pattern was observed for the second co-primary outcome of CV death, MI, or IDR, but the effect was more pronounced because of the increased benefit driven by IDR (Supplementary Figure 1, Supplementary Figure 2). A cubic spline analysis was performed to assess the interaction between treatment effect and LVEF as a continuous variable (Supplementary Figure 3). The hazard ratio for both co-primary outcomes remained stable across the LVEF spectrum. The interaction p-values between treatment group and LVEF were 0.388 for CV death or MI and 0.369 for CV death, MI, or IDR, indicating no significant effect modification by LVEF. The subpopulation treatment effect pattern plot analysis demonstrated that the relative benefit of complete revascularisation remained consistent across the different subgroups, reinforcing the findings from the spline analysis (Supplementary Figure 4). 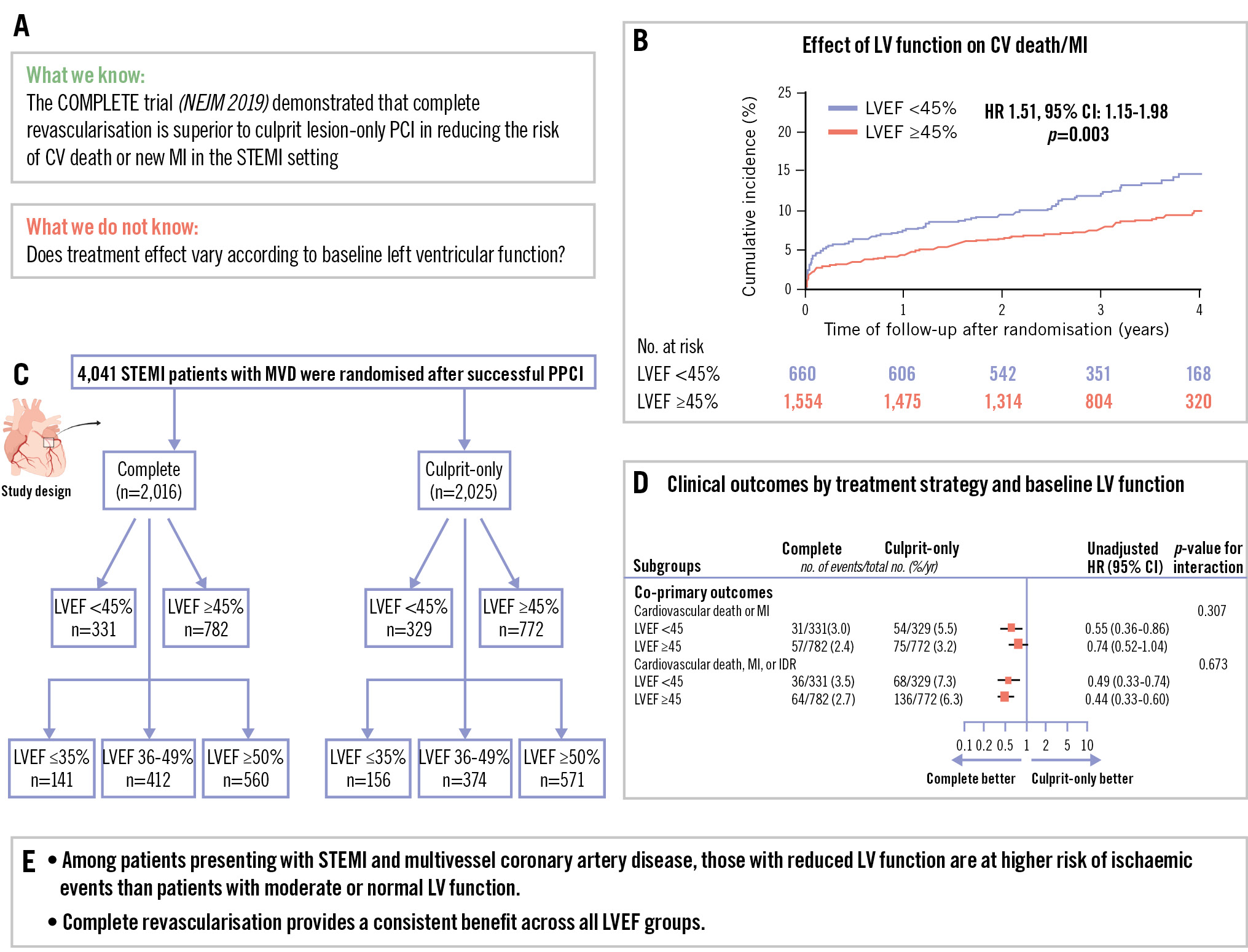
Central illustration. Complete versus culprit lesion-only PCI in STEMI: does LV function matter? A) Study rationale. B) Cumulative incidence of CV death/MI by LV function. C) Study design. D) Clinical outcomes according to treatment strategy and baseline LVEF. E) Summary of the trial’s main finding: there is a consistent benefit of complete revascularisation across LVEF categories. In patients presenting with STEMI and multivessel disease, complete revascularisation reduces major cardiovascular events, regardless of LVEF. CI: confidence interval; HR: hazard ratio; LV: left ventricular; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; PPCI: primary PCI; STEMI: ST-segment elevation myocardial infarction
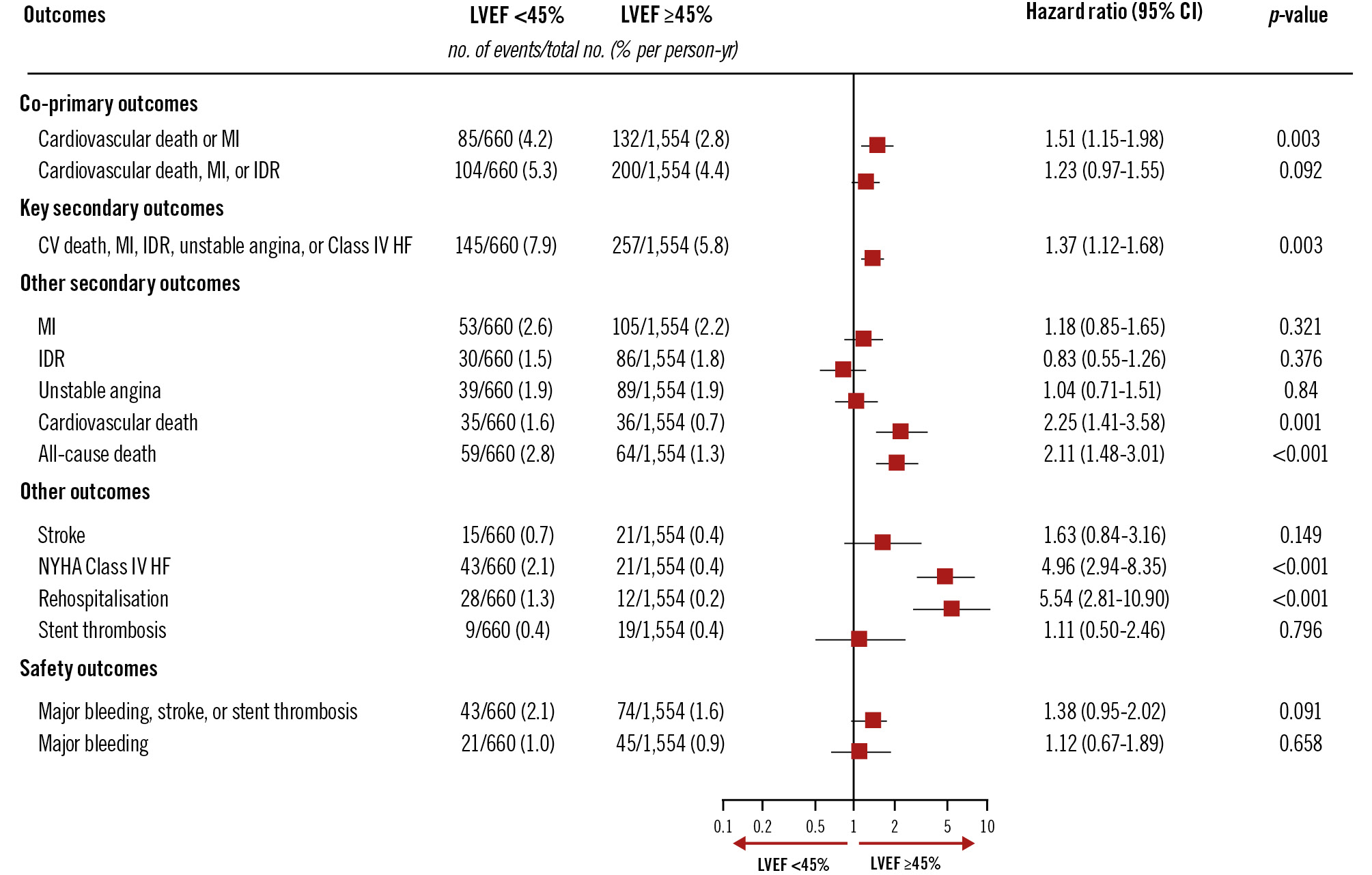
Figure 1. Analysis of the effect of LV function category on clinical outcomes. Clinical outcomes according to baseline LVEF category (LVEF <45% vs ≥45%). Patients with LVEF <45% had a higher incidence of CV death or new MI (4.2%/year vs 2.8%/year; p=0.003), as well as higher rates of NYHA Class IV heart failure and rehospitalisation. This highlights the greater risk among those with reduced LVEF. CI: confidence interval; CV: cardiovascular; HF: heart failure; IDR: ischaemia-driven revascularisation; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association
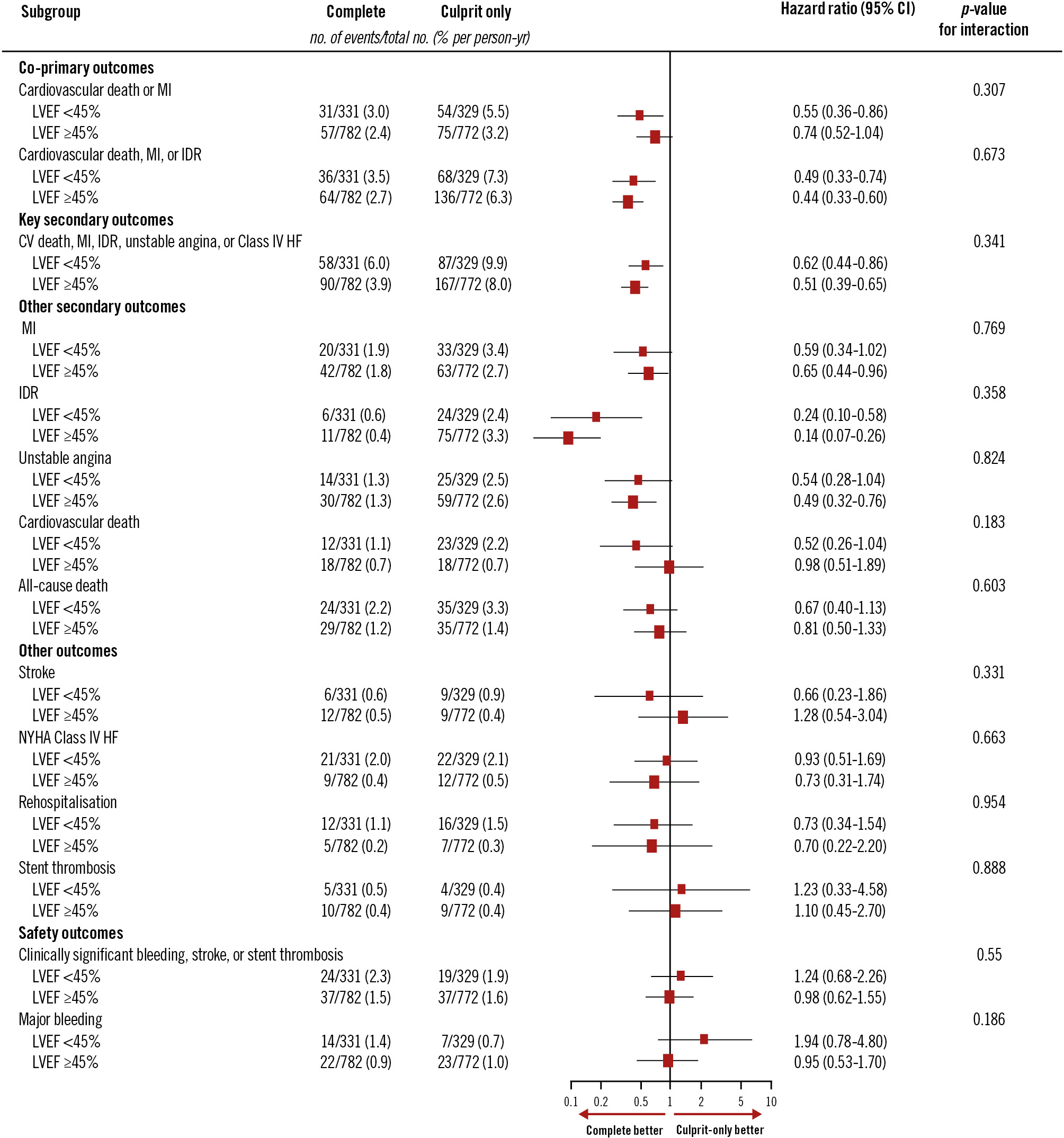
Figure 2. Subgroup analysis of clinical outcomes in left ventricular ejection fraction <45% versus ≥45%. Effect of complete versus culprit-only revascularisation on co-primary outcomes by LVEF subgroup (LVEF <45% vs ≥45%). In patients with LVEF <45%, complete revascularisation reduced the risk of CV death or MI (3.0%/year vs 5.5%/year; HR 0.55 [95% CI: 0.36-0.86]). In those with LVEF ≥45%, event rates were 2.4%/year versus 3.2%/year; HR 0.74 (95% CI: 0.52-1.04); interaction p=0.31. Results were consistent for the second co-primary outcome. CI: confidence interval; CV: cardiovascular; HF: heart failure; HR: hazard ratio; IDR: ischaemia-driven revascularisation; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association
Discussion
The COMPLETE trial is the largest randomised trial comparing complete versus culprit lesion-only revascularisation in patients presenting with STEMI and multivessel coronary artery disease. It demonstrated superiority of complete revascularisation in reducing the composite of CV death or new MI. There is, however, a paucity of data on the effect of complete revascularisation in patients with left ventricular dysfunction. Here, we present the trial results according to baseline LVEF. We found that in patients with STEMI and multivessel disease, mortality and major cardiovascular events are substantially higher in patients with LVEF <45% compared with LVEF ≥45%. However, regardless of LVEF, complete revascularisation consistently reduced the incidence of both co-primary outcomes compared with a culprit lesion-only revascularisation strategy, with no evidence of a differential treatment effect in the two cohorts (Figure 2). In sensitivity analyses, these results were consistent when LVEF was further divided into categories of LVEF ≤35%, LVEF 36-49% and LVEF ≥50% (Supplementary Figure 1). Halkin et al previously showed that 30-day and 1-year survival rates of patients whose LVEF was <40% were significantly lower than in those whose LVEF was ≥40%, and that the effect of baseline LV dysfunction on mortality was predominantly seen in the first 3 months after acute MI6. We also observed a notably steeper increase in adverse events early in the trial among participants with LVEF <45% in whom complete revascularisation was not achieved (Figure 3) and this was predominantly seen in those with LVEF ≤35% (Supplementary Figure 2). Because patients with LVEF <45% had a higher risk of cardiovascular events, the absolute benefit of complete revascularisation was higher in these patients. There was an absolute risk reduction of 2.5% in those who had complete revascularisation, with a number needed to treat of 40 for reducing the risk of CV death or MI. COMPLETE differed from the CULPRIT-SHOCK trial7, which demonstrated a reduction in all-cause death at 30 days with culprit lesion-only PCI. CULPRIT-SHOCK randomised patients with cardiogenic shock to culprit vessel-only PCI or immediate multivessel PCI. Direct comparisons on the effects of complete revascularisation are therefore not possible given the different patient populations. With an early mortality rate of 40% at 30 days, this limited the ability of CULPRIT-SHOCK to show longer-term effects. In the COMPLETE trial, we found that the benefit of complete revascularisation emerged over several years8. A significant observation from a subgroup analysis of the CULPRIT-SHOCK trial9 revealed that only a quarter of the participants from the multivessel PCI arm truly achieved complete revascularisation, compared with >90% in the COMPLETE trial. Three prior randomised trials that demonstrated benefits of complete over culprit vessel-only revascularisation included cardiac magnetic resonance (CMR) substudies101112. Patients had a baseline LVEF of >45% and an overall improvement in LVEF was observed, although there was no significant difference in LV function between both groups at follow-up. This suggests that the benefits of complete revascularisation are driven by mechanisms beyond simply improving LVEF. In the REVIVED-BCIS2 trial, patients with ischaemic cardiomyopathy were enrolled, and PCI did not result in a reduction of death from any cause or hospitalisation for heart failure over 2 years compared with optimal medical therapy alone13. These findings are consistent with other trials on stable coronary artery disease, such as the ISCHEMIA trial14, which demonstrated that an invasive strategy did not reduce the risk of cardiac events or death compared to conservative management in patients with chronic coronary disease and evidence of at least moderate ischaemia.
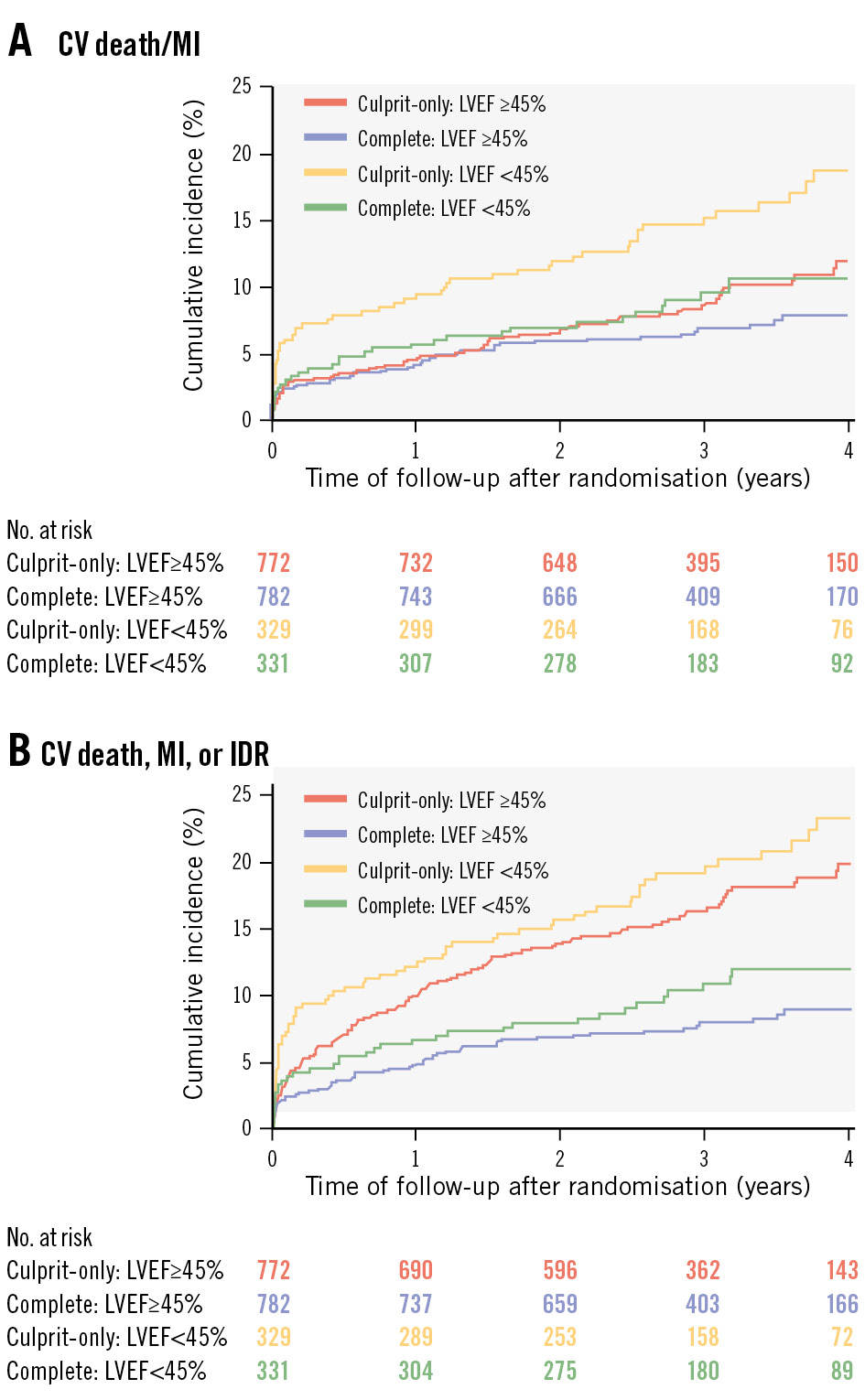
Figure 3. Cumulative incidence of the co-primary outcomes for left ventricular ejection fraction <45% versus ≥45% in the complete and culprit-only revascularisation groups. Kaplan-Meier curves of the co-primary outcomes by LVEF category (LVEF <45% vs ≥45%): (A) CV death or MI and (B) CV death, MI, or IDR. Patients with LVEF <45% showed higher event rates for both outcomes, but complete revascularisation consistently lowered the risk across both groups. CV: cardiovascular; IDR: ischaemia-driven revascularisation; LVEF: left ventricular ejection fraction; MI: myocardial infarction
Limitations
This study has some limitations. First, baseline LV function data was only available in 2,214 of the 4,041 patients who were recruited for the main trial. During data collection, LV function could be reported either qualitatively (as mild, moderate or severe) or quantitatively. For the purpose of this analysis, only patients with numerical LVEF values were included. Despite this limitation, our study represents the largest analysis to date of complete versus culprit lesion-only revascularisation in patients with reduced LVEF. We performed sensitivity analyses and did not find differences in baseline characteristics or clinical outcomes between patients with and without baseline LV function data (Supplementary Table 1). Second, although prespecified, this is a subgroup analysis of the COMPLETE trial and is therefore not powered to determine a definite cause and effect. Third, while unadjusted analyses are presented in the main paper, we acknowledge the potential for confounders. Adjusted analyses accounting for key variables were performed, which demonstrated similar results and therefore support the robustness of our findings (Supplementary Figure 5-Supplementary Figure 6-Supplementary Figure 7).
Conclusions
This prespecified analysis of the COMPLETE trial provides further insight into the effects of complete versus culprit-only revascularisation stratified according to baseline LV function. It showed that patients with STEMI and multivessel coronary artery disease who present with reduced LV function have a substantially higher risk of mortality and major cardiovascular events. As a result of the inherently higher baseline risks, those with reduced LVEF exhibited a numerically greater absolute risk reduction. More importantly, it demonstrated that the relative benefit of complete revascularisation is consistent across all LVEF subgroups, with no significant heterogeneity in the treatment effect.
Impact on daily practice
In patients with ST-segment elevation myocardial infarction and multivessel disease, those with reduced left ventricular ejection fraction (LVEF) are at higher risk of ischaemic events. Our study demonstrates that the benefit of complete revascularisation remains consistent across all LVEF groups. This reinforces the importance of complete revascularisation as the standard of care regardless of left ventricular function at baseline.
Conflict of interest statement
D.A. Wood declares research grants from Edwards Lifesciences, Abbott, and Boston Scientific. R. Mehran discloses institutional research grants from Abbott, Alleviant Medical, Beth Israel Deaconess Medical Center, Concept Medical, CPC Clinical Research, Cordis, Elixir Medical, Faraday Pharmaceuticals, Idorsia Pharmaceuticals, Janssen, MedAlliance, Mediasphere Medical, Medtronic, Novartis, Protembis GmbH, RM Global BioAccess Fund Management, and Sanofi US Services, Inc.; received personal consulting fees from Elixir Medical, IQVIA, Medtronic, Medscape/WebMD Global, and Novo Nordisk; received honoraria from American College of Cardiology (ACC) Board of Trustees, StC member, AMA (JAMA Associate Editor); holds the following leadership or fiduciary roles: member of the scientific advisory board and JAMA Cardiology Associate Editor for American Medical Association, Board of Trustees Member, Scientific Committee Member, CTR Program for American College of Cardiology; Women in Innovations Committee Member (no fees) for Society for Cardiovascular Angiography & Interventions; holds stocks in Elixir Medical, Stel, and ControlRad (spouse); serves as faculty in Cardiovascular Research Foundation (no fees); and founding director of Women as One (no fees). R.F. Storey reports institutional research grants from AstraZeneca and Cytosorbents; received consulting fees from Abbott, Alfasigma, AstraZeneca, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb/Johnson & Johnson, Pfizer, Daiichi Sankyo, Chiesi, Cytosorbents, Idorsia, Novartis, Novo Nordisk, and PhaseBio; received payment or honoraria from AstraZeneca, Pfizer, and Tabuk; and declares participation on a Data Safety Monitoring Board or Advisory Board for Afortiori Development/Thrombolytic Science (personal fees). R. Moreno declares consulting fees for Abbott, Boston Scientific, and Medtronic; received payment or honoraria from Abbott, Boston Scientific, Medtronic, Biosensors, Daiichi Sankyo, AstraZeneca, Bayer, Meril Life Sciences, Biotronik, Terumo, and Shockwave Medical. S. Rao declares participation on a Data Safety Monitoring Board or Advisory Board for FORWARD CAD and SPYRAL GEMINI. R. Welsh discloses consulting fees from GSK and AstraZeneca; received payment or honoraria from Bayer, Novartis, AstraZeneca, and Edwards Lifesciences. K.R. Bainey declares consulting fees from Novartis. E.A. Cohen declares consulting fees from Medtronic; and participation on a Data Safety Monitoring Board for COMPLETE-2. M.B. Tsang reports grants or contracts from Abiomed as a participant site for DTU STEMI; received reimbursements for meetings/travel from Abiomed and Penumbra; and received Impella devices from Abiomed for DTU STEMI. M. Sibbald declares support for the present manuscript from Abbott; and received payment or honoraria from Abbott. J.A. Cairns received support for the present manuscript from Boston Scientific, and AstraZeneca (McMaster University); received grants from Edwards Lifesciences (University of British Columbia); and declares participation on a Data Safety Monitoring Board or Advisory Board for the following trials: ARTESiA, ATLAS-AF, LAAOS-4, and OCEAN-AF. S.R. Mehta declares grants from Abbott (COMPLETE-2), Janssen (LIBREXIA-ACS), and Amgen (OCEAN-[a]); received consulting fees from Amgen, Novo Nordisk, Novartis, and Diapin; and declares participation on a Data Safety Monitoring Board or Advisory Board for CORALreef Programme (Merck). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

