Abstract
BACKGROUND: Complete revascularisation is supported by recent trials in patients with ST-elevation myocardial infarction (STEMI) and multivessel disease (MVD) without cardiogenic shock. However, the optimal timing of non-culprit lesion revascularisation is currently debated.
AIMS: This prespecified analysis of the BioVasc trial aims to determine the effect of immediate complete revascularisation (ICR) compared to staged complete revascularisation (SCR) on clinical outcomes in patients with STEMI.
METHODS: Patients presenting with STEMI and MVD were randomly assigned to ICR or SCR. The primary endpoint was the composite of all-cause mortality, myocardial infarction, any unplanned ischaemia-driven revascularisation, or cerebrovascular events at 1-year post-index procedure.
RESULTS: Between June 2018 and October 2021, 608 (ICR: 305, SCR: 303) STEMI patients were enrolled. No significant differences between ICR and SCR were observed at 1-year follow-up in terms of the primary endpoint (7.0% vs 8.3%, hazard ratio [HR] 0.84, 95% confidence interval [CI]: 0.47-1.50; p=0.55): all-cause mortality (2.3% vs 1.3%, HR 1.77, 95% CI: 0.52-6.04; p=0.36), myocardial infarction (1.7% vs 3.3%, HR 0.50, 95% CI: 0.17-1.47; p=0.21), unplanned ischaemia-driven revascularisation (4.1% vs 5.0%, HR 0.80, 95% CI: 0.38-1.71; p=0.57) and cerebrovascular events (1.4% vs 1.3%, HR 1.01, 95% CI: 0.25-4.03; p=0.99). At 30-day follow-up, a trend towards a reduction of the primary endpoint in the ICR group was observed (ICR: 3.0% vs SCR: 6.0%, HR 0.50, 95% CI: 0.22-1.11; p=0.09). ICR was associated with a reduction in overall hospital stay (ICR: median 3 [interquartile range {IQR} 2-5] days vs SCR: median 4 [IQR 3-6] days; p<0.001).
CONCLUSIONS: Clinical outcomes at 1 year were similar for STEMI patients who had undergone ICR and those who had undergone SCR.
Multivessel coronary artery disease is observed in up to 40% of patients with ST-elevation myocardial infarction (STEMI) undergoing primary percuÂtaneous coronary intervention (PCI)12. Although primary PCI of the culprit lesion is the established standard of care to restore blood flow in the infarct-related artery, the timing for treatment of non-culprit lesions in patients with STEMI without cardiogenic shock is still a matter of debate.
The current European guidelines recommend complete revascularisation either during the index PCI procedure or within 45 days in patients presenting with STEMI and multivessel disease (MVD) without cardiogenic shock (Class of Recommendation I, Level of Evidence A)3.
The COMPLETE trial demonstrated a reduction in cardiovascular death and myocardial infarction when a staged complete revascularisation (SCR) strategy was adopted compared with a culprit lesion-only PCI approach4. The benefit of the SCR strategy was observed regardless of the timing of the staged procedure, either during the index hospitalisation or after hospital discharge, within 45 days from the randomisation4. However, immediate complete revascularisation (ICR) was not evaluated, and the timing for the SCR procedure was based on clinical judgement.
The recently published MULTISTARS AMI Trial demonstrated that ICR was non-inferior to SCR in a selected population of STEMI patients with respect to the risk of death from any cause, non-fatal myocardial infarction, stroke, unplanned ischaemia-driven revascularisation, or hospitalisation for heart failure at 1 year; any differences were mostly driven by non-fatal myocardial infarction and early unplanned ischaemia-driven revascularisation5.
Similarly, the BioVasc trial demonstrated the non-inferiority of ICR compared with SCR in patients presenting with acute coronary syndrome (ACS) with or without ST-segment elevation. In addition, ICR was associated with a risk reduction in terms of myocardial infarction and unplanned ischaemia-driven revascularisation6.
The purpose of this prespecified analysis of the randomised BioVasc trial was to provide further insights into the STEMI subpopulation and to determine the effect of ICR compared to SCR on clinical outcomes in patients with STEMI and MVD.
Methods
STUDY DESIGN
This is a prespecified subanalysis of the BioVasc trial (Percutaneous Complete Revascularization Strategies Using Sirolimus Eluting Biodegradable Polymer Coated Stents in Patients Presenting With Acute Coronary Syndrome and Multivessel Disease)6.
The study design of the BioVasc trial has been previously published7. In brief, the BioVasc study is an investigator-initiated, prospective, open-label, randomised, multicentre, non-inferiority trial that enrolled 1,525 patients presenting with ACS and MVD. Only patients with ACS and a clear culprit lesion were eligible for randomisation8. Inclusion and exclusion criteria have been previously described7.
All 608 patients admitted with STEMI and enrolled in the BioVasc study were included in the present analysis. All patients were randomised in a 1:1 ratio to ICR or SCR, consisting of immediate culprit lesion treatment and PCI of all significant non-culprit lesions either during the index hospitalisation or through an elective readmission within 6 weeks after the index procedure.
Although the timing of the staged procedure was left to the operator’s discretion, per protocol, it needed to be documented at the end of the index procedure.
A non-culprit lesion with at least 70% stenosis by visual estimation or positive coronary physiology testing in a coronary vessel ≥2.5 mm was defined as significant. The use of intravascular imaging was left to the operator’s discretion.
A patient was considered completely revascularised if all significant lesions with a vessel diameter of 2.5 mm or more were treated, and they showed a final Thrombolysis in Myocardial Infarction grade 3 flow. One patient in the staged complete revascularisation group withdrew consent before the staged procedure, so completeness of the revascularisation could not be ascertained.
Optimal medical therapy according to current guidelines was recommended to all the patients.
Clinical follow-up was performed at 30 days and 12 months after the index PCI; telemedicine follow-up was allowed during the COVID-19 pandemic.
The ethics committee of each participating centre reviewed and approved the study protocol. All the enrolled patients provided written informed consent. The trial was conducted according to the principles outlined in the Declaration of Helsinki.
ENDPOINTS AND DEFINITIONS
The primary endpoint was defined as the composite of all-cause mortality, myocardial infarction, any unplanned ischaemia-driven revascularisation, or cerebrovascular events at 1 year post-index procedure7.
Secondary endpoints comprised the primary endpoint at 30 days, individual components of the composite primary endpoint at 30 days and at 1 year, probable or definite stent thrombosis, target vessel revascularisation, and major bleeding (Bleeding Academic Research Consortium types 3 and 5) at 30 days and 1 year7.
Myocardial infarction was defined according to the third universal definition9, with modification for the ACS setting as reported in the COMPLETE trial4.
In particular, in patients whose cardiac troponin values were already elevated or were recently elevated, new ischaemic symptoms for at least 20 minutes and evidence of unequivocally new ischaemic electrocardiogram (ECG) changes were required6.
Ischaemia-driven revascularisation was defined as follows: (1) any repeat revascularisation associated with either ischaemic symptoms, a positive non-invasive functional test, or both, and a lesion with diameter stenosis ≥50% on angiography; (2) any revascularisation of lesions with a diameter stenosis ≥70%; (3) any revascularisation of lesions with fractional flow reserve ≤0.80 or instant wave-free ratio ≤0.89; or (4) any culprit lesion of a new acute myocardial infarction with/without ST-segment elevation7.
In the SCR group, revascularisations performed before the planned date for the staged procedure were consiÂdered unplanned ischaemia-driven revascularisations only if prompted by a new acute coronary syndrome, with new ECG changes and/or new elevation of cardiac enzymes. After the staged procedure and in the ICR group, any ischaemia-driven revascularisations were considered unplanned7.
STATISTICAL ANALYSIS
Comparisons were performed in the intention-to-treat population, including all randomised patients.
The superiority of ICR versus SCR was assessed for the primary and the secondary endpoints. Categorical data are presented as counts and percentages, and comparisons were conducted with use of the chi-square or Fisher’s exact test, as appropriate. Continuous data were tested for normality with the Shapiro-Wilk test. These data are presented as means with standard deviations and were tested by the independent-samples t-test or are presented as medians with interquartile ranges (IQR) and were tested by the Mann-Whitney U test, if appropriate.
The method of Kaplan-Meier was used to estimate the cumulative incidences of the primary and the secondary endpoints during follow-up. If an endpoint had not occurred, patients were censored at the time they were last known to be alive.
Cox proportional hazard models were used to determine differences between the randomly allocated treatment groups, which were expressed as hazard ratios (HR) and corresponding 95% confidence intervals (CI). The proportionality assumption was assessed comparing the log-minus-log plots of the incidence of the primary endpoint in the randomly allocated treatment groups and indicated no suspicion of a violation. A 2-sided p-value<0.05 was considered statistically significant. All statistical analyses were performed by using R, version 4.2.1 (packages used: data.table, dplyr, ggplot2, ggpubr, graphics, lubridate, stats, survival, survminer, tidycmprsk [R Foundation for Statistical Computing]).
Results
Between June 2018 and October 2021, a total of 608 patients presenting with STEMI were enrolled in the BioVasc trial; 305 were randomised to immediate complete revascularisation and 303 to staged complete revascularisation.
In the ICR arm, 3 (0.98%) patients received an SCR per operator decision. In the SCR arm, 2 (0.66%) patients received an ICR per operator decision.
The baseline characteristics were well balanced between the 2 treatment groups (Table 1). The procedural characteristics are presented in Table 2, and the distribution of the time to the staged procedure is illustrated in Figure 1. Intravascular imaging was used in 23 (7.5%) patients in the ICR group and in 42 (13.9%) patients in the SCR group (p=0.012) (Table 2). Physiological assessment of the non-culprit lesion was performed in 41 (13.4%) patients in the ICR group and in 55 (18.2%) patients in the SCR group (p=0.11). In the SCR group, 9 (1.5%) patients underwent physiological assessment during the index procedure and 46 (7.6%) patients during the staged procedure (Table 2).
Non-culprit lesions were located in the target vessel in 94/304 (30.9%) patients in the ICR and in 92/299 (30.8%) patients in the SCR group (p=0.968). In the ICR group, 93/94 (98.9%) patients had an additional lesion in another major vessel, whereas in the SCR group, 90/92 (97.8%) patients had another diseased major vessel.
The median overall amount of contrast dye used was 200 (IQR 150-250) ml in the ICR group and 258 (IQR 200-330) ml in the SCR group (p<0.001) (Table 2). The total procedure duration was 60 (IQR 46-80) minutes in the ICR group and 88 (IQR 64-114) minutes in the SCR group (p<0.001) (Table 2). PCI procedures were performed during off-hours in 140 (45.9%) patients undergoing ICR and in 144 (47.5%) patients undergoing SCR (p=0.69).
The median length of the hospital stay was shorter in the ICR group compared with the SCR group (3 [IQR 2-5] days vs 4 [IQR 3-6] days; p<0.001) (Table 2). In the staged group, PCI was performed as an elective procedure in 202 patients (66.7%).
Table 1. Characteristics of the patients at baseline.
| Characteristics | Immediate complete revascularisation (N=305) | Staged complete revascularisation (N=303) | p-value |
|---|---|---|---|
| Age, yrs | 62.7 (56.0-57.2) | 63.3 (57.2-70.8) | 0.873 |
| Male sex | 248 (81.3) | 234 (77.2) | 0.214 |
| BMI, kg/m² | 27.3 (24.8-29.7) | 26.9 (24.6-29.4) | 0.332 |
| Medical history | |||
| Previous PCI | 22 (7.2) | 39 (9.6) | 0.020 |
| History of MI | 16 (5.2) | 24 (7.9) | 0.183 |
| Peripheral artery disease | 8/304 (2.6) | 10/303 (3.3) | 0.627 |
| Valve disease | 6/305 (2.0) | 4/302 (1.3) | 0.752 |
| Chronic obstructive pulmonary disease | 16/304 (5.3) | 12/303 (4.0) | 0.444 |
| Atrial fibrillation or flutter | 10 (3.3) | 5 (1.7) | 0.196 |
| Renal insufficiency | 9 (3.0) | 6 (2.0) | 0.440 |
| History of stroke | 12/304 (3.9) | 9/303 (3.0) | 0.510 |
| Hypertension | 137 (44.9) | 129 (42.6) | 0.560 |
| Diabetes | 51 (16.7) | 46 (15.2) | 0.604 |
| Hypercholesterolaemia | 124/305 (59.4) | 129/302 (42.6) | 0.607 |
| Family history of CVD | 87/304 (28.6) | 99/303 (32.7) | 0.665 |
| Smoking behaviour | 0.724 | ||
| Never | 145 (47.5) | 151 (49.8) | |
| Current | 110 (36.1) | 109 (36.0) | |
| Former | 50 (16.4) | 43 (14.2) | |
| Data are presented as median (IQR), n (%) or n/N (%). BMI: body mass index; CVD: cardiovascular disease; IQR: interquartile range; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||
Table 2. Procedural characteristics.
| Characteristics | Immediate complete revascularisation (N=305) | Staged complete revascularisation (N=303) | p-value |
|---|---|---|---|
| Systolic blood pressure, mmHg | 122 (108-137) | 121 (110-140) | 0.56 |
| Diastolic blood pressure, mmHg | 72 (64-84) | 72 (62-81) | 0.45 |
| Radial access | 291 (95.4) | 296 (97.7) | 0.12 |
| Location of culprit lesion | 0.43 | ||
| Left main coronary artery | 1 (0.3) | 0 (0) | |
| Left anterior descending artery | 112 (36.7) | 115 (38.0) | |
| Circumflex artery | 69 (22.6) | 56 (18.5) | |
| Right coronary artery | 123 (40.3) | 132 (43.6) | |
| No. of vessels with significant non-culprit lesions* | 0.08 | ||
| 1 | 246/299 (82.3) | 221/289 (76.5) | |
| ≥2 | 53/299 (17.7) | 68/289 (23.5) | |
| Lesion complexity§ | 0.25 | ||
| Type A | 63/648 (9.7) | 49/633 (7.7) | |
| Type B1 | 140/648 (21.6) | 163/633 (25.8) | |
| Type B2 | 137/648 (21.1) | 125/633 (19.7) | |
| Type C | 308/648 (47.5) | 296/633 (46.8) | |
| Procedure performed off-hours† | 140 (45.9) | 144 (47.5) | 0.69 |
| Complete revascularisation¶ | 292 (95.7) | 291 (96.0) | 0.85 |
| FFR/iFR | 41 (13.4) | 55 (18.2) | 0.11 |
| IVUS/OCT | 23 (7.5) | 42 (13.9) | 0.012 |
| Total hospital stay, days | 3 (2-5) | 4 (3-6) | <0.001 |
| Time to staged procedure, days | NA | 16 (3-29) | |
| No. of stents used per patient | |||
| Index procedure | 3 (2-4) | 1 (1-2) | <0.001 |
| Index+staged procedure | 3 (2-4) | 3 (2-4) | 0.12 |
| Length of stents, mm | |||
| Index procedure | 64 (48-90) | 30 (22-49) | <0.001 |
| Index+staged procedure | 64 (48-90) | 70 (48-99) | 0.21 |
| Index procedure duration, mins | 60 (46-80) | 42 (31-57) | <0.001 |
| Index+staged procedure duration, mins | 60 (46-80) | 88 (64-114) | <0.001 |
| Index procedure contrast use, mL | 200 (150-250) | 128 (100-179) | <0.001 |
| Index+staged procedure contrast use, mL | 200 (150-250) | 258 (200-330) | <0.001 |
| Index procedure total area dose, cGy∙cm2 | 4,547 (2,608-8,573) | 2,563 (1,393-4,930) | <0.001 |
| Index+staged procedure total area dose, cGy∙cm2 | 4,547 (2,608-8,573) | 5,649 (3,190-10,182) | 0.053 |
| P2Y12 inhibitor at discharge‡ | 0.65 | ||
| Ticagrelor | 198/303 (65.3) | 208/303 (68.6) | |
| Prasugrel | 73/303 (24.1) | 64/303 (21.1) | |
| Clopidogrel | 31/303 (10.6) | 31/303 (10.2) | |
| Data are median (IQR), n (%), or n/N (%). *In total, 20 patients had no significant multivessel disease when physiological assessment was performed after randomisation. §The total number of vessels with significant lesions (with vessel diameter ≥2.5 mm) was 1,329. The lesion complexity was not reported for 48 lesions (3.6%). †On-hours was defined as a procedure performed from Monday to Friday between 8 AM and 6 PM. A procedure outside this interval was considered off-hours. ¶A patient was considered completely revascularised if all significant lesions with a vessel diameter ≥2.5 mm were treated and showed a final Thrombolysis in Myocardial Infarction grade 3. ‡Two patients died before discharge, so no medication was prescribed. FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; IQR: interquartile range; IVUS: intravascular ultrasound; NA: not applicable; OCT: optical coherence tomography | |||
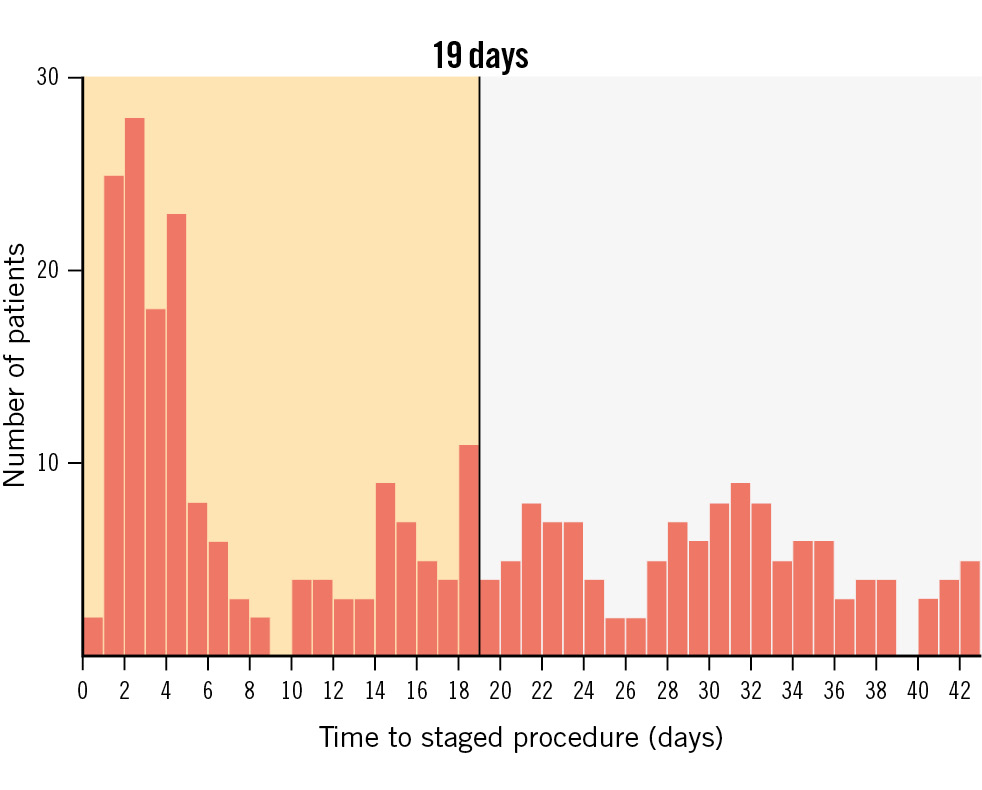
Figure 1. Distribution of the time to the staged procedure. Distribution of the time to the scheduled staged PCI. The planned staged revascularisation was completed in a substantial number of patients before the 19th day. PCI: percutaneous coronary intervention
PRIMARY AND SECONDARY OUTCOMES
In the ICR group, 4 patients were lost to follow-up, and 4 withdrew their informed consent. In the SCR group, no patients were lost to follow-up, but 2 withdrew their informed consent.
At 1-year follow-up, the primary composite outcome occurred in 21 (7.0%) patients in the ICR group and in 25 (8.3%) patients in the SCR group (HR 0.84, 95% CI: 0.47-1.50; p=0.55) (Central illustration, Table 3). No statistically significant differences were observed between the 2 groups in terms of all-cause mortality (ICR: 2.3% vs SCR: 1.3%, HR 1.77, 95% CI: 0.52-6.04; p=0.36), myocardial infarction (ICR: 1.7% vs SCR: 3.3%, HR 0.50, 95% CI: 0.17-1.47; p=0.21), unplanned ischaemia-driven revascularisation (ICR: 4.1% vs SCR: 5.0%, HR 0.80, 95% CI: 0.38-1.71; p=0.57), or cerebrovascular events (ICR: 1.4% vs SCR: 1.3%, HR 1.01, 95% CI: 0.25-4.03; p=0.99) (Figure 2). Any revascularisation was significantly lower in the ICR group compared with the SCR group (4.4% vs 8.6%, HR 0.49, 95% CI: 0.24-0.96; p=0.036) (Table 3).
At 30-day follow-up, the primary composite outcome occurred in 9 (3.0%) of the ICR group and in 18 (6.0%) of the SCR group (HR 0.50, 95% CI: 0.22-1.11; p=0.09).
No statistically significant differences between the groups were found regarding the individual components of the primary outcome at 30 days (all-cause mortality: HR 2.01, 95% CI: 0.37-10.97; p=0.42; myocardial infarction: HR 0.57, 95% CI: 0.17-1.96; p=0.38; unplanned ischaemia-driven revascularisation: HR 0.40, 95% CI: 0.12-1.27; p=0.12; cerebrovascular events: HR 0.50, 95% CI: 0.09-2.74; p=0.43).
Any revascularisation occurred in 5 (1.7%) patients in the ICR group and in 20 (6.6%) patients in the SCR group (HR 0.25, 95% CI: 0.09-0.66; p=0.005) (Table 4).
Since procedure-related myocardial infarction (myocardial infarction type 4a) could have been underdiagnosed in the ICR group, an exploratory analysis was conducted excluding all procedure-related myocardial infarctions occurring during the index or staged procedures. This analysis corroborated the main findings of the study, and the composite primary outcome at 1-year follow-up occurred in 20 (6.7%) patients in the ICR group and in 23 (7.6%) patients in the SCR group (HR 0.87, 95% CI: 0.48-1.58; p=0.65) (Table 5).
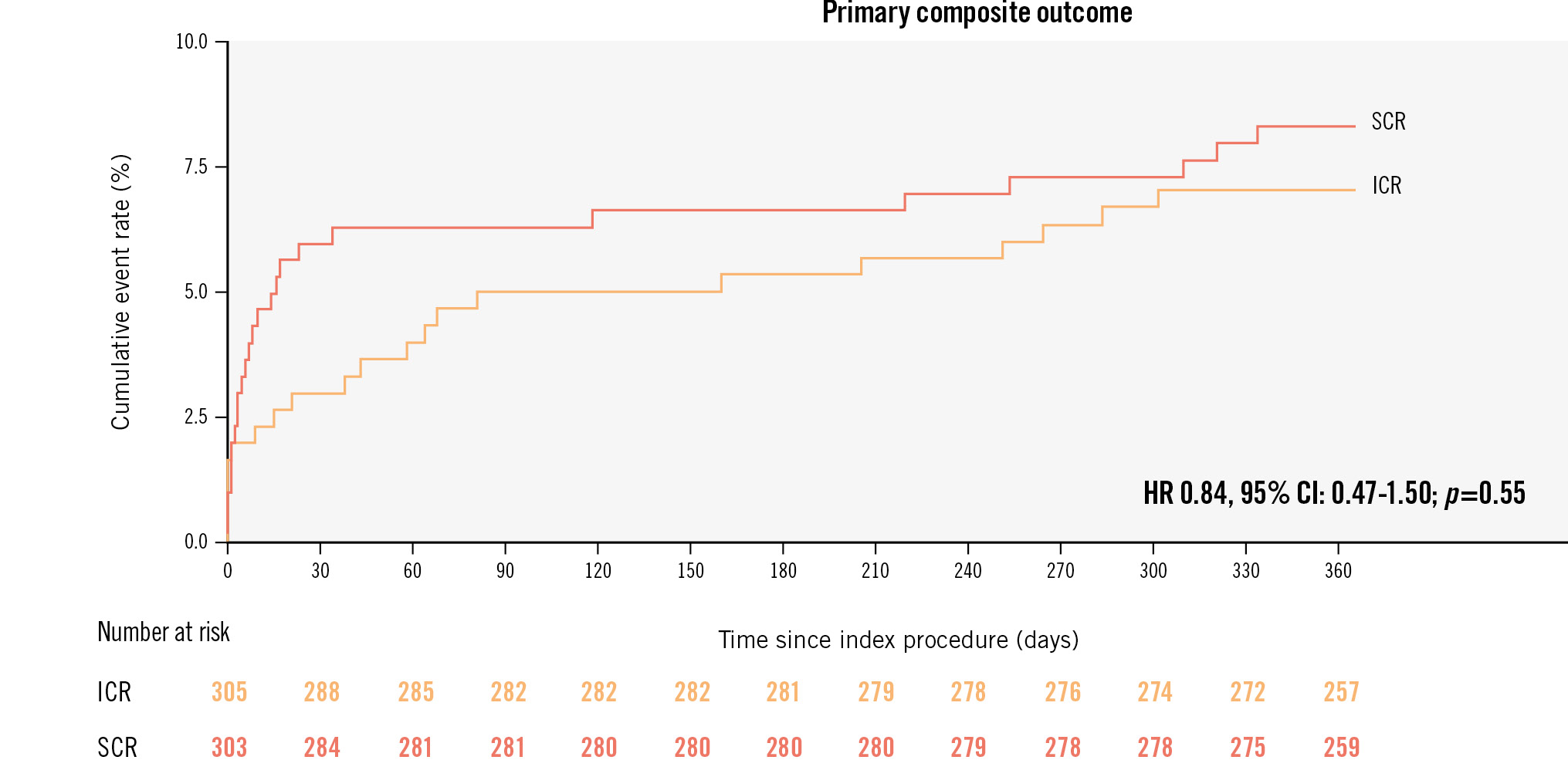
Central illustration. Primary composite outcome at 1 year. Kaplan-Meier estimates of the primary composite outcome at 1 year. The primary outcome was a composite of all-cause mortality, myocardial infarction, unplanned ischaemia-driven revascularisation, or cerebrovascular events at 1 year. CI: confidence interval; HR: hazard ratio; ICR immediate complete revascularisation; SCR: staged complete revascularisation
Table 3. Primary and secondary outcomes.
| Outcome | Immediate complete revascularisation (N=764) | Staged complete revascularisation (N=761) | Hazard ratio (95% CI) | p-value | Risk difference (95% CI)† | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of events | %* | No. of events | %* | ||||||
| Primary outcome | |||||||||
| All-cause mortality, any MI, unplanned ischaemia-driven revascularisation or cerebrovascular event | 21 | 7.0 | 25 | 8.3 | 0.84 (0.47 to 1.50) | 0.55 | 1.3 (−3.0 to 5.5) | ||
| Secondary outcomes | |||||||||
| Cardiovascular mortality or MI | 8 | 2.7 | 13 | 4.3 | 0.62 (0.26 to 1.49) | 0.29 | 1.6 (−1.3 to 4.6) | ||
| All-cause mortality | 7 | 2.3 | 4 | 1.3 | 1.77 (0.52 to 6.04) | 0.36 | −1.0 (−3.1 to 1.1) | ||
| Cardiovascular mortality | 5 | 1.7 | 3 | 1.0 | 1.68 (0.40 to 7.04) | 0.48 | −0.7 (−2.5 to 1.2) | ||
| Any MI | 5 | 1.7 | 10 | 3.3 | 0.50 (0.17 to 1.47) | 0.21 | 1.7 (−0.8 to 4.2) | ||
| Unplanned ischaemia-driven revascularisation | 12 | 4.1 | 15 | 5.0 | 0.80 (0.38 to 1.71) | 0.57 | 0.9 (−2.4 to 4.3) | ||
| Cerebrovascular event | 4 | 1.4 | 4 | 1.3 | 1.01 (0.25 to 4.03) | 0.99 | 0.0 (−1.9 to 1.8) | ||
| Probable or definite stent thrombosis | 4 | 1.3 | 3 | 1.0 | 1.33 (0.30 to 5.96) | 0.71 | −0.3 (−2.0 to 1.4) | ||
| Target vessel revascularisation | 10 | 3.4 | 13 | 4.3 | 0.77 (0.34 to 1.76) | 0.54 | 0.9 (−2.2 to 4.0) | ||
| Target lesion revascularisation | 8 | 2.7 | 12 | 4.0 | 0.67 (0.27 to 1.63) | 0.38 | 1.3 (−1.6 to 4.2) | ||
| Any revascularisation | 13 | 4.4 | 26 | 8.6 | 0.49 (0.24 to 0.96) | 0.036 | 4.2 (0.3 to 8.2) | ||
| All-cause mortality, any MI, any revascularisation or cerebrovascular event | 22 | 7.4 | 34 | 11.3 | 0.63 (0.37 to 1.08) | 0.09 | 3.9 (−0.7 to 8.6) | ||
| All-cause mortality, MI, stroke or major bleeding (BARC 3 or 5) | 19 | 6.3 | 22 | 7.3 | 0.87 (0.47 to 1.60) | 0.64 | 1.0 (−3.1 to 5.0) | ||
| Major bleeding (BARC 3 or 5) | 9 | 2.7 | 8 | 3.0 | 1.15 (0.44 to 2.97) | 0.78 | −0.4 (−3.0 to 2.3) | ||
| *Cumulative incidence at 365 days according to the Kaplan-Meier method. †Based on the Kaplan-Meier estimates. A difference in favour of immediate complete revascularisation is presented as a positive value. BARC: Bleeding Academic Research Consortium; CI: confidence interval; MI: myocardial infarction | |||||||||
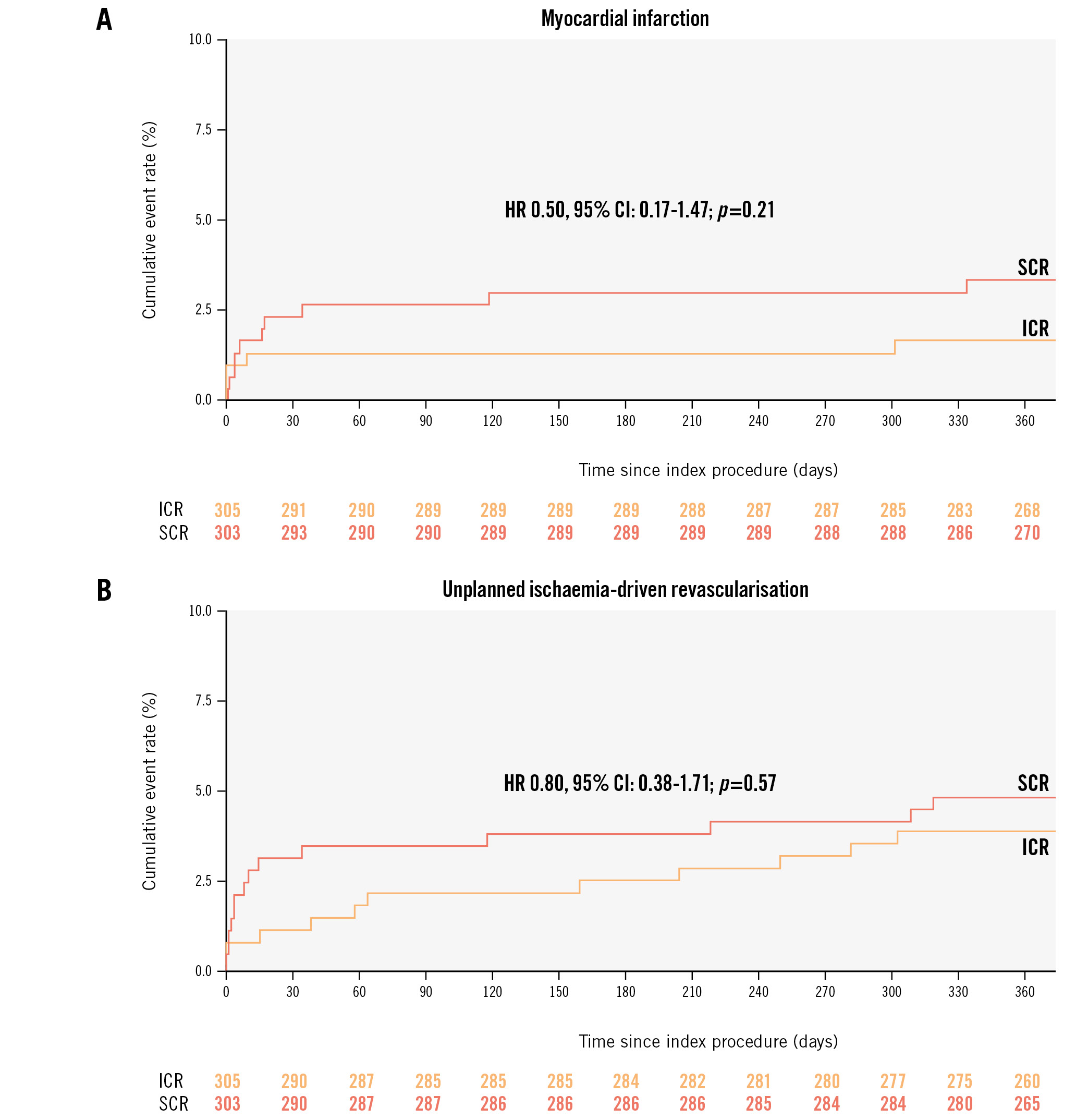
Figure 2. Myocardial infarction and unplanned ischaemia-driven revascularisation at 1 year. A) Kaplan-Meier estimates of myocardial infarction at 1 year. B) Kaplan-Meier estimates of unplanned ischaemia-driven revascularisation at 1 year. CI: confidence interval; HR: hazard ratio; ICR: immediate complete revascularisation; SCR: staged complete revascularisation
Table 4. Primary and secondary outcomes at 30 days.
| Outcome | ICR (N=305) | SCR (N=303) | Hazard ratio (95% CI) | p-value | Risk difference (95% CI)
|
||
|---|---|---|---|---|---|---|---|
| No. events | %* | No. events | %* | ||||
| Primary outcome | |||||||
| All-cause mortality, any MI, unplanned ischaemia-driven revascularisation or cerebrovascular event |
9 | 3.0 | 18 | 6.0 | 0.50 (0.22 to 1.11) | 0.09 | 3.0 (−0.3 to 6.3) |
| Secondary outcomes | |||||||
| Cardiovascular mortality or MI | 6 | 2.0 | 9 | 3.0 | 0.67 (0.24 to 1.88) | 0.45 | 1.0 (−1.5 to 3.5) |
| All-cause mortality | 4 | 1.3 | 2 | 0.7 | 2.01 (0.37 to 10.97) | 0.42 | −0.7 (−2.2 to 0.9) |
| Cardiovascular mortality | 4 | 1.3 | 2 | 0.7 | 2.01 (0.37 to 10.97) | 0.42 | −0.7 (−2.2 to 0.9) |
| Any MI | 4 | 1.3 | 7 | 2.3 | 0.57 (0.17 to 1.96) | 0.38 | 1.0 (−1.1 to 3.1) |
| Unplanned ischaemia-driven revascularisation | 4 | 1.3 | 10 | 3.3 | 0.40 (0.12 to 1.27) | 0.12 | 2.0 (−0.4 to 4.4) |
| Cerebrovascular event | 2 | 0.7 | 4 | 1.3 | 0.50 (0.09 to 2.74) | 0.43 | 0.7 (−0.9 to 2.2) |
| Probable or definite stent thrombosis | 4 | 1.3 | 3 | 1.0 | 1.33 (0.30 to 5.96) | 0.71 | −0.3(−2.0 to 1.4) |
| Target vessel revascularisation | 4 | 1.3 | 9 | 3.0 | 0.44 (0.14 to 1.44) | 0.18 | 1.7 (−0.7 to 4.0) |
| Target lesion revascularisation | 4 | 1.3 | 9 | 3.0 | 0.44 (0.14 to 1.44) | 0.18 | 1.7 (−0.7 to 4.0) |
| Any revascularisation | 5 | 1.7 | 20 | 6.6 | 0.25 (0.09 to 0.66) | 0.005 | 5.0 (1.8 to 8.1) |
| All-cause mortality, any MI, any revascularisation or cerebrovascular event | 10 | 3.3 | 28 | 9.3 | 0.35 (0.17 to 0.72) | 0.004 | 6.0 (2.1 to 9.8) |
| All-cause mortality, MI, stroke or major bleeding (BARC 3 or 5) | 11 | 3.6 | 16 | 5.3 | 0.69 (0.32 to 1.49) | 0.34 | 1.7 (−1.6 to 5.0) |
| Major bleeding (BARC 3 or 5) | 5 | 1.7 | 5 | 1.7 | 1.01 (0.29 to 3.50) | 0.98 | 0.0 (−2.1 to 2.0) |
| *Cumulative incidence at 365 days according to the Kaplan-Meier method. †Based on the Kaplan-Meier estimates. A difference in favour of immediate complete revascularisation is presented as a positive value. BARC: Bleeding Academic Research Consortium; CI: confidence interval; ICR: immediate complete revascularisation; MI: myocardial infarction; SCR: staged complete revascularisation | |||||||
Table 5. Clinical outcomes excluding periprocedural myocardial infarctions.
| Outcome | Immediate complete revascularisation (N=305) | Staged complete revascularisation (N=303) | Hazard ratio (95% CI) | p-value* | Risk difference (95% CI)‡ | ||
|---|---|---|---|---|---|---|---|
| No. events | %† | No. events | %† | ||||
| All-cause mortality, MI, unplanned ischaemia-driven revascularisation or cerebrovascular event | 20 | 6.7 | 23 | 7.6 | 0.87 (0.48 to 1.58) | 0.65 | 0.9 (−3.2 to 5.1) |
| Cardiovascular mortality or MI | 7 | 2.3 | 8 | 2.7 | 0.89 (0.32 to 2.45) | 0.82 | 0.3 (−2.2 to 2.8) |
| MI | 4 | 1.3 | 5 | 1.7 | 0.81 (0.22 to 3.01) | 0.75 | 0.3 (−1.6 to 2.3) |
| All-cause mortality, MI, any revascularisation or cerebrovascular event | 21 | 7.0 | 32 | 10.6 | 0.64 (0.37 to 1.11) | 0.11 | 3.6 (−1.0 to 8.1) |
| All-cause mortality, MI, stroke or major bleeding (BARC 3 and 5) | 18 | 6.0 | 17 | 5.6 | 1.07(0.55 to 2.08) | 0.84 | −0.4 (−4.1 to 3.4) |
| *This p-value was obtained from a test of superiority. †Cumulative incidence at 365 days according to the Kaplan-Meier method. ‡Based on the Kaplan-Meier estimates. A difference in favour of immediate complete revascularisation is presented as a positive value. BARC: Bleeding Academic Research Consortium; CI: confidence interval; MI: myocardial infarction | |||||||
Discussion
In the present analysis of the BioVasc trial, undertaken in STEMI patients with multivessel disease, the primary composite endpoint occurred numerically less often with ICR at 30 days, although at 1 year there was no difference in the endpoint rate between ICR and SCR. Secondary outcomes at 1 year, including all-cause mortality, myocardial infarction and any unplanned ischaemia-driven revascularisation, were also similar between groups.
Current European guidelines do not provide recommendations in favour of an immediate versus a staged non-infarct-related artery revascularisation in patients with STEMI, whilst a routine revascularisation of non-culprit lesions is recommended within 45 days3.
The substudy of the COMPLETE trial focused on the timing of staged procedures and suggested that the benefit of complete revascularisation over a culprit-only strategy is maintained with staged procedures both in-hospital or after discharge10. However, in this analysis the in-hospital or post-discharge approaches were not directly compared, and ICR was not performed.
The results of the MULTISTARS AMI trial showed that in patients with STEMI and multivessel disease, ICR was not inferior to SCR for the composite primary endpoint of death from any cause, non-fatal myocardial infarction, stroke, unplanned ischaemia-driven revascularisation, or hospitalisation for heart failure at 1 year. This result was mainly driven by non-fatal myocardial infarction and early unplanned ischaemia-driven revascularisation5.
In this subanalysis of the BioVasc trial, the adoption of an ICR approach in STEMI patients did not show a significant benefit compared with an SCR strategy. However, given a 50% risk reduction in the ICR group compared with the SCR group in terms of the primary endpoint at 30-day follow-up, we cannot exclude a lack of statistical power in detecting significant differences. Nonetheless, there was a trend towards fewer events in favour of ICR, in line with the short-term findings of the CvLPRIT, BioVasc and MULTISTARS AMI trials5611.
Although the study population of the MULTISTARS AMI Trial is numerically similar to ours, interestingly, the event rate in the staged arm of the MULTISTARS AMI Trial was much higher than in our study, while the event rate in the immediate arm was similar.
This might be explained by the use of a different composite endpoint but also by the different definitions of ischaemia-driven revascularisation.
In the MULTISTARS AMI Trial, ischaemia-driven revascularisation was defined as revascularisation performed because of angina symptoms, ischaemic changes on ECG, or signs of reversible myocardial ischaemia on non-invasive imaging. In contrast, in the BioVasc trial, ischaemia-driven revascularisation was defined as revascularisation performed because of prompted dynamic ECG changes, a new rise in cardiac enzymes, or both. In MULTISTARS AMI, 13 of the 23 patients who underwent revascularisation before the planned staged procedure had isolated angina. This might have generated a bias toward non-inferiority. However, when comparing these results with ours that included angina, the total event rate is similar (9.3% vs 8.6%).
Another aspect that needs to be considered is the timing of the planned stage procedure. In the MULTISTARS AMI Trial, SCR was performed in the time window between 19 and 45 days following the index procedure, while in the STEMI subpopulation of the BioVasc trial revascularisation was completed in a substantial number of patients before the 19th day. In patients with acute coronary syndrome, increased plaque vulnerability is commonly observed in non-culprit lesions12131415. The Optical Coherence Tomography (OCT) substudy of the COMPLETE trial showed that approximately 50% of the patients had at least 1 obstructive non-culprit lesion containing complex vulnerable plaque morphology16. In this scenario, earlier staged procedures might have prevented early events due to non-culprit lesion instability.
Finally, given the observed shorter total length of hospital stay in the ICR arm, the possible advantages associated with the adoption of this strategy might not be limited to clinical events but may include potential health economics benefits.
Limitations
This prespecified analysis of the randomised BioVasc trial provides further insights into the STEMI subpopulation. STEMI as a stratification criterion resulted in a good balance of confounders. However, for this analysis, no formal power calculation was performed. Due to the relatively small sample size, the present subgroup analysis might lack in statistical power to capture the true-positive interactions and, thus, be prone to false-negative results. For this reason, it should be considered as hypothesis-generating for future randomised trials.
Patients with cardiogenic shock were not included in the trial, and results cannot be generalised to those patients. Case-selection bias cannot be excluded, considering the acute STEMI setting in which enrolment might have been performed, depending on the clinical situation.
Coronary chronic total occlusion was an exclusion criterion, but we cannot rule out that patients with complex lesions might also have been excluded per the operator’s decision.
Intravascular imaging was not mandatory to assess culprit or non-culprit lesions and was left to the operator’s discretion. Consequently, the use of intracoronary imaging was low, reflecting current European clinical practice.
Finally, it is important to acknowledge that only a quarter of the patients had 3-vessel disease, thus our findings might not be generalised for this subgroup of patients.
Conclusions
In patients with STEMI and MVD, the adoption of either an immediate or staged complete revascularisation translated into similar clinical outcomes at 1 year.
Impact on daily practice
In patients with ST-elevation myocardial infarction (STEMI) without cardiogenic shock, immediate complete revascularisation might be considered. Immediate and staged complete revascularisation showed similar results in terms of clinical outcomes. Immediate complete revascularisation in patients with STEMI might be associated with a shorter hospital stay.
Funding
This study was funded by Erasmus University Medical Center and Biotronik AG.
Conflict of interest statement
M. Sabaté has received consulting fees from Abbott and iVascular. N.M. Van Mieghem has received institutional research grants from Biotronik, Abbott, Medtronic, Edwards Lifesciences, PulseCath, Abiomed, and Daiichi Sankyo; speaker fees from Abiomed and Amgen; and a travel grant from JenaValve. R. Diletti has received institutional research grants from Biotronik, Medtronic, ACIST Medical Systems, and Boston Scientific. W.K. den Dekker has received institutional research grants from Biotronik. The other authors have no conflicts of interest to declare.

