Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Trials assessing the prognostic influence of the completeness, timing, and guidance of percutaneous coronary intervention (PCI) for haemodynamically stable acute myocardial infarction (MI) and multivessel coronary artery disease (MV-CAD) have provided heterogeneous results.
Aims: We aimed to comprehensively and simultaneously assess the available evidence on the completeness, timing, and guidance of PCI for acute MI and MV-CAD.
Methods: Major electronic databases were screened to identify randomised trials comparing at least two PCI strategies for acute MI and MV-CAD. Recurrent MI and cardiac death were the primary and co-primary outcomes. Frequentist and Bayesian 5- and 3-node network meta-analyses were conducted along with complementary analyses to explore potential sources of heterogeneity.
Results: Fourteen trials, including 14,433 patients, were pooled. In the frequentist 5-node analysis, angiography-guided immediate complete revascularisation (CR) reduced MI compared with infarct-related artery (IRA)-only revascularisation (hazard ratio [HR] 0.42, 95% confidence interval [CI]: 0.27-0.66), angiography-guided staged CR (HR 0.56, 95% CI: 0.36-0.87), and functionally guided staged CR (HR 0.37, 95% CI: 0.20-0.69). Functionally guided immediate CR was associated with reduced MI compared with IRA-only revascularisation (HR 0.53, 95% CI 0.34-0.82). The Bayesian analysis confirmed only an advantage of angiography-guided immediate CR over IRA-only revascularisation. In frequentist 3-node analysis, immediate CR reduced MI (HR 0.51, 95% CI: 0.37-0.70) and cardiac death (HR 0.68, 95% CI: 0.50-0.93) compared with IRA-only revascularisation and MI compared with staged CR (HR 0.55, 95% CI: 0.38-0.79). The Bayesian analysis did not confirm the reduction in cardiac death. CR, regardless of the type of guidance and especially when immediate, reduced the rate of any revascularisation compared with IRA-only revascularisation.
Conclusions: In haemodynamically stable patients with acute MI and non-complex MV-CAD undergoing PCI, immediate CR following successful culprit lesion treatment reduces recurrent MI compared with IRA-only revascularisation and staged CR. Whether CR is associated with reduced cardiovascular death remains uncertain.
Multivessel coronary artery disease (MV-CAD) − defined as the presence of significant narrowing in multiple epicardial vessels − is observed in approximately 50% of patients presenting with acute coronary syndrome1. This condition is associated with worse clinical outcomes compared with single-vessel disease, especially in the context of ST-segment elevation myocardial infarction (STEMI)234. Complete revascularisation (CR) in patients with haemodynamically stable STEMI and MV-CAD undergoing primary percutaneous coronary intervention (PCI) has been associated with improved clinical outcomes compared with infarct-related artery (IRA)-only revascularisation56789.
Nevertheless, in some pivotal trials, the improvement associated with CR compared with IRA-only revascularisation was primarily driven by recurrent revascularisation in the context of open-label designs, and only the COMPLETE and FIRE trials provided robust and unequivocal evidence on hard endpoints678910. Yet, the FULL REVASC trial recently challenged previous conclusions, as no significant differences between CR and culprit lesion-only revascularisation were found, while relevant complementary questions about the optimal timing for non-IRA lesion revascularisation (i.e., during the index or staged PCI) and type of guidance (i.e., functional or exclusively angiographic) have remained underexplored for a long time67811.
Over the last year, several trials comparing different PCI strategies in terms of completeness11, timing10121314 and guidance1115 for the treatment of patients with acute MI and MV-CAD have yielded mixed results. Given the residual uncertainty surrounding this complex topic and the need for a comprehensive view accounting for the completeness of myocardial revascularisation, the timing of the intervention and the role of functional assessment in guiding PCI of non-IRA lesions, we decided to conduct comprehensive, updated, frequentist and Bayesian network meta-analyses comparing different revascularisation strategies for PCI in patients with acute MI and MV-CAD.
Methods
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements (Supplementary Table 1) and Cochrane Collaboration recommendations1617. The protocol was registered with PROSPERO (CRD42023474321).
Eligibility criteria
Studies satisfying the following criteria were included: (i) random allocation to at least two of the strategies among IRA-only revascularisation, angiography-guided immediate CR, angiography-guided staged CR, functionally guided immediate CR, and functionally guided staged CR; (ii) inclusion of patients with acute MI and MV-CAD; (iii) predominant implantation of drug-eluting stents; and (iv) clinical follow-up of at least 12 months.
Search, data extraction, and qualitative assessment
Supplementary Table 2 illustrates the full search strategy. Randomised trials were systematically searched in PubMed/Medline, Scopus, Web of Science and Cochrane. Reports were independently screened at the title and abstract levels and subsequently at the full-text level by three reviewers under the supervision of a senior reviewer. The quality of each trial was collegially evaluated by the Risk of Bias (RoB) 2 tool18. A list of included trials is provided in Supplementary Appendix 1.
Outcomes
The prespecified primary and co-primary outcomes of interest were MI and cardiac death, respectively. Secondary outcomes included all-cause death, definite or probable stent thrombosis, any revascularisation, ischaemia-driven revascularisation, a composite of major adverse cardiac events (MACE) as defined in the original trials, and periprocedural events, including stroke of any type, contrast-induced acute kidney injury and major bleeding.
Statistical analysis
Categorical variables are reported as counts and proportions and continuous variables as means or medians.
The analyses were primarily conducted in a frequentist framework and secondarily replicated in a Bayesian framework to assess the robustness of conclusions1719. Network meta-analyses allow the combination of evidence from both direct and indirect comparisons17. The analyses were based on a comprehensive 5-node network (i.e., IRA-only revascularisation, angiography-guided immediate CR, functionally guided immediate CR, angiography-guided staged CR, and functionally guided staged CR) and were subsequently replicated in a simplified 3-node closed-loop network (i.e., IRA-only revascularisation, immediate CR and staged CR) to gain statistical power.
Comparisons between the revascularisation strategies at the longest available follow-up, as defined in the original intention-to-treat analyses, were reported by hazard ratios (HR) and 95% confidence intervals (CI) or credible intervals (CrI), for frequentist and Bayesian frameworks, respectively, computed by hierarchical random-effects consistency models17. In-hospital outcomes were evaluated by using odds ratios (OR) and 95% CI or CrI, depending on the framework. For each outcome of interest, the network of evidence was visually and numerically assessed in terms of weights, comparisons, and individual trial influence20. Specifications on the priors used for Bayesian analyses, as well as the number of iterations, Gibbs sampling procedure, and assessment of chain convergence are reported in Supplementary Appendix 2.
The results are displayed by using relative-effect tables and forest plots. Each strategy was ranked according to its probability of having a certain rank (i.e., rankogram) and the surface under the cumulative ranking curve (SUCRA)21. Consistency between direct and indirect evidence within the network was assessed by node-splitting1922. Between-trial heterogeneity was assessed by I2 and τ2 statistics17.
Sensitivity analyses were conducted by excluding the largest and most impactful trial (COMPLETE9), trials including non-STEMI (NSTEMI) patients, trials employing non-invasive guidance to achieve staged CR, trials deemed at high risk for bias according to the RoB 2 tool, trials aiming at culprit lesion-only instead of IRA-only revascularisation, trials with inconsistent revascularisation timing, and trials mostly contributing to heterogeneity based on common statistics. Meta-regression analyses were used to explore the influence of diabetes, 3-vessel disease and year of publication on outcomes.
Finally, the impact of small-study effects and publication bias were assessed by comparison-adjusted funnel plots complemented with Egger’s tests1722. Statistical analyses were conducted using R 4.3.2 (R Foundation for Statistical Computing) and Stata 18 (StataCorp).
Results
Study selection and network geometry
The selection process is summarised in Supplementary Figure 1. Fourteen eligible trials encompassing a total of 14,433 patients were included56789101112131415232425. The FRAME-AMI15, FLOWER-MI24, and CROSS-AMI25 trials were excluded from the complementary 3-node network meta-analysis (10 trials, 10,883 patients) as they did not compare strategies employed at different times.
Figure 1 displays the geometry of the networks. The inclusion and exclusion criteria of each trial are reported in Supplementary Table 3. The definitions of CR used in each trial and the proportion of patients who eventually received a treatment strategy are reported in Supplementary Table 4.
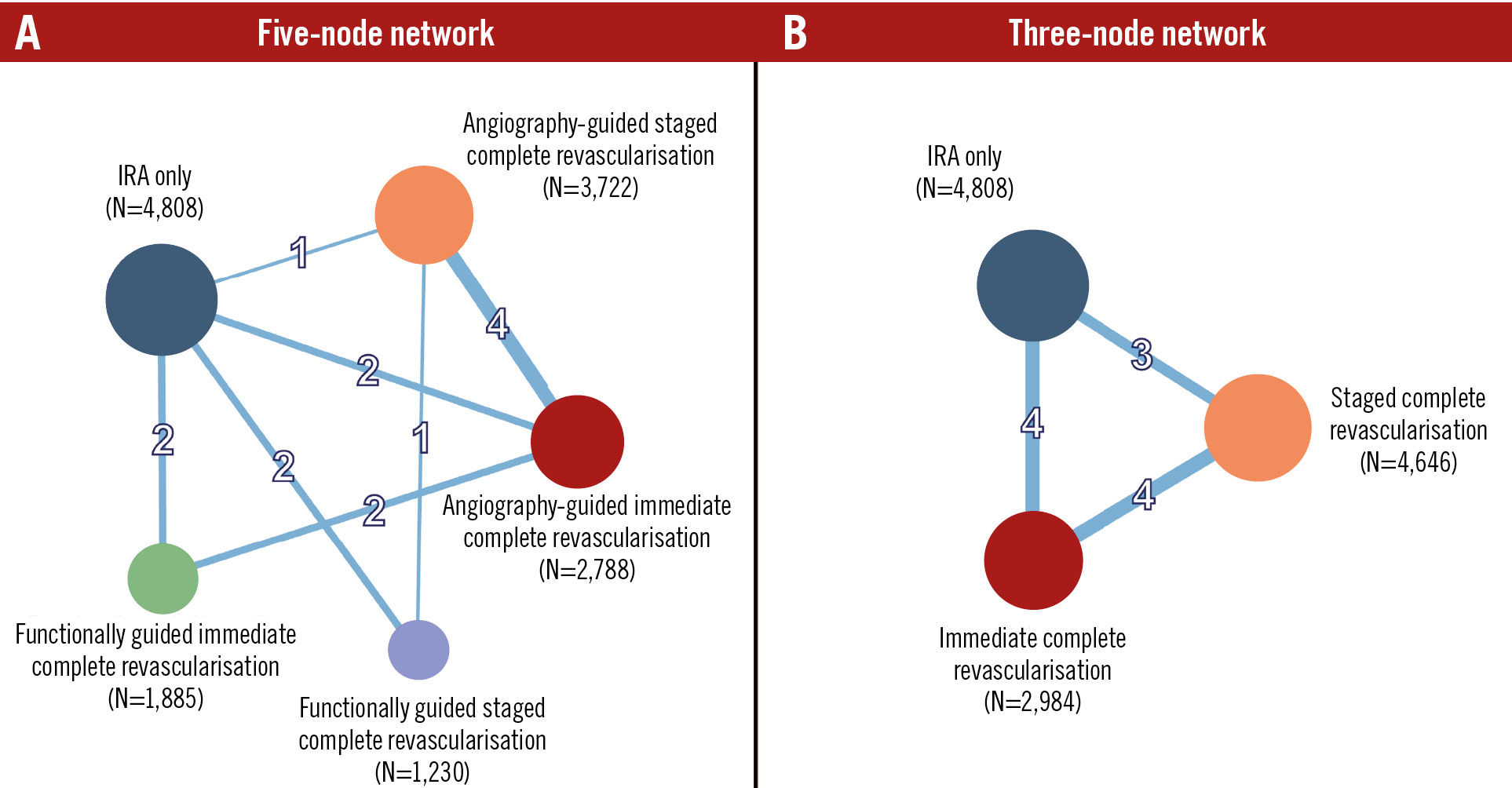
Figure 1. Network geometry. Network geometry of the 5- (A) and 3-node (B) analyses for the primary endpoint of myocardial infarction. Node size is proportional to the number of patients, and connection thickness is proportional to the number of trials contributing to the comparison. IRA: infarct-related artery
Study characteristics
Table 1 summarises the key trial characteristics. Nine trials included only patients with STEMI5678913142425, 4 trials included patients with acute MI undergoing emergency or urgent PCI of the culprit lesion10111215, and 1 trial included only patients with NSTEMI23. Overall, the mean age was 65.6 years, 20.8% were females, 19.7% had diabetes, and 25.1% had 3-vessel disease. The median time to revascularisation in trials of staged CR ranged from 1 to 37 days. Outcomes reported by each trial are illustrated in Supplementary Table 5, while trial-specific definitions of MI and MACE are reported in Supplementary Table 6.
Table 1. Main characteristics of the included trials.
| Trial (year) | Age, years | Female | Clinical presentation | Diabetes | Previous PCI | Previous myocardial infarction | Three-vessel disease | Chronic kidney disease | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|
| PRAMI (2013)5 | 62.0 | 102 (21.9) | STEMI: 465 (100) | 83 (17.9) | NA | 35 (7.5) | 167 (35.9) | NA | 23 |
| CvLPRIT (2015)631 | 64.9 | 56 (18.9) | STEMI: 296 (100) | 39 (13.2) | 9 (3.0) | 12 (4.1) | 67 (22.6) | 2 (0.7) | 67a |
| DANAMI-3-PRIMULTI (2015)7 | 63.5 | 121 (19.3) | STEMI: 627 (100) | 71 (11.3) | NA | 44 (7.0) | 197 (31.4) | 0 (0) | 27a |
| SMILE (2016)23 | 72.5 | 111 (20.5) | NSTEMI: 542 (100) | 19 (4.1) | 85 (15.7) | 133 (24.5) | NA | NA | 12b |
| COMPARE-ACUTE (2017)832 | 62.5 | 202 (22.8) | STEMI: 885 (100) | 137 (15.5) | 69 (7.8) | 70 (7.9) | 285 (32.2) | 10 (1.1) | 36b |
| CROSS-AMI (2019)24 | 62.0 | 48 (15.7) | STEMI: 306 (100) | 44 (14.4) | NA | 16 (5.3) | 133 (43.5) | NA | 12b |
| COMPLETE (2019)9 | 62.0 | 816 (20.2) | STEMI: 4,041 (100) | 787 (19.5) | 28 3(7.0) | 302 (7.5) | 901 (22.5) | 81 (2.0) | 36a |
| FLOWER-MI (2021)2533 | 61.5 | 205 (17.5) | STEMI: 1,171 (100) | 189 (16.1) | 103 (8.8) | 76 (6.5) | 266 (22.7) | 23 (2.0) | 42a |
| FRAME-AMI (2023)15 | 63.3 | 88 (15.7) | STEMI: 265 (47.2)NSTEMI: 297 (52.8) | 183 (32.6) | 37 (6.6) | 14 (2.5) | 217 (38.6) | 16 (2.8) | 42a |
| BIOVASC (2023)12 | 65.2 | 169 (22.2) | STEMI: 608 (39.9)NSTEMI: 790 (51.8)UA: 127 (8.3) | 321 (21.0) | 204 (13.4) | 158 (10.4) | 265 (17.4) | 78 (5.1) | 12b |
| FIRE(2023)10 | 80.5 | 528 (36.5) | STEMI: 509 (35.2)NSTEMI: 936 (64.8) | 463 (32.0) | 257 (17.8) | 220 (15.2) | 432 (29.9) | 662 (45.8) | 12b |
| COCUA (2023)14 | 62.7 | 41 (19.6) | STEMI: 209 (100) | 79 (37.8) | 2 (1.0) | 2 (1.0) | 39 (18.7) | NA | 12a |
| MULTISTARS AMI(2023)13 | 65.0 | 178 (21.2) | STEMI: 840 (100) | 131 (15.6) | 56 (6.7) | 48 (5.7) | 131 (15.6) | NA | 12b |
| FULL REVASC (2024)11 | 65.4 | 365 (23.7) | STEMI: 1,410 (91.4)NSTEMI: 132 (8.6) | 249 (16.2) | 134 (8.7) | 125 (8.1) | 487 (31.6) | NA | 57a |
| Data are presented as n (%) or mean value unless otherwise specified. aMedian, bmaximum. NA: not available; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; UA: unstable angina | |||||||||
Risk of bias
Supplementary Figure 2 and Supplementary Figure 3 display the RoB 2 assessment, with detection of significant risk of bias in the outcomes assessment in two trials1314. Some concerns surrounded the remaining trials, mostly because of the unfeasibility of a blinded design.
Five-node network meta-analyses: primary and co-primary outcomes
Angiography-guided immediate CR significantly reduced MI compared with IRA-only revascularisation in both the frequentist (HR 0.42, 95% CI: 0.27-0.66) and Bayesian (HR 0.47, 95% CrI: 0.25-0.89) frameworks (Table 2, Central illustration, Supplementary Table 7). In the frequentist analysis, MI was also reduced by functionally guided immediate CR compared with IRA-only revascularisation (HR 0.53, 95% CI: 0.34-0.82), by angiography-guided immediate CR compared with angiography-guided staged CR (HR 0.56, 95% CI: 0.36-0.87) and functionally guided staged CR (HR 0.37, 95% CI: 0.20-0.69), and by functionally guided immediate CR compared with functionally guided staged CR (HR 0.47, 95% CI: 0.25-0.88) (Table 2, Central illustration). The overall heterogeneity was moderate (I2=39%, τ2=0.074) (Table 2, Central illustration). Angiography-guided immediate CR showed the highest probability of ranking first among all strategies (Supplementary Table 8). No sign of inconsistency was detected in either frequentist or Bayesian analysis (Figure 2, Supplementary Table 9).
The co-primary outcome of cardiovascular death did not significantly differ between revascularisation strategies in either the frequentist analysis or the Bayesian analysis (Table 2, Central illustration, Supplementary Table 7), and moderate heterogeneity was detected (I2=42%, τ2=0.166). Functionally guided immediate CR ranked first in both the frequentist and Bayesian frameworks (Supplementary Table 9). Significant inconsistency was detected for the comparisons of angiography-guided immediate CR versus IRA-only revascularisation (frequentist: p=0.028, Bayesian: p=0.040), functionally guided immediate CR versus IRA-only revascularisation (frequentist: p=0.021, Bayesian: p=0.025), and angiography-guided immediate CR versus functionally guided immediate CR (frequentist: p=0.027, Bayesian: p=0.025) (Figure 2, Supplementary Table 9).
Table 2. Frequentist random-effects network meta-analysis – 5-node analysis.
| Myocardial infarction | |||||
|---|---|---|---|---|---|
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 2.36 [1.51-3.71]* | 1.32 [0.85-2.03] | 1.89 [1.22-2.92]* | 0.88 [0.55-1.41] |
| Angio immediate CR | 0.42 [0.27-0.66]* | – | 0.56 [0.36-0.87]* | 0.80 [0.49-1.30] | 0.37 [0.20-0.69]* |
| Angio staged CR | 0.76 [0.49-1.18] | 1.80 [1.15-2.82]* | – | 1.43 [0.82-2.50] | 0.67 [0.37-1.20] |
| Functional immediate CR | 0.53 [0.34-0.82]* | 1.25 [0.77-2.04] | 0.70 [0.40-1.21] | – | 0.47 [0.25-0.88]* |
| Functional staged CR | 1.14 [0.71-1.83] | 2.69 [1.44-5.03]* | 1.50 [0.84-2.68] | 2.15 [1.14-4.05]* | – |
| Heterogeneity: I2=39%; t2=0.074 | |||||
| Cardiac death | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 1.29 [0.65-2.57] | 1.30 [0.67-2.52] | 1.82 [0.92-3.62] | 1.25 [0.60-2.59] |
| Angio immediate CR | 0.77 [0.39-1.54] | – | 1.01 [0.56-1.80] | 1.41 [0.60-3.34] | 0.97 [0.36-2.57] |
| Angio staged CR | 0.77 [0.40-1.50] | 0.99 [0.56-1.78] | – | 1.41 [0.58-3.42] | 0.96 [0.37-2.48] |
| Functional immediate CR | 0.55 [0.28-1.09] | 0.71 [0.30-1.67] | 0.71 [0.29-1.73] | – | 0.68 [0.25-1.85] |
| Functional staged CR | 0.80 [0.39-1.67] | 1.04 [0.39-2.76] | 1.04 [0.40-2.69] | 1.46 [0.54-3.97] | – |
| Heterogeneity: I2=42%; t2=0.166 | |||||
| Death | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 1.21 [0.86-1.71] | 1.17 [0.85-1.61] | 1.47 [1.07-2.04]* | 0.82 [0.57-1.18] |
| Angio immediate CR | 0.83 [0.58-1.17] | – | 0.97 [0.69-1.36] | 1.22 [0.83-1.79] | 0.68 [0.41-1.12] |
| Angio staged CR | 0.85 [0.62-1.18] | 1.03 [0.73-1.46] | – | 1.26 [0.83-1.91] | 0.70 [0.43-1.13] |
| Functional immediate CR | 0.68 [0.49-0.94]* | 0.82 [0.56-1.21] | 0.79 [0.52-1.21] | – | 0.56 [0.34-0.91]* |
| Functional staged CR | 1.22 [0.85-1.76] | 1.48 [0.89-2.44] | 1.43 [0.88-2.31] | 1.80 [1.10-2.93]* | – |
| Heterogeneity: I2=34%; t2=0.018 | |||||
| Definite or probable stent thrombosis | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 0.81 [0.37-1.79] | 0.72 [0.41-1.26] | 1.39 [0.67-2.87] | 0.18 [0.03-0.96]* |
| Angio immediate CR | 1.24 [0.56-2.74] | – | 0.89 [0.46-1.72] | 1.72 [0.71-4.14] | 0.22 [0.04-1.23] |
| Angio staged CR | 1.39 [0.80-2.43] | 1.13 [0.58-2.18] | – | 1.93 [0.84-4.43] | 0.25 [0.05-1.21] |
| Functional immediate CR | 0.72 [0.35-1.49] | 0.58 [0.24-1.40] | 0.52 [0.23-1.18] | – | 0.13 [0.02-0.77]* |
| Functional staged CR | 5.57 [1.05-29.66]* | 4.50 [0.81-24.89] | 4.00 [0.83-19.35] | 7.73 [1.30-45.89]* | – |
| Heterogeneity: I2=0%; t2=0 | |||||
| Any revascularisation | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 2.99 [1.70-5.27]* | 1.97 [1.06-3.69]* | 2.40 [1.29-4.46]* | 2.27 [1.15-4.45]* |
| Angio immediate CR | 0.33 [0.19-0.59]* | – | 0.66 [0.39-1.12] | 0.80 [0.42-1.52] | 0.76 [0.34-1.70] |
| Angio staged CR | 0.51 [0.27-0.95]* | 1.51 [0.89-2.58] | – | 1.21 [0.57-2.59] | 1.15 [0.52-2.52] |
| Functional immediate CR | 0.42 [0.22-0.78]* | 1.25 [0.66-2.37] | 0.82 [0.39-1.76] | – | 0.95 [0.39-2.29] |
| Functional staged CR | 0.44 [0.22-0.87]* | 1.32 [0.59-2.96] | 0.87 [0.40-1.92] | 1.06 [0.44-2.56] | – |
| Heterogeneity: I2=81%; t2=0.263 | |||||
| Ischaemia-driven revascularisation | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 3.00 [1.36-6.64]* | 2.42 [1.10-5.36]* | 2.06 [0.97-4.39] | 3.02 [1.13-8.08]* |
| Angio immediate CR | 0.33 [0.15-0.74]* | – | 0.81 [0.39-1.67] | 0.69 [0.28-1.69] | 1.01 [0.33-3.08] |
| Angio staged CR | 0.41 [0.19-0.91]* | 1.24 [0.6-2.56] | – | 0.85 [0.32-2.29] | 1.25 [0.46-3.39] |
| Functional immediate CR | 0.49 [0.23-1.03] | 1.46 [0.59-3.59] | 1.18 [0.44-3.16] | – | 1.47 [0.44-4.85] |
| Functional staged CR | 0.33 [0.12-0.88]* | 0.99 [0.32-3.04] | 0.80 [0.29-2.18] | 0.68 [0.21-2.26] | – |
| Heterogeneity: I2=84%; t2=0.331 | |||||
| Major adverse cardiac events | |||||
| IRA only | Angio immediate CR | Angio staged CR | Functional immediate CR | Functional staged CR | |
| IRA only | – | 2.02 [1.38-2.96]* | 1.51 [1.00-2.29] | 2.08 [1.37-3.15]* | 1.37 [0.87-2.17] |
| Angio immediate CR | 0.49 [0.34-0.72]* | – | 0.75 [0.53-1.06] | 1.03 [0.67-1.59] | 0.68 [0.39-1.18] |
| Angio staged CR | 0.66 [0.44-1.00] | 1.34 [0.94-1.90] | – | 1.38 [0.83-2.29] | 0.91 [0.53-1.56] |
| Functional immediate CR | 0.48 [0.32-0.73]* | 0.97 [0.63-1.50] | 0.73 [0.44-1.21] | – | 0.66 [0.36-1.20] |
| Functional staged CR | 0.73 [0.46-1.15] | 1.47 [0.85-2.56] | 1.10 [0.64-1.90] | 1.52 [0.83-2.76] | – |
| Heterogeneity: I2=76%; t2=0.112 | |||||
| Values are HRs [95% CIs]. *Indicates a significant result when comparing treatment 1 (row) vs treatment 2 (column). CI: confidence interval; CR: complete revascularisation; HR: hazard ratio; IRA: infarct-related artery | |||||
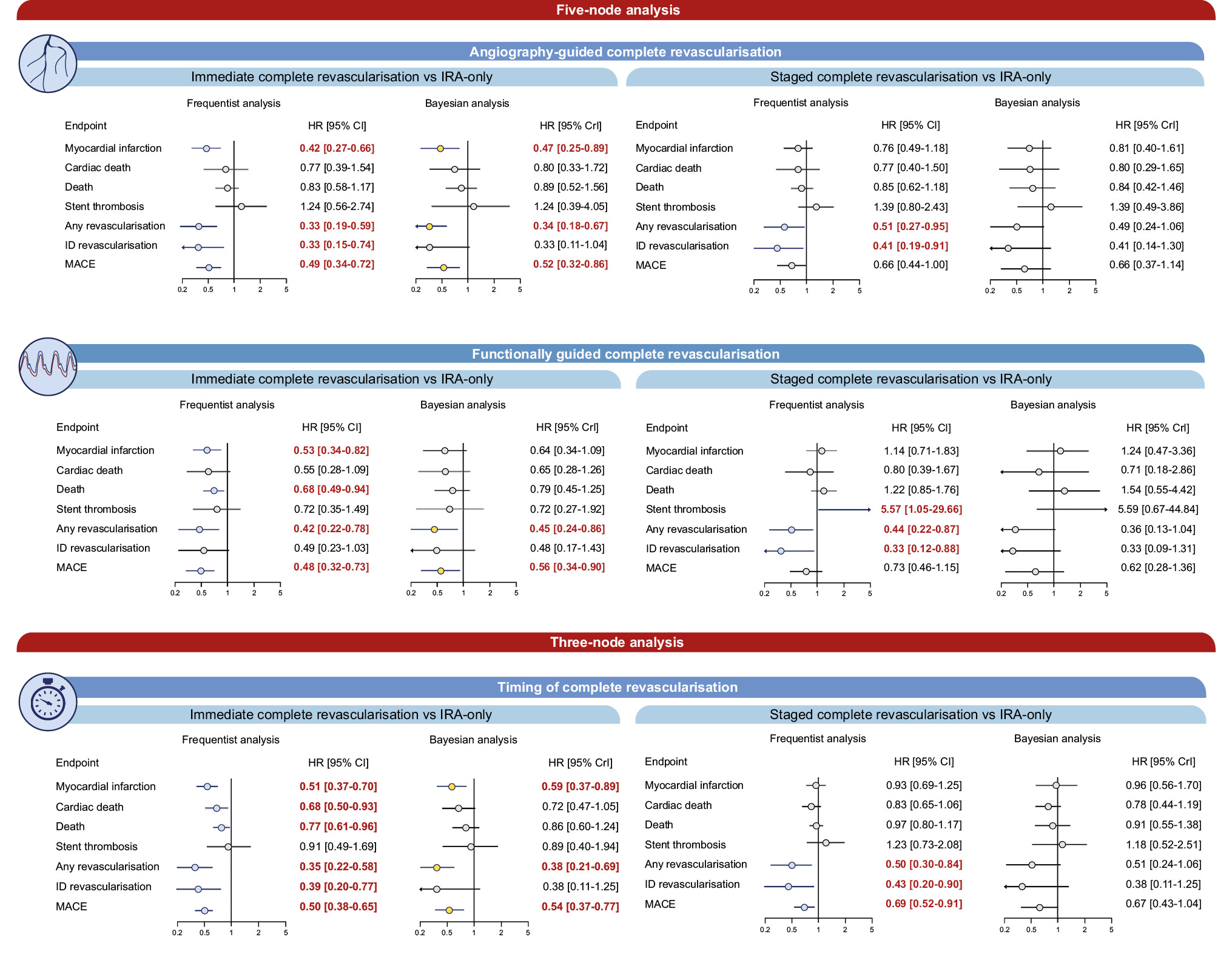
Central illustration. Comparison between CR strategies and IRA-only revascularisation. Risk estimates for the comparisons between CR strategies and IRA-only revascularisation. In the five-node analyses, both the timing (immediate or staged) and guidance (angiographic or functional) for achieving CR revascularisation were considered. In the three-node analyses, the timing for achieving CR was considered. CI: confidence interval; CR: complete revascularisation; CrI: credible interval; HR: hazard ratio; ID: ischaemia-driven; IRA: infarct-related artery; MACE: major adverse cardiac events
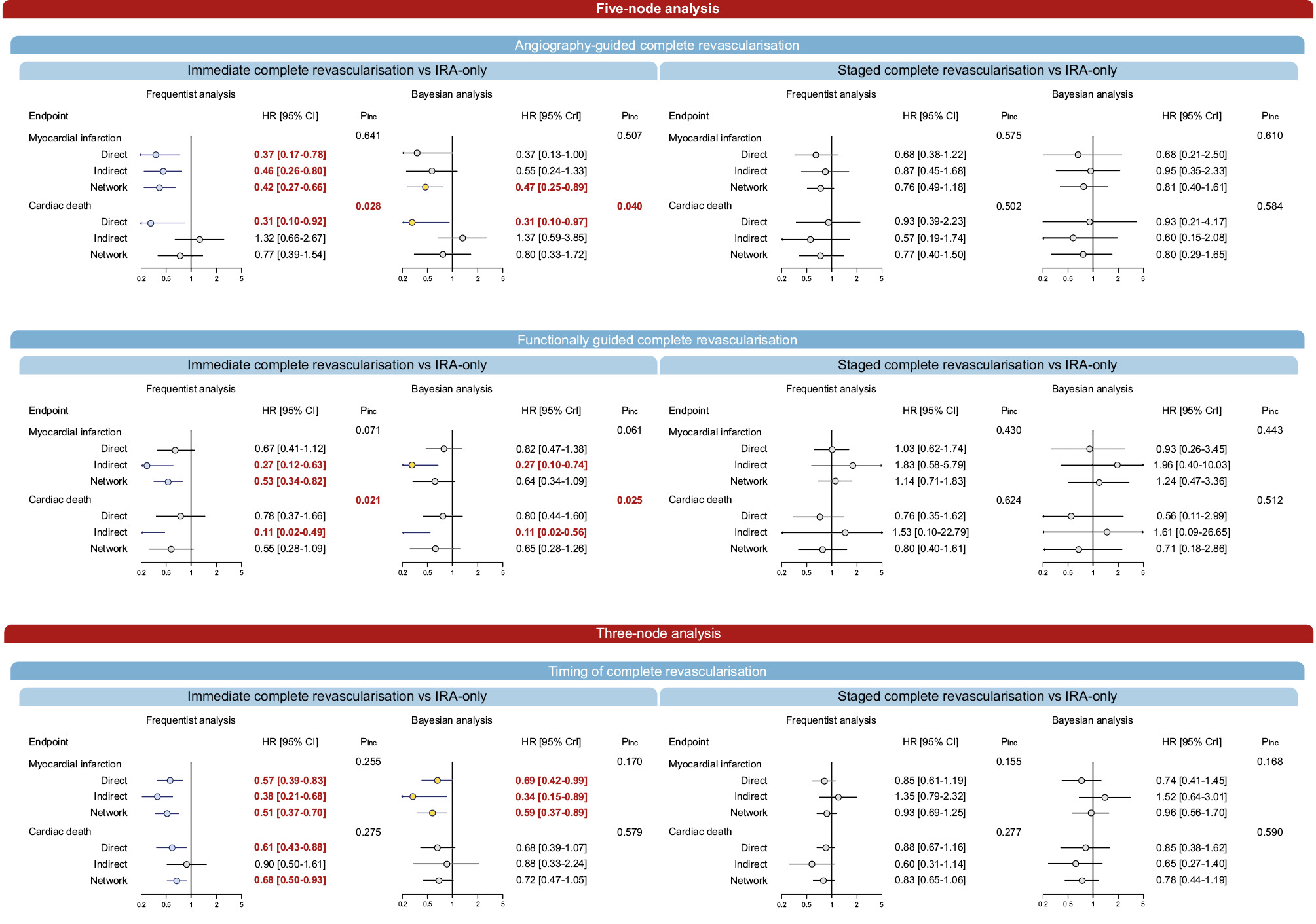
Figure 2. Node-splitting analysis for different revascularisation strategies compared with IRA-only revascularisation. Relative estimates for direct and indirect comparisons of revascularisation strategies compared with IRA-only revascularisation.CI: confidence interval; CR: complete revascularisation; CrI: credible interval; HR: hazard ratio; IRA: infarct-related artery; Pinc: P for inconsistency
Five-node network meta-analyses: secondary outcomes
Functionally guided immediate CR was the only strategy that reduced mortality compared to IRA-only revascularisation in the frequentist analysis (HR 0.68, 95% CI: 0.49-0.94), but this was not observed in the Bayesian analysis (Table 2, Central illustration, Supplementary Table 7). Moderate heterogeneity was detected (I2=34%, τ2=0.018).
The incidence of stent thrombosis increased with functionally guided staged CR compared with IRA-only revascularisation (HR 5.57, 95% CI: 1.07-29.66) and functionally guided immediate CR (HR 7.73, 95% CI: 1.30-45.89) (Table 2). However, these effects were not detected in the Bayesian framework (Central illustration, Supplementary Table 7).
In the frequentist framework, compared with IRA-only revascularisation, any revascularisation was significantly lower with each of the four CR strategies (Central illustration, Table 2). Ischaemia-driven revascularisation was reduced by angiography-guided immediate CR, angiography-guided staged CR, and functionally guided staged CR (Table 2, Central illustration). In the Bayesian framework, angiography-guided and functionally guided immediate revascularisation reduced any revascularisation when compared with IRA-only revascularisation, and no significant differences were detected for ischaemia-driven revascularisation (Supplementary Table 7). High heterogeneity was detected for any revascularisation (I2=81%, τ2=0.263) and ischaemia-driven revascularisation (I2=84%, τ2=0.331). Significant inconsistency was detected for any revascularisation in the comparisons of angiography-guided staged CR versus IRA-only revascularisation (frequentist: p=0.008, Bayesian: p=0.007) and angiography-guided immediate versus angiography-guided staged CR (frequentist: p=0.011, Bayesian: p=0.002), as well as for ischaemia-driven revascularisation in the comparisons of angiography-guided staged CR versus IRA-only revascularisation (frequentist: p=0.029, Bayesian: p=0.049) and angiography-guided immediate versus angiography-guided staged CR (frequentist: p=0.002, Bayesian: p=0.022) (Supplementary Table 9). MACE was reduced with angiography-guided and functionally guided immediate CR (Table 2, Central illustration). In the Bayesian framework, the results in terms of MACE were consistent (Supplementary Table 7). High heterogeneity was detected for MACE (I2=76%, τ2=0.112).
Three-node network analyses: primary and co-primary outcomes
In the 3-node analysis, immediate CR significantly reduced MI incidence compared with both IRA-only revascularisation (HR 0.51, 95% CI: 0.37-0.70) and staged CR (HR 0.55, 95% CI: 0.38-0.79) (Table 3, Central illustration). Heterogeneity was low (I2=28%, τ2=0.046) (Table 3). Bayesian analysis reported consistent results for immediate CR versus IRA-only revascularisation (HR 0.59, 95% CrI: 0.37-0.89) (Supplementary Table 10). Immediate CR was associated with the highest probability of ranking first (Supplementary Table 11), and no evidence of inconsistency was detected (Figure 2, Supplementary Table 12).
Immediate CR significantly reduced the incidence of cardiac death compared with IRA-only revascularisation in the frequentist analysis (HR 0.68, 95% CI: 0.50-0.93), but not in the Bayesian analysis, with undetectable heterogeneity (I2=0%, τ2<0.001) (Table 3, Central illustration, Supplementary Table 10). Regardless of the statistical framework, immediate CR had the highest probability of ranking first (Supplementary Table 11). No significant inconsistency was detected (Figure 2, Supplementary Table 12).
Table 3. Frequentist random-effects network meta-analysis – 3-node analysis.
| Myocardial infarction | |||
|---|---|---|---|
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 1.97 [1.43-2.73]* | 1.08 [0.80-1.45] |
| Immediate CR | 0.51 [0.37-0.70]* | – | 0.55 [0.38-0.79]* |
| Staged CR | 0.93 [0.69-1.25] | 1.83 [1.27-2.63]* | – |
| Heterogeneity: I2=28%; t²=0.046 | |||
| Cardiac death | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 1.46 [1.08-1.99]* | 1.21 [0.94-1.55] |
| Immediate CR | 0.68 [0.50-0.93]* | – | 0.82 [0.59-1.16] |
| Staged CR | 0.83 [0.65-1.06] | 1.21 [0.86-1.70] | – |
| Heterogeneity: I2=2%; t²<0.001 | |||
| Death | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 1.30 [1.04-1.63]* | 1.03 [0.85-1.24] |
| Immediate CR | 0.77 [0.61-0.96]* | – | 0.79 [0.61-1.02] |
| Staged CR | 0.97 [0.80-1.17] | 1.26 [0.98-1.63] | – |
| Heterogeneity: I2=31%; t²=0.001 | |||
| Definite or probable stent thrombosis | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 1.10 [0.59-2.04] | 0.81 [0.48-1.37] |
| Immediate CR | 0.91 [0.49-1.69] | – | 0.74 [0.41-1.34] |
| Staged CR | 1.23 [0.73-2.08] | 1.35 [0.75-2.44] | – |
| Heterogeneity: I2=0%; t²=0 | |||
| Any revascularisation | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 2.82 [1.71-4.64]* | 2.00 [1.18-3.38]* |
| Immediate CR | 0.35 [0.22-0.58]* | – | 0.71 [0.42-1.19] |
| Staged CR | 0.50 [0.30-0.84]* | 1.41 [0.84-2.36] | – |
| Heterogeneity: I2=84%; t²=0.296 | |||
| Ischaemia-driven revascularisation | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 2.53 [1.30-4.95]* | 2.34 [1.12-4.91]* |
| Immediate CR | 0.39 [0.20-0.77]* | – | 0.92 [0.44-1.93] |
| Staged CR | 0.43 [0.20-0.90]* | 1.08 [0.52-2.26] | – |
| Heterogeneity: I2=89%; t²=0.400 | |||
| Major adverse cardiac events | |||
| IRA only | Immediate CR | Staged CR | |
| IRA only | – | 2.01 [1.53-2.63]* | 1.45 [1.11-1.91]* |
| Immediate CR | 0.50 [0.38-0.65]* | – | 0.72 [0.55-0.96]* |
| Staged CR | 0.69 [0.52-0.91]* | 1.38 [1.05-1.83]* | – |
| Heterogeneity: I2=77%; t²=0.072 | |||
| Values are HRs [95% CIs]. *Indicates a significant result when comparing treatment 1 (row) vs treatment 2 (column). CI: confidence interval; CR: complete revascularisation; HR: hazard ratio; IRA: infarct-related artery | |||
Three-node network analyses: secondary outcomes
Consistently with MI and cardiac death, immediate CR was associated with a significant reduction in mortality compared with IRA-only revascularisation in the frequentist framework (Table 3, Supplementary Table 10). While stent thrombosis was comparable between strategies, both immediate and staged CR reduced ischaemia-driven revascularisation compared with IRA-only revascularisation in the frequentist analysis but not in the Bayesian framework (Table 3, Supplementary Table 10). High between-trial heterogeneity was detected for any (I2=84%, τ2=0.296) and ischaemia-driven revascularisation (I2=89%, τ2=0.400). Significant inconsistency was detected for both outcomes (Supplementary Table 12). Immediate CR reduced MACE compared with both IRA-only revascularisation and staged CR, and staged CR reduced MACE compared to IRA-only revascularisation.
Periprocedural outcomes
No significant differences between strategies in terms of periprocedural stroke, major bleeding, or contrast-induced acute kidney injury were detected in either the 5- or 3-node analysis, independently of the framework adopted, with no sign of heterogeneity or inconsistency (Supplementary Table 13-Supplementary Table 14-Supplementary Table 15-Supplementary Table 16-Supplementary Table 17-Supplementary Table 18).
Sensitivity analyses
The leave-one-out analysis revealed that most of the heteroÂgeneity for MI, cardiac death and all-cause mortality was introduced by the FRAME-AMI trial (Supplementary Table 19-Supplementary Table 20-Supplementary Table 21-Supplementary Table 22-Supplementary Table 23-Supplementary Table 24-Supplementary Table 25-Supplementary Table 26-Supplementary Table 27-Supplementary Table 28-Supplementary Table 29-Supplementary Table 30-Supplementary Table 31-Supplementary Table 32-Supplementary Table 33-Supplementary Table 34-Supplementary Table 35-Supplementary Table 36-Supplementary Table 37-Supplementary Table 38). Sensitivity analyses, after excluding the largest trial, trials with NSTEMI patients, the only trial employing non-invasive guidance, trials deemed at high risk of bias, trials comparing CR with culprit lesion-only revascularisation, trials with inconsistent revascularisation timing and the trial introducing most heterogeneity, were generally consistent with the main analysis, and only limited variations in estimates were observed (Supplementary Table 39-Supplementary Table 40-Supplementary Table 41-Supplementary Table 42-Supplementary Table 43-Supplementary Table 44-Supplementary Table 45-Supplementary Table 46-Supplementary Table 47-Supplementary Table 48-Supplementary Table 49-Supplementary Table 50-Supplementary Table 51-Supplementary Table 52-Supplementary Table 53-Supplementary Table 54-Supplementary Table 55-Supplementary Table 56-Supplementary Table 57-Supplementary Table 58-Supplementary Table 59-Supplementary Table 60-Supplementary Table 61-Supplementary Table 62-Supplementary Table 63-Supplementary Table 64). Meta-regressions showed no signifiÂcant influence of key variables on treatment effects (Supplementary Table 65-Supplementary Table 66-Supplementary Table 67-Supplementary Table 68-Supplementary Table 69-Supplementary Table 70). Pairwise comÂÂÂparisons of trials focusing on CR versus IRA-only revascularisation showed significant reductions in MI, any revascularisation and MACE, with consistent results between the frequentist and Bayesian frameworks (Supplementary Table 71-Supplementary Table 72-Supplementary Table 73-Supplementary Table 74).
Discussion
This meta-analysis of randomised clinical trials comparing different PCI-based revascularisation strategies in patients with acute MI and MV-CAD without haemodynamic instability consistently shows that CR is associated with a significant reduction in recurrent MI compared with IRA-only revascularisation. In a 5-node analysis attempting to simultaneously address questions about the optimal timing and guidance to achieve CR, both angiography-guided and functionally guided immediate CR were associated with 58% and 47% relative risk reductions in MI, respectively, compared with IRA-only revascularisation. Conversely, no decrease in MI was found with staged CR, regardless of the type of guidance. A 3-node analysis not only confirmed a reduction in MI after immediate CR compared with IRA-only revascularisation but also showed a 45% relative risk reduction after immediate CR compared with staged CR.
Based on these results, it is reasonable to hypothesise that preventive treatment of significant non-culprit lesions reduces the occurrence of spontaneous MI compared with IRA-only revascularisation, though evidence from available trials has yielded mixed results5678913142425. In particular, the large-scale randomised COMPLETE trial showed that CR reduces cardiovascular death or recurrent MI compared with culprit lesion-only revascularisation (HR 0.74, 95% CI: 0.60-0.91), primarily driven by a reduced incidence of recurrent MI (HR 0.68, 95% CI: 0.53-0.86), as cardiovascular death did not significantly differ between groups9. Later, in the FIRE trial10, patients assigned to CR exhibited a lower incidence of MACE as a result of consistent reductions in mortality (HR 0.70, 95% CI: 0.51-0.96), recurrent MI (HR 0.62, 95% CI: 0.40-0.97), and ischaemia-driven revascularisation (HR 0.63, 95% CI: 0.40-0.98). However, in the DANAMI-3-PRIMULTI and COMPARE-ACUTE trials, the significant reductions in MACE after CR compared with IRA-only revascularisation were essentially due to reduced repeat revascularisation, which is a softer endpoint (HR 0.31, 95% CI: 0.18-0.53 and HR 0.32, 95% CI: 0.20-0.54, respectively)78. More importantly, the large-scale FULL REVASC trial did not find significant benefits with functionally guided staged CR compared with IRA-only revascularisation, apart from a reduction in repeat revascularisation (HR 0.59, 95% CI: 0.45-0.78), at the expense of higher risk of stent thrombosis (HR 2.80, 95% CI: 1.18-6.67)11.
The timing for achieving CR may partially explain the differences across trials, and immediate CR seems to align with the mechanisms of acute MI, wherein the improved collateral circulation provided by CR reduces the extent of myocardial tissue transitioning into necrosis in areas supplied by the culprit lesion. In this regard, the present meta-analysis not only indicates a significant reduction in recurrent MI associated with immediate CR but also reveals that staged CR may be associated with fewer benefits compared with IRA-only revascularisation. Yet there is heterogeneity surrounding this result, and some considerations are required. In particular, the MULTISTARS AMI and BIOVASC trials − specifically designed to address the question of CR timing − have indicated advantages of immediate compared to staged CR in patients presenting with acute MI and MV-CAD1213. However, in both trials the primary composite endpoints were composed of a broad number of outcomes with mixed specificity, and the conclusions in terms of MI and repeat revascularisation were associated with some areas of uncertainty. More specifically, in MULTISTARS AMI, 56.7% of non-fatal MIs in the two groups were periprocedural, only 31.8% of non-fatal MIs in the staged CR group occurred before achieving CR, and the difference in non-fatal MIs did not significantly differ between CR strategies after excluding periprocedural events (HR 0.62, 95% CI: 0.20-1.89)13. In the BIOVASC trial, there was a significant reduction in MI in patients assigned to immediate CR compared to those assigned to staged CR12. However, the difference was driven by an excess of periprocedural events in the staged CR group compared with the immediate CR group (0.1% vs 1.4%), and in the staged CR group only 44.1% of events occurred in the time window between the index and staged revascularisations12. Adjudicating periprocedural MI associated with immediate CR performed in the context of acute MI, primarily STEMI, may be challenging as the diagnosis relies on the definition used, and the index event may mask recurrences26. In this context, the difference in MI between immediate and complete CRs may reflect the dissimilar preprocedural troponin levels between the index and staged revascularisation times. This uncertainty is further amplified by a prespecified mechanistic substudy of the CvLPRIT trial showing that CR not only failed to show significant reductions in total (12.6% vs 13.5%; p=0.57) and IRA infarct size (12.1% vs 12.2%; p=0.68) on 48- to 72-hour cardiac magnetic resonance imaging, compared with IRA-only revascularisation, but was also associated with increased periprocedural MI (22.4% vs 10.5%; p=0.02)27. For these reasons, considering the limited statistical power for hard individual endpoints of BIOVASC and MULTISTARS AMI as well as the heterogeneity in the population of interest and some inconsistent findings between the two trials, larger trials and high-quality mechanistic studies are still required to draw more robust conclusions on the benefits of different timings to achieve CR.
Against this background, demonstrating a significant reduction in cardiac death associated with different CR strategies is essential. Our 3-node meta-analysis showed that immediate CR is associated with a 32% relative risk reduction in cardiac death compared with IRA-only revascularisation, without signals of inconsistency, but the Bayesian 3-node analysis showed less marked effects, and the 5-node analysis did not reveal significant differences between strategies. Importantly, signs of network inconsistency were found in the 5-node analyses. In network meta-analyses, direct and indirect comparisons between treatments are assumed to derive from studies with reasonable similarity, ensuring the transitivity of information across the network1620. When direct and indirect evidence show significant disagreement for a given comparison, the transitivity is locally violated1620. In light of the inconsistency observed, there are conflicting conclusions, possibly deriving from trials employing functionally guided CR. Indeed, the COMPARE-ACUTE, FULL REVASC, FLOWER-MI, and FRAME-AMI trials showed conflicting results for functionally guided CR. Specifically, functionally guided immediate CR reduced repeat revascularisation compared with IRA-only revascularisation in the COMPARE-ACUTE trial8. FULL REVASC showed no benefit of predominantly staged functionally guided CR compared with culprit lesion-only revascularisation11. While FLOWER-MI showed no benefit of functionally guided staged CR compared with angiography-guided CR, FRAME-AMI indicated reduced cardiovascular mortality and MI by functionally guided revascularisation compared with angiography-guided CR1525. The absence of uniform and objective methods to define the significance of non-culprit disease, with some trials using angiographic (essentially visual) and others functional assessment, has introduced heterogeneity across studies and influenced the identification of lesions requiring treatment28.
The present meta-analysis also indicates a substantial reduction in repeat revascularisation associated with CR compared with IRA-only revascularisation. The 5-node analysis revealed that these results occur regardless of the timing and type of guidance of CR, with relative risk reductions ranging from 49% to 67%, no significant differences between CR strategies, and consistent results in the 3-node and pairwise analyses. However, the amount of heterogeneity detected was not negligible. In the context of trials with unblinded treatment allocation, the awareness of residual non-culprit lesions may have magnified the incidence of repeat revascularisation over time in patients assigned to IRA-only revascularisation compared with those assigned to CR. Consistently, the results in terms of ischaemia-driven revascularisation were less marked compared with those in terms of any revascularisation. In addition, although the similar trends in terms of MI and ischaemia-driven revascularisation support an increased occurrence of MI in patients assigned to IRA-only revascularisation compared to CR, other results across analyses revealed inconsistency between the two outcomes due to significant reductions in MI without a concomitant reduction in ischaemia-driven revascularisation. These findings require more analysis, as they cannot be further properly inspected at the aggregate level.
Finally, the increased incidence of stent thrombosis associated with functionally guided staged CR was driven by the observation of a significant excess of events in the FULL REVASC trial. However, this finding has no clear procedure- or strategy-related explanation.
Limitations
Some considerations are required when interpreting the results of this meta-analysis. First, the absence of individual patient data did not allow for an evaluation of confounders on the overall results, though sensitivity analyses and meta-regressions mitigated some concerns. Second, some approximations were required in the definition of the strategies employed across trials. Indeed, the proportions of immediate and staged revascularisation across trials that did not impose a specific timing showed some heterogeneity. However, this condition influenced only the 5-node analysis, and, except for FRAME-AMI, it was possible in all the other trials to identify a clearly predominant timing of CR. Similarly, per-protocol analyses reported across trials did not present overall sufficient granularity to account for the patients who eventually did not receive the allocated strategy in the context of a per-protocol sensitivity analysis. Third, the timing for achieving CR was physician driven and was dissimilar across trials. Individual patient data would be necessary to properly address these latter two points. Fourth, the use of intravascular imaging was limited, with possible implications on procedural outcomes29. Lastly, the coronary artery disease patterns included across trials may not be representative of more complex conditions, limiting the generalisability of some results30.
Conclusions
In haemodynamically stable patients with non-complex MV-CAD undergoing urgent PCI for acute MI, immediate CR following successful culprit-lesion treatment, whether angiographically or functionally guided, reduces recurrent MI and repeat revascularisation compared with IRA-only revascularisation and staged CR. Staged CR is associated with reduced benefits compared with IRA-only revascularisation, generally limited to reduced repeat revascularisation. Although functionally guided immediate CR may reduce mortality compared with IRA-only revascularisation, this conclusion was highly inconsistent across analyses.
Impact on daily practice
In patients with haemodynamically stable acute myocardial infarction and non-complex multivessel coronary artery disease, immediate complete revascularisation (CR) reduces recurrent myocardial infarction and repeat revascularisation compared with infarct-related artery-only revascularisation and staged CR, regardless of the type of guidance. These findings suggest that immediate angiography- or functionally guided CR should be pursued, when feasible, to improve long-term outcomes in this population.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
The study was supported by the Department of General Surgery and Surgical-Medical Specialties of the University of Catania, Catania, Italy.
Conflict of interest statement
The authors have no conflicts of interest relevant to the contents of this paper to declare. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; reports speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens, and Amgen; and research grants paid to his institution from Boston Scientific and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

