Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Comparative data between self-expanding Navitor (NAV) and balloon-expandable SAPIEN 3 Ultra (ULTRA) transcatheter heart valves (THVs) in patients with small aortic annuli are lacking.
Aims: This study sought to evaluate outcomes of transcatheter aortic valve implantation (TAVI) using the intra-annular NAV and the ULTRA THVs in severe aortic stenosis patients with small annuli.
Methods: Patients with an aortic annulus area ≤430 mm2 undergoing TAVI with either NAV or ULTRA from the NAVULTRA registry were included. Propensity-matched analysis was performed for adjustment. Primary endpoints included 1-year mortality, a composite endpoint (all-cause mortality, disabling stroke, or heart failure hospitalisation), and 30-day device-oriented outcomes (severe prosthesis-patient mismatch, moderate or greater paravalvular leak [PVL], mean gradient ≥20 mmHg).
Results: Among 1,617 patients, 524 propensity score-matched pairs were analysed. At 1 year, all-cause mortality was 8.8% with NAV versus 9.0% with ULTRA (adjusted p=0.585), and the composite endpoint occurred in 11.3% versus 11.8%, respectively (adjusted p=0.149). The device-oriented endpoint favoured NAV compared to ULTRA (6.0% vs 29.3%; adjusted p<0.01), with a lower residual transvalvular gradient (7.3 mmHg vs 12.7 mmHg; adjusted p<0.01), and reduced incidence of any prosthesis-patient mismatch (odds ratio 0.27, 95% confidence interval: 0.18-0.43; adjusted p<0.01). However, NAV was associated with higher rates of mild paravalvular leak (NAV 33.5% vs ULTRA 23.2%; adjusted p<0.05) and permanent pacemaker implantation (PPI; NAV 20.1% vs 11.9% ULTRA; adjusted p<0.01).
Conclusions: In patients with small aortic annuli, TAVI with both NAV and ULTRA provided comparable 1-year clinical outcomes, but NAV showed better haemodynamic performance at the cost of higher rates of mild PVL and PPI.
Over the past several years, transcatheter aortic valve implantation (TAVI) has become the standard treatment for elderly patients with severe aortic stenosis across a wide spectrum of surgical risk1. Different types of transcatheter heart valves (THVs) are now available, with supra-annular self-expanding (SE) valves demonstrating superior haemodynamic performance compared to balloon-expandable (BE) valves, possibly due to the supra-annular positioning of their leaflets23. These haemodynamic advantages are particularly important for patients with small annuli, who are at higher risk of residual elevated gradients, prosthesis-patient mismatch, and reduced exercise capacity45. The randomised SMART trial (Small Annuli Randomized to Evolut or SAPIEN Trial)6 recently confirmed the superior haemodynamic performance of supra-annular self-expanding valves compared with intra-annular balloon-expandable valves in small annuli. However, data on the performance of intra-annular self-expanding valves in this population are scarce78. The aim of this study was therefore to evaluate, in real-world practice, the clinical outcomes and valve performance at 30 days and 1 year of the intra-annular self-expanding Navitor (NAV; Abbott) THV compared with the intra-annular balloon-expandable SAPIEN 3 Ultra (ULTRA; Edwards Lifesciences) THV in patients with small aortic valve (AV) anatomy.
Methods
Study population
NAVULTRA is a multicentre, observational, investigator-initiated registry that enrolled consecutive patients with symptomatic severe aortic stenosis (AS) who underwent transfemoral TAVI using SE Navitor and BE SAPIEN 3 Ultra THVs at 16 high-volume centres across Europe and the United States. Details of the registry have been previously reported9. The present analysis included consecutive patients with an aortic valve annulus area of 430 mm2 or less as determined on the pre-TAVI computed tomography (CT) scan. For the purposes of the present study, patients with a previous surgical aortic valve replacement, incomplete follow-up, missing THV identification (ID), or incomplete CT data were excluded (Figure 1). The study was approved by the local ethics committee of the coordinating institution and was conducted in accordance with the Declaration of Helsinki.
Figure 1. Study flowchart. Study flowchart showing the derivation of unmatched and propensity-matched patient cohorts with small aortic annuli from the NAVULTRA registry. AS: aortic stenosis; CT: computed tomography; ID: identification; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Definitions and study outcomes
A small aortic valve annulus was defined as an aortic valve area of 430 mm2 or less as measured on computed tomography. The device-oriented endpoint was defined as haemodynamic structural valve dysfunction (HSVD) if the mean gradient was ≥20 mmHg or non-structural valve dysfunction (NSVD) if there was a severe prosthesis-patient mismatch (PPM) according to Valve Academic Research Consortium 3 (VARC-3) guidelines or the presence of moderate to severe paravalvular leak (PVL). The primary outcomes of this analysis were the rate of all-cause mortality, the composite of all-cause death, disabling stroke, and repeat hospitalisation for heart failure at 1 year, as well as the composite device-oriented endpoint of HSVD and NSVD. Secondary outcomes of interest were technical success, 30-day device success, and 30-day early safety. All clinical outcomes, procedural complications, and PPM were defined according to VARC-3 criteria10.
Statistical analysis
All continuous variables are expressed as the mean±standard deviation (SD) and compared using the unpaired Student’s t-test. All categorical variables were compared using the chi-square test or Fisher’s exact test. Missing baseline covariates were estimated using the multiple imputation by chained equations method (n=5)11. The propensity score (PS) was used to adjust for differences in baseline characteristics and potential confounders that may lead to biased estimates of treatment outcomes. A 1-to-1 nearest-neighbour matching algorithm without replacement (calliper=0.2) was performed to identify PS-matched pairs. This was done by means of a non-parsimonious multivariable logistic regression model including the following 38 covariates: age, sex, body mass index, hypertension, Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score, New York Heart Association Functional Class III or IV, diabetes, chronic obstructive pulmonary disease, severe liver disease, atrial fibrillation, peripheral vascular disease, prior stroke, coronary artery disease, prior myocardial infarction, prior percutaneous coronary intervention, previous coronary artery bypass graft, other previous cardiac surgery, estimated glomerular filtration rate, dialysis, porcelain aorta, prior permanent pacemaker implantation (PPI), baseline left bundle branch block, baseline right bundle branch block, baseline first-degree atrioventricular block, left ventricular ejection fraction, transaortic maximum gradient, transaortic mean gradient, aortic valve area, moderate to severe mitral regurgitation, moderate to severe tricuspid regurgitation, moderate to severe aortic regurgitation, severe pulmonary hypertension, anaesthesia type, aortic valve perimeter, sinus of Valsalva mean diameter, eccentric annulus index, left ventricular outflow tract (LVOT), and aortic valve calcium distribution at the pre-TAVI CT. Matching was performed within each imputed dataset using the observed and imputed covariate values. The balance in the matched datasets was assessed by computing the standardised mean difference for each covariate. Finally, the treatment effects estimated in each of the matched datasets were pooled together using Rubin’s rules12.
Prespecified primary and secondary outcomes were compared between the NAV and ULTRA valve groups in both the overall and PS-matched cohorts. The risk of adverse events 1 year after TAVI was compared for both cohorts using Cox proportional hazards regression and Kaplan-Meier analysis. The impact of the competing risk of death on disabling stroke incidence and heart failure (HF) rehospitalisation rates was assessed using cumulative incidence function analysis.
Interaction p-values between valve type and annulus size for clinical and echocardiographic outcomes were also calculated.
Statistical analysis was performed using R version 4.2.0 (R Foundation for Statistical Computing) and SPSS Statistics version 25 for Macintosh (IBM). Propensity score and matching procedures were conducted using the MatchThem package in R12.
Results
Study population and baseline characteristics
A total of 4,878 patients who underwent transfemoral TAVI were included in the NAVULTRA registry between November 2018 and April 2024; 1,617 patients with small annuli met the inclusion criteria and were analysed in the present study. Among these, 787 patients underwent TAVI with NAV and 830 with ULTRA (Figure 1). The overall cohort was predominantly female (75.4%), with a mean age of 80.7 years and a mean STS-PROM score of 4.5%. The mean±SD aortic annulus area was 377±38 mm2. Baseline characteristics of the unmatched population are reported in Table 1 and Supplementary Table 1.
From the entire cohort, a 1-to-1 propensity score-matching analysis based on clinical and anatomical characteristics and anaesthesia type resulted in 524 matched pairs. There were no significant differences in baseline characteristics between the propensity score-matched NAV and ULTRA groups, including the mean aortic annular area, the degree of AV and LVOT calcification (Supplementary Figure 1).
Table 1. Baseline characteristics of registry patients before PS matching.
| Missing data,% | Overall (n=1,617) | NAV (n=787) | ULTRA (n=830) | p-value | |
|---|---|---|---|---|---|
| Age, years | - | 80.7±6.7 | 81.0±6.0 | 80.0±7.3 | <0.01 |
| Female, n | - | 1,219 (75.4) | 635 (80.7) | 584 (70.4) | <0.01 |
| Body mass index, kg/m2 | 1.4 | 26.80±5.22 | 26.20±4.58 | 27.36±5.70 | <0.01 |
| Body surface area, m2 | 1.4 | 1.74±0.20 | 1.73±0.18 | 1.76±0.22 | <0.01 |
| STS-PROM score | 25.3 | 4.55±3.29 | 4.98±3.54 | 4.34±3.14 | 0.01 |
| NYHA Class III or IV | 2.8 | 873 (55.5) | 358 (46.0) | 515 (65.0) | <0.01 |
| Hypertension | - | 1,294 (80.0) | 638 (81.0) | 656 (79.1) | 0.330 |
| Diabetes mellitus | - | 530 (32.8) | 239 (30.3) | 291 (35.1) | 0.04 |
| COPD | 0.1 | 233 (14.4) | 126 (16.0) | 107 (12.9) | 0.076 |
| Severe liver disease | 1.7 | 22 (1.4) | 8 (1.0) | 14 (1.7) | 0.235 |
| Porcelain aorta | 7.1 | 38 (2.0) | 19 (2.8) | 19 (2.3) | 0.506 |
| Atrial fibrillation | - | 312 (19.2) | 124 (15.7) | 188 (22.6) | <0.01 |
| Prior PCI | 1.6 | 299 (18.8) | 158 (20.0) | 141 (17.6) | 0.199 |
| Peripheral vascular disease | 0.5 | 180 (11.2) | 91 (11.6) | 89 (10.7) | 0.566 |
| Previous stroke | - | 121 (7.5) | 60 (7.6) | 61 (7.3) | 0.834 |
| CAD | 0.1 | 569 (35.2) | 244 (31.0) | 325 (39.2) | <0.01 |
| Prior MI | 0.1 | 200 (12.4) | 85 (10.8) | 115 (13.8) | 0.06 |
| Prior CABG | 0.1 | 68 (4.2) | 23 (2.9) | 45 (5.4) | 0.01 |
| Other prior cardiac surgery | 7.9 | 41 (2.7) | 16 (2.1) | 25 (3.4) | 0.145 |
| Dialysis | - | 30 (1.8) | 13 (1.6) | 17 (2.0) | 0.551 |
| eGFR <30 mL/min/1.73m2 | 2.8 | 151 (9.6) | 58 (7.4) | 93 (11.8) | 0.03 |
| eGFR, mL/min/1.73m2 | 2.8 | 58.72±22.81 | 60.50±22.73 | 56.94±22.76 | <0.01 |
| Haemoglobin, g/dL | 5.4 | 12.00±2.62 | 12.16±1.71 | 11.85±3.30 | 0.02 |
| Severe pulmonary hypertension | 22.5 | 119 (9.5) | 61 (9.6) | 58 (9.9) | 0.657 |
| Previous pacemaker | - | 128 (7.9) | 84 (10.7) | 44 (5.3) | <0.01 |
| Values are n, n (%), or mean±standard deviation. CABG: coronary artery bypass graft; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; MI: myocardial infarction; NAV: Navitor; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PS: propensity score; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; ULTRA: SAPIEN 3 Ultra | |||||
Procedural details, in-hospital and 30-day outcomes
Procedural characteristics and in-hospital outcomes for the unadjusted and PS-matched populations are presented in Table 2, Supplementary Table 2, Supplementary Table 3, Supplementary Figure 2, and Supplementary Figure 3. In the PS-matched population, both predilatation and post-dilatation were more frequently performed with NAV compared with ULTRA (predilatation: odds ratio [OR] 17.32, 95% confidence interval [CI]: 10.98-27.31; p<0.01; post-dilatation: OR 3.09, 95% CI: 2.06-4.62; p<0.01). Procedural complications were rare with no significant differences between the two groups. The incidence of new left bundle branch block (OR 1.73, 95% CI: 1.18-2.56; p<0.01) and new permanent pacemaker implantation (OR 2.14, 95% CI: 1.40-3.25; p<0.01) were significantly higher in NAV recipients compared to those receiving ULTRA in both the unmatched and matched populations.
At 30 days, there were no significant differences between patients treated with the BE and SE valves in terms of all-cause mortality, disabling or non-disabling stroke, or rehospitalisation for heart failure. However, the incidence of new PPI was significantly higher in the SE group (Supplementary Table 4).
Table 2. Procedural and in-hospital outcomes of unadjusted and propensity-matched cohorts.
| NAV (n=787) | ULTRA (n=830) | Unadjusted | Propensity-matched | |||
|---|---|---|---|---|---|---|
| Mean change/OR (95% CI) | p-value | Mean change/OR (95% CI) | p-value | |||
| General anaesthesia | 47 (6.0) | 130 (15.7) | 0.34 (0.24-0.48) | <0.01 | 0.96 (0.58-1.49) | 0.872 |
| Predilatation | 592/747 (79.2) | 156/740 (21) | 14.30 (11.17-18.41) | <0.01 | 17.32 (10.98-27.31) | <0.01 |
| Post-dilatation | 210/746 (28.1) | 81/740 (10.9) | 3.19 (2.42-4.24) | <0.01 | 3.09 (2.06-4.62) | <0.01 |
| Contrast dye, mL | 134±77 | 136±81 | –2.23 (–11.33 to 6.77) | 0.622 | –4.10 (–14.19 to 5.99) | 0.425 |
| In-hospital death | 3 (0.3) | 8 (0.9) | 0.30 (0.08-1.36) | 0.169 | 1.28 (0.08-21.07) | 0.858 |
| Cardiac tamponade | 2 (0.2) | 4 (0.5) | 0.71 (0.10-3.63) | 0.689 | 0.61 (0.5-7.64) | 0.690 |
| Conversion to open-heart surgery | 1 (0.1) | 4 (0.5) | 0.26 (0.01-1.78) | 0.232 | 0.46 (0.04-5.17) | 0.528 |
| Second THV implanted | 8 (1.0) | 8 (0.9) | 1.05 (0.39-2.88) | 0.915 | 0.80 (0.22-2.91) | 0.739 |
| Major vascular complications | 6 (0.8) | 12 (1.4) | 0.52 (0.18-1.35) | 0.198 | 0.74 (0.17-1.74) | 0.683 |
| Major bleeding (type 2) | 3 (0.4) | 15 (1.8) | 0.21 (0.05-0.63) | 0.01 | 0.47 (0.10-2.20) | 0.340 |
| New pacemaker | 138 (17.5) | 76 (9.1) | 2.10 (1.56-2.85) | <0.01 | 2.14 (1.40-3.25) | <0.01 |
| New onset of AF | 13 (1.6) | 10 (1.2) | 1.37 (0.60-3.24) | 0.450 | 1.40 (0.44-4.52) | 0.565 |
| New LBBB | 143/555 (25.8) | 124/813 (15.2) | 1.92 (1.47-2.52) | <0.01 | 1.73 (1.18-2.56) | <0.01 |
| New dialysis | 3 (0.4) | 4 (0.5) | 0.790 (0.15-3.59) | 0.758 | 0.85 (0.03-22.46) | 0.919 |
| VARC-3 technical success | 745 (94.7) | 796 (95.9) | 0.76 (0.47-1.20) | 0.240 | 0.65 (0.31-1.37) | 0.245 |
| LOS, days | 4.1±4.9 | 3.8±6.7 | 0.33 (–0.25 to 0.91)* | 0.265 | 0.66 (–0.10 to 1.43)* | 0.09 |
| Values are n (%), n/N (%), or mean±standard deviation, unless otherwise indicated. *Indicates mean change. AF: atrial fibrillation; CI: confidence interval; LBBB: left bundle branch block; LOS: length of stay; NAV: Navitor; OR: odds ratio; THV: transcatheter heart valve; ULTRA: SAPIEN 3 Ultra; VARC-3: Valve Academic Research Consortium 3 | ||||||
Study endpoints
The study outcomes of both unadjusted and propensity score-matched populations are presented in Table 3. The rate of the coprimary composite endpoint of death from any cause, disabling stroke, or HF rehospitalisation at 1 year after the procedure was similar between the two groups (11.3% NAV vs 11.8% ULTRA; p=0.463) (Central illustration). The estimates for each component of the clinical coprimary endpoint in the SE NAV and the BE ULTRA groups were as follows: the rates of death from any cause were 8.8% in patients receiving an SE NAV and 9.0% in those with a BE ULTRA (p=0.449); the rates of disabling stroke were 1.3% for NAV and 1.6% for ULTRA (p=0.963); rehospitalisation for heart failure rates were, respectively, 3.9% and 3.0% (p=0.122) (Supplementary Figure 4). These findings were consistent after accounting for the competing risk of all-cause death. The rate of a repeat procedure at 1 year was low and comparable between NAV and ULTRA groups, with only 1 and 2 cases, respectively.
The propensity-matched analysis confirmed that there were no significant differences in the rates of any death (hazard ratio [HR] 1.36, 95% CI: 0.89-2.08; p=0.152), cardiac death (HR 1.17, 95% CI: 0.70-1.98; p=0.543), disabling stroke (HR 1.20, 95% CI: 0.37-3.90; p=0.755), non-disabling stroke (HR 1.03, 95% CI: 0.33-3.21; p=0.961) or HF hospitalisation (HR 1.69, 95% CI: 0.84-3.38; p=0.137). However, the rate of new PPI at 1 year (HR 1.97, 95% CI: 1.36-2.85; p<0.01) was significantly higher in the NAV group compared with the ULTRA group in both unmatched and matched populations (Table 3).
In the unadjusted population, the composite device-oriented endpoint (Table 3, Central illustration) occurred more frequently with the BE ULTRA (29.3%) than with SE NAV (6.0%; OR 0.15, 95% CI: 0.08-0.26; p<0.01). The rate of HSVD at 30 days was 0.6% with NAV and 10.4% with ULTRA (p<0.01). Similarly, NSVD was higher in the ULTRA group (4.4% NAV vs 19.6% ULTRA; p<0.01) (Figure 2). The SE NAV yielded lower mean postprocedural aortic valve gradients than ULTRA (7.35 mmHg vs 12.71 mmHg, respectively; p<0.01) and larger effective orifice areas (EOAs; 2.09 cm2 vs 1.64 cm2; p<0.01). These differences corresponded to a significantly lower incidence of moderate PPM (NAV 11.9% vs ULTRA 30.8%; p<0.01) and severe PPM (NAV 2.5% vs ULTRA 18.8%; p<0.01) at 30 days in the NAV group. However, ULTRA more frequently achieved none/trace PVL compared to NAV (OR 0.63, 95% CI: 0.44-0.90; p=0.01), whereas the rate of mild PVL was higher in the NAV group (OR 1.63, 95% CI: 1.14-2.38; p<0.01) (Figure 3).
In the propensity-matched analysis (Table 3), the SE NAV confirmed having more favourable haemodynamic performance at 30 days (device-oriented endpoint: OR 0.34, 95% CI: 0.18-0.63; p<0.01) with lower residual mean gradients (mean difference: –5.03, 95% CI: –5.73 to 0.435; p<0.01), a larger effective orifice area (mean difference: 0.37, 95% CI: 0.24-0.50; p<0.01) and a lower incidence of any PPM, including moderate and severe (moderate: OR 0.45, 95% CI: 0.25-0.78; p<0.05; severe: OR 0.38, 95% CI: 0.18-0.80; p<0.05). The ULTRA remained associated with a lower incidence of PVL, both none/trace and mild (none/trace: OR 0.66, 95% CI: 0.50-0.94; p<0.05; mild: OR 1.56, 95% CI: 1.01-2.39; p<0.05) (Figure 3, Supplementary Table 5). These results were consistent at 1 year post-procedure (Supplementary Table 6).
Among the secondary outcomes (Figure 4, Table 3), the rate of technical success was high and comparable between the two groups (94.7% for NAV vs 95.9% for ULTRA; p=0.240). The device success rate was also high in both groups, with a statistically significant difference favouring the NAV group (92.9% for NAV vs 84.7% for ULTRA; p<0.01). However, the rate of the early safety endpoint was significantly higher with the ULTRA THV (82.6%) compared to the NAV THV (75.6%; OR 0.65, 95% CI: 0.51-0.83; p<0.01).
Table 3. Study outcomes/endpoints of unadjusted and propensity-matched cohorts.
| Unadjusted | Propensity-matched | |||||
|---|---|---|---|---|---|---|
| NAV | ULTRA | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Primary clinical endpoints | n=787 | n=830 | ||||
| All-cause death | 50 (8.8) | 54 (9.0) | 1.13 (0.81-1.57) | 0.449 | 1.36 (0.89-2.08) | 0.152 |
| Composite endpoint | 66 (11.3) | 72 (11.8) | 1.11 (0.83-1.49) | 0.463 | 1.33 (0.90-1.98) | 0.149 |
| Primary echocardiography endpoint | n=249 | n=470 | ||||
| Device-oriented endpoint at 30 days | 15 (6.0) | 138 (29.3) | 0.15 (0.08-0.26) | <0.01 | 0.34 (0.18-0.63) | <0.01 |
| Secondary endpoints | ||||||
| 30-day HSVD* | 4 (0.6) | 69 (10.4) | 0.05 (0.02-0.13) | <0.01 | 0.11 (0.03-0.35) | <0.01 |
| 30-day NSVD* | 11 (4.4) | 87 (19.6) | 0.19 (0.09-0.35) | <0.01 | 0.33 (0.21-0.52) | <0.01 |
| 30-day moderate PPM** | 29 (11.9) | 136 (30.8) | 0.30 (0.19-0.45) | <0.01 | 0.45 (0.25-0.78) | 0.01 |
| 30-day severe PPM** | 6 (2.5) | 83 (18.8) | 0.08 (0.02-0.21) | <0.01 | 0.38 (0.18-0.80) | 0.02 |
| 30-day any PPM** | 35 (14.4) | 219 (49.6) | 0.17 (0.11-0.25) | <0.01 | 0.28 (0.18-0.43) | <0.01 |
| VARC-3 technical success | 745 (94.7) | 796 (95.9) | 0.76 (0.47-1.20) | 0.240 | 0.64 (0.30-1.37) | 0.245 |
| VARC-3 device success | 731 (92.9) | 703 (84.7) | 2.36 (1.70-3.30) | <0.01 | 1.88 (1.23-2.88) | <0.01 |
| VARC-3 early safety | 595 (75.6) | 686 (82.6) | 0.65 (0.51-0.83) | <0.01 | 0.61 (0.44-0.83) | <0.01 |
| At 1 year | ||||||
| Cardiac death | 31 (5.5) | 35 (5.7) | 0.95 (0.63-1.44) | 0.820 | 1.17 (0.70-1.98) | 0.543 |
| Disabling stroke | 9 (1.3) | 11 (1.6) | 1.02 (0.45-2.32) | 0.963 | 1.20 (0.37-3.90) | 0.755 |
| Non-disabling stroke | 8 (1.1) | 6 (0.8) | 1.22 (0.44-3.38) | 0.694 | 1.03 (0.33-3.21) | 0.961 |
| Hospitalisation for HF | 23 (3.9) | 17 (3.0) | 1.54 (0.89-2.67) | 0.122 | 1.69 (0.84-3.38) | 0.137 |
| New PPI | 152 (20.1) | 90 (11.2) | 1.88 (1.45-2.44) | <0.01 | 1.97 (1.36-2.85) | <0.01 |
| Values are n (%) unless otherwise indicated. Clinical outcomes are reported as Kaplan-Meier estimates at the specific timepoint. *Echo data were available for 641 patients with NAV and 662 with ULTRA. **Echo data were available for 243 with NAV and 444 with ULTRA. CI: confidence interval; HF: heart failure; HR: hazard ratio; HSVD: haemodynamic structural valve dysfunction; NAV: Navitor; NSVD: non-structural valve dysfunction; OR: odds ratio; PPI: permanent pacemaker implantation; PPM: prosthesis-patient mismatch; ULTRA: SAPIEN 3 Ultra; VARC-3: Valve Academic Research Consortium 3 | ||||||
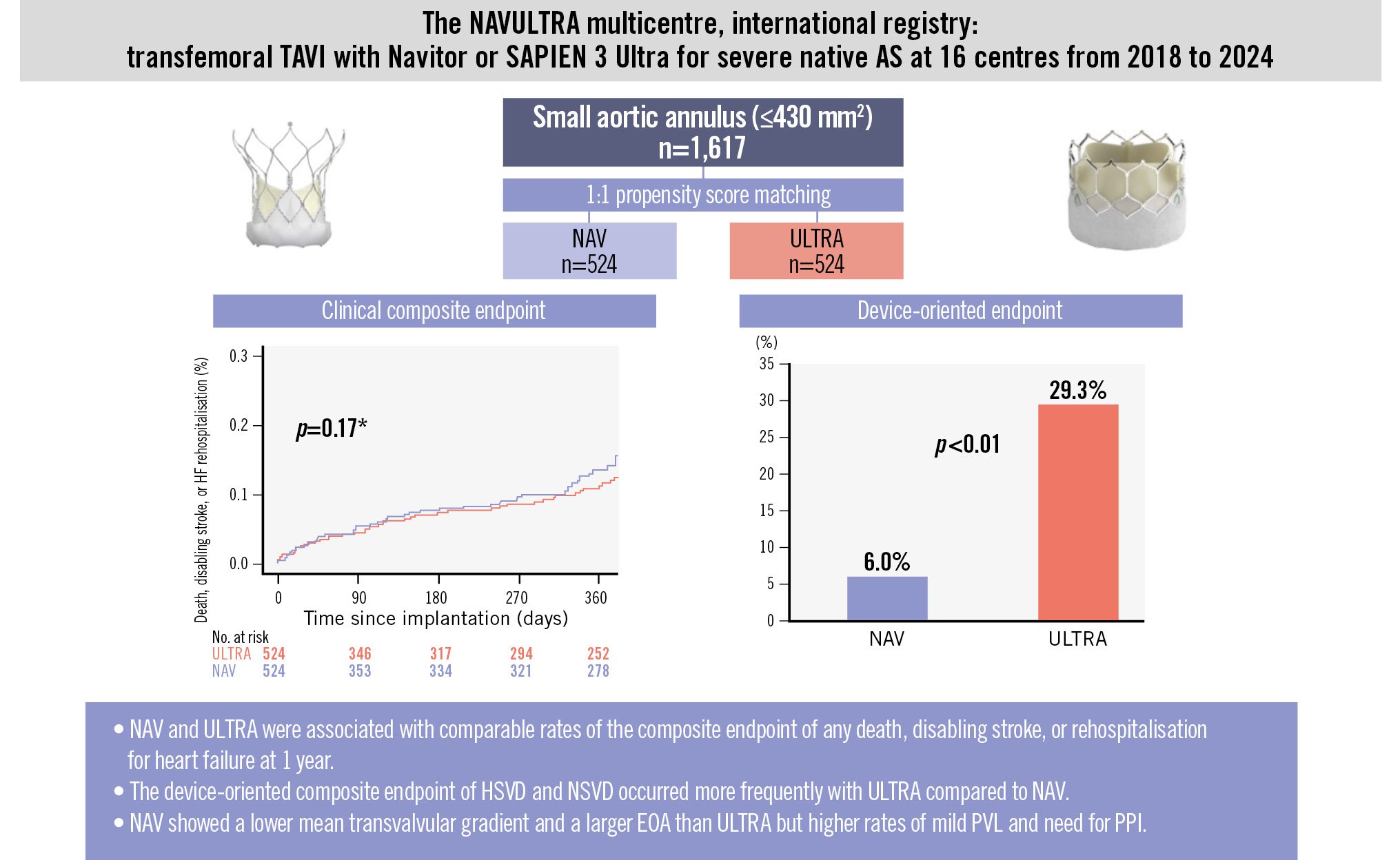
Central illustration. Primary outcomes of TAVI with Navitor or SAPIEN 3 Ultra in patients with small aortic annuli. Kaplan-Meier curves show the clinical composite endpoint at 1 year, and the device-oriented composite endpoint is presented in a bar chart. *The Kaplan-Meier curves in the figure are derived from a single imputed dataset and should be considered representative of the main results presented in the paper. AS: aortic stenosis; EOA: effective orifice area; HF: heart failure; HSVD: haemodynamic structural valve dysfunction; NAV: Navitor; NSVD: non-structural valve dysfunction; PPI: permanent pacemaker implantation; PVL: paravalvular leak; TAVI: transcatheter aortic valve implantation; ULTRA: SAPIEN 3 Ultra
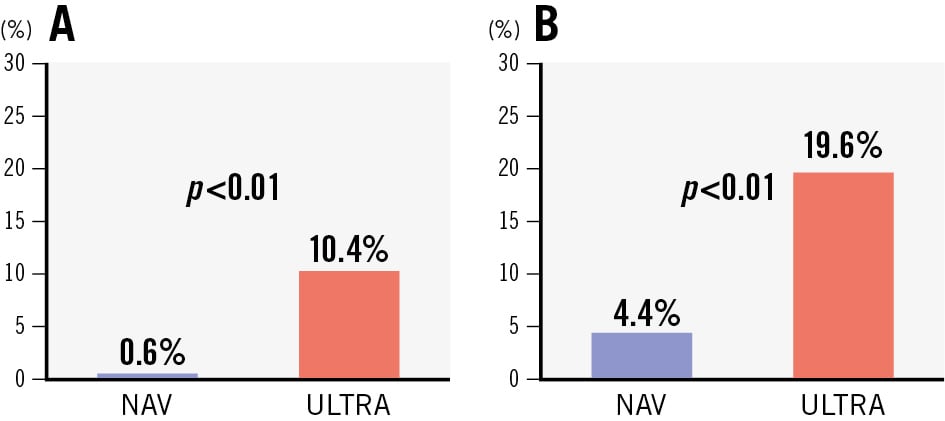
Figure 2. Components of the device-oriented composite endpoint. The components of the device-oriented composite endpoint at 30 days post-procedure. A) Haemodynamic structural valve dysfunction (MG ≥20 mmHg). B) Non-structural valve dysfunction (severe PPM or PVL ≥2). MG: mean gradient; NAV: Navitor; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; ULTRA: SAPIEN 3 Ultra
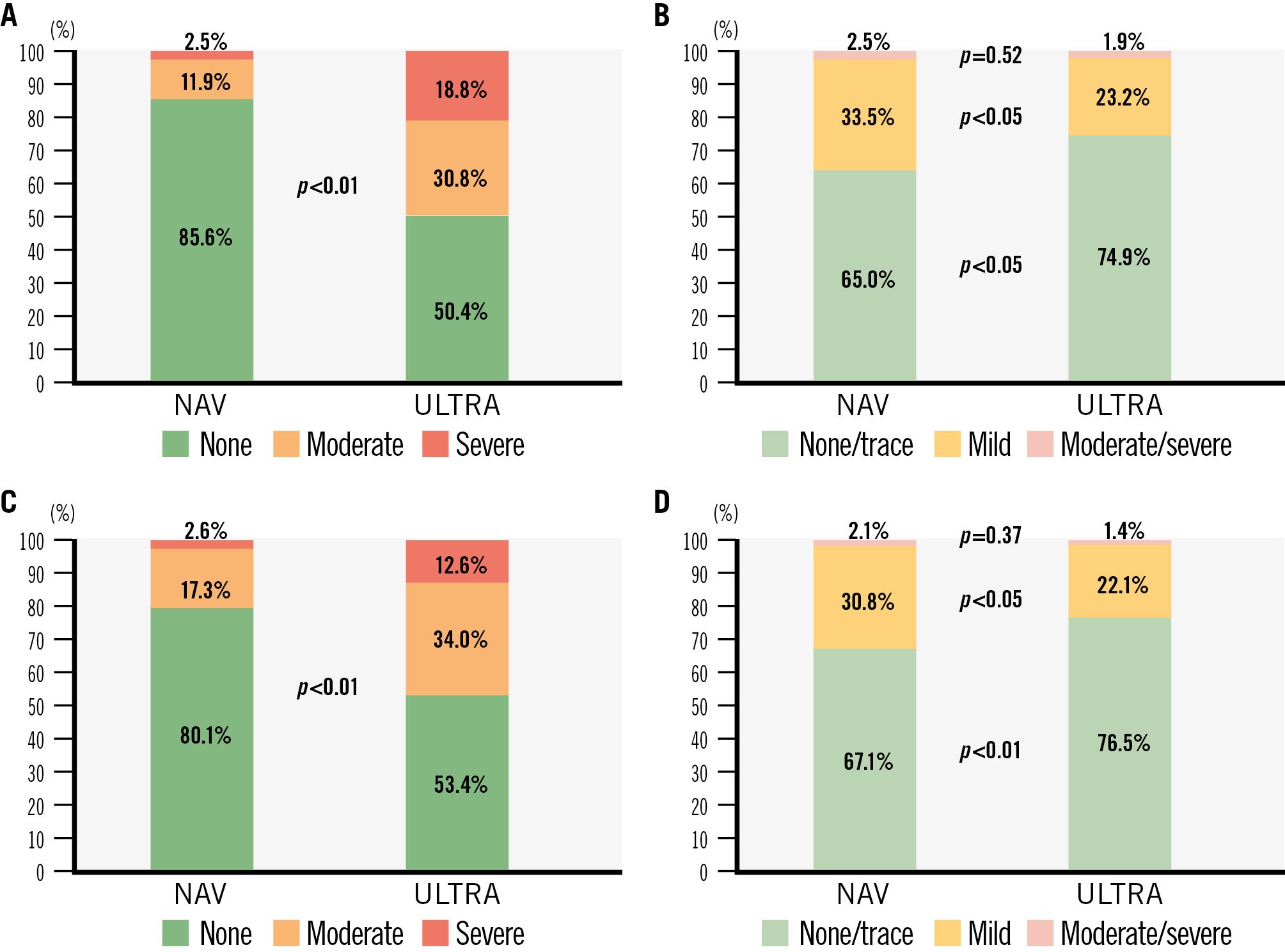
Figure 3. Prosthesis-patient mismatch and paravalvular leak with Navitor and SAPIEN 3 Ultra in small aortic annuli. The bar charts represent the rates of prosthesis-patient mismatch and paravalvular leak at 30 days and 1 year in patients with small annuli undergoing TAVI with NAV and ULTRA: (A) prosthesis-patient mismatch at 30 days; (B) paravalvular leak at 30 days; (C) prosthesis-patient mismatch at 1 year; (D) paravalvular leak at 1 year. Echocardiographic data missing at 1 year were imputed using the last observation carried forward method. NAV: Navitor; TAVI: transcatheter aortic valve implantation; ULTRA: SAPIEN 3 Ultra
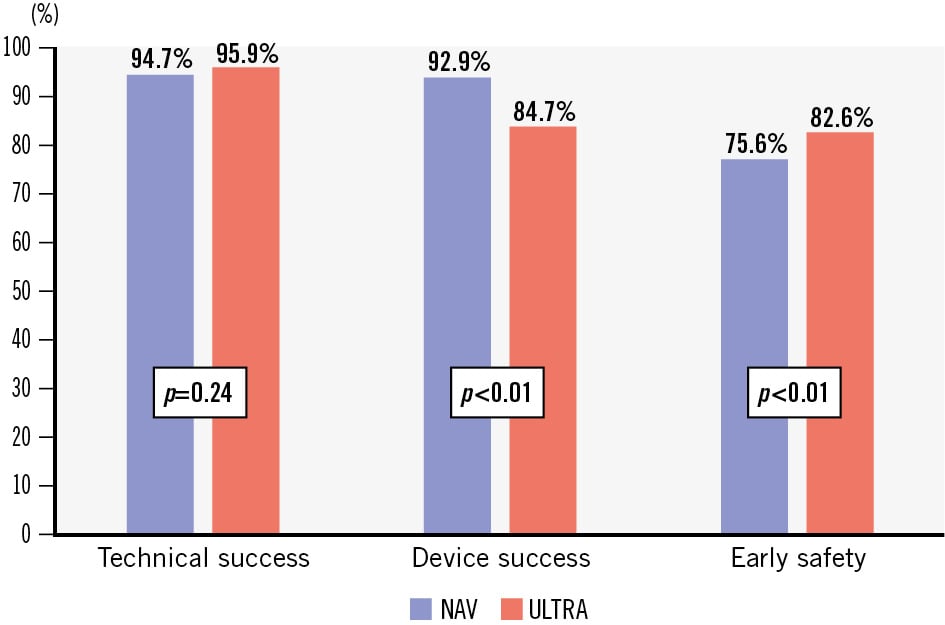
Figure 4. Early VARC-3 endpoints comparing intra-annular self-expanding versus balloon-expandable transcatheter heart valves in small annuli. Comparison of VARC-3 early composite endpoints between intra-annular NAV and ULTRA devices in small annuli. NAV: Navitor; ULTRA: SAPIEN 3 Ultra; VARC-3: Valve Academic Research Consortium-3
Interaction analyses
In the extended cohort, which also included patients with larger annuli (>430 mm2), clinical and haemodynamic performance of the two devices was similar for both large and small annuli (all interaction p-values>0.05).
Discussion
The main findings of the present analysis comparing intra-annular SE Navitor and BE SAPIEN 3 Ultra THVs in an unselected real-world population with small annuli are as follows: (1) there were no significant differences between the SE and BE THVs in the rate of all-cause mortality or in the composite endpoint of death, disabling stroke, and repeat hospitalisation for heart failure at 1 year; (2) the SE device was superior to the BE platform with respect to the device-oriented composite endpoint of HSVD and NSVD; (3) the SE device demonstrated lower incidences of HSVD, NSVD, and any prosthesis-patient mismatch at 30 days owing to a lower mean residual transvalvular gradient and a larger EOA than with the BE device; (4) the VARC-3 technical success rate was achieved in >90% of patients for both devices, with no significant difference between groups; (5) the BE device had a lower rate of VARC-3 device success, mainly due to a higher residual mean transprosthetic gradient; (6) the BE device was associated with a lower rate of PPI at 1 year and less occurrence of any PVL.
Patients with small annuli represent a challenging subset of aortic stenosis patients as they are at higher risk of residual elevated gradients and prosthesis-patient mismatch. These haemodynamic considerations may also have implications for clinical outcomes and valve durability1314.
In the present analysis from the unselected, real-world NAVULTRA registry, the rates of all-cause mortality and the composite endpoint at 1 year were similar between patients with small aortic annuli undergoing TAVI with intra-annular NAV and ULTRA THVs. Similarly, no significant differences were observed in the incidence of cardiac death, any stroke, disabling stroke, or repeat procedures between the two groups at 1 year. However, the rate of new PPI at 1 year was lower in the ULTRA group.
The SE NAV, despite its intra-annular design – which is often considered haemodynamically less favourable, particularly in patients with small aortic annuli – demonstrated superior haemodynamic performance compared with the intra-annular BE ULTRA due to the significantly lower rate of patients with mean residual transvalvular gradients ≥20 mmHg and less incidence of moderate or severe PPM. These outcomes are comparable to those reported for supra-annular self-expanding devices151617.
The clinical relevance of elevated residual gradients and moderate to severe PPM in patients with small aortic annuli undergoing TAVI remains a subject of debate. Data from the FRANCE-2 registry and the National Echo Database Australia demonstrated increased mortality at both 1 and 5 years among patients with persistently elevated transprosthetic gradients1819. Previous studies have also shown increased risks of mortality and heart failure hospitalisation in patients with moderate to severe PPM following surgical aortic valve replacement and TAVI, particularly in those with severe PPM52021. Conversely, other investigations have reported no significant association between severe PPM and clinical outcomes142223. Few prospective, randomised studies comparing THV platforms have demonstrated superior haemodynamic performance of supra-annular self-expanding valves, yet they show no significant difference in clinical outcomes up to 5 years34. Most recently, the SMART randomised trial also confirmed that although supra-annular self-expanding valves offer improved haemodynamic performance in patients with small annuli, there was no difference in the composite clinical endpoint of death, stroke, and heart failure hospitalisation at 2 years6. This conflicting evidence on the impact of high residual gradients and PPM may reflect differences in study populations, definitions of PPM (measured EOA vs predicted EOA), and the variety of bioprostheses used across studies. Furthermore, echocardiographic assessment of gradients may be influenced by factors such as Doppler misalignment, fluid viscosity, and the pressure recovery phenomenon. Notably, discordance between echocardiographic and invasive measurements for haemodynamic performance of bioprostheses has been shown in several studies2425, with higher transprosthetic gradients and smaller EOAs observed on echocardiography compared to catheter-based assessments.
In our study, the observed differences in residual mean gradients and rates of PPM did not appear to translate into differences in 1-year clinical outcomes between the two THV platforms. Specifically, there were no significant differences in mortality, heart failure rehospitalisation, any stroke, or reintervention at 1 year. However, impaired forward haemodynamics may become apparent in long-term outcomes, potentially accelerating bioprosthetic degeneration and the need for reintervention. Extended follow-up is therefore warranted.
In terms of paravalvular leak, the incidence of moderate or greater PVL was very low across both cohorts at 30 days and at 1 year. However, mild PVL was less frequent in patients treated with ULTRA compared to those treated with NAV. While the association between moderate PVL and increased mortality is well established, a recent meta-analysis has also suggested that even mild PVL may negatively affect mortality and rehospitalisation, regardless of the type of THV, although the data remain controversial2627.
Among the secondary outcomes, although VARC-3 technical success rates were high and comparable between groups, VARC-3 device success favoured NAV in our analysis, primarily due to the higher residual transprosthetic gradients observed in the ULTRA group. Conversely, the VARC-3 early safety composite endpoint significantly favoured ULTRA, driven by the higher incidence of new PPI in the NAV group. New PPI remains a concern following TAVI, as it has been associated with adverse clinical outcomes, including increased mortality and HF hospitalisations28.
Of note, regarding in-hospital and 30-day outcomes, the rates of complications – including all-cause mortality, any stroke, annular rupture, or coronary occlusion – were very low for both devices, suggesting that both platforms are safe in patients with small aortic anatomy.
Finally, in the extended cohort, which included patients with larger annuli (>430 mm2), clinical and haemodynamic performance between the two devices remained consistent across annulus sizes, with no significant heterogeneity in treatment effect observed.
This study demonstrated that both intra-annular devices yielded comparable clinical outcomes at 1 year. However, the NAV device showed superior haemodynamic performance, with lower rates of PPM and residual high gradients, albeit at the cost of a higher incidence of mild paravalvular leak and need for PPI. As TAVI continues to expand to younger and lower-risk patient populations, haemodynamic performance becomes increasingly relevant, as it may influence long-term valve durability and the need for reintervention – particularly in patients with small aortic annuli, where reintervention poses technical challenges and is associated with increased procedural risks such as coronary occlusion and sinus of Valsalva sequestration. Nevertheless, treatment decisions must also take into account other key clinical factors, including the risk of PVL, which is known to be associated with increased mortality and rehospitalisation for HF, along with the need for permanent pacemaker implantation, which may adversely affect long-term outcomes28. Therefore, transcatheter heart valve selection in patients with small aortic annuli should not rely solely on early haemodynamic parameters but rather be guided by a comprehensive, patient-specific approach including clinical and anatomical characteristics. This should incorporate life expectancy, body size, anatomical characteristics and calcium burden, risk of PVL and PPI, and the feasibility of future coronary access and repeat TAVI procedures. Further randomised investigations are warranted to compare different THV platforms in this challenging subset of patients with severe aortic stenosis.
Limitations
This study has the inherent limitations of non-randomised, observational, retrospective studies without an independent adjudication of clinical events or an independent core laboratory to assess PVL severity and transprosthetic gradients. Although a propensity-matched approach based on 38 variables was applied to overcome differences in baseline characteristics and potential confounders, residual confounding remains a source of bias that cannot be excluded. Moreover, including a large number of variables may have reduced the number of matched pairs and negatively impacted the precision of the estimates. Selection bias in THV choice should also be acknowledged. It should be recognised that some missing echocardiographic data may have increased the risk of a type II error; however, this appears unlikely given the significant differences observed in the device-oriented endpoint and rate of prosthesis-patient mismatch. Lastly, this analysis is limited to 1-year outcomes, whereas haemodynamic differences may have an impact on longer-term outcomes.
Conclusions
This subanalysis from the NAVULTRA registry demonstrated that, among patients with aortic stenosis and small annuli undergoing TAVI, the NAV and ULTRA devices were comparable with respect to the 1-year composite endpoint of mortality, heart failure rehospitalisation, or disabling stroke. However, the intra-annular NAV was associated with superior haemodynamic performance, showing a reduced risk of prosthesis-patient mismatch and residual high gradients, albeit with a higher rate of mild paravalvular leaks and PPI. These findings warrant further investigation and extended follow-up in dedicated randomised clinical trials directly comparing these intra-annular devices in this challenging patient population.
Impact on daily practice
In this real-world, multicentre study, we found that the two transcatheter aortic valve implantation platforms, Navitor (NAV) and SAPIEN 3 Ultra, were associated with similar 1-year clinical outcomes, but the NAV device showed better haemodynamic performance and a lower incidence of moderate to severe prosthesis-patient mismatch, as well as higher rates of mild paravalvular leak and new permanent pacemaker implantation. Transprosthetic gradients were significantly lower in patients receiving NAV. Randomised clinical trials with longer follow-up are needed to explore the differences between the two devices, aiming for a patient-specific approach to ensure optimised patient outcomes in this challenging population.
Conflict of interest statement
N.M. Van Mieghem has received research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril, Pie Medical Imaging, PulseCath BV, and Teleflex; and is a consultant for Abbott, Abiomed, Alleviant Medical Inc., AnchorValve, Anteris, Approxima Srl, Bolt Medical, Boston Scientific, Daiichi Sankyo, LUMA Vision, Materialise, Medtronic, Pie Medical Imaging, Polares, PulseCath BV, and Siemens. O. De Backer has received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. J. Byrne has served on advisory boards or as a physician proctor for Abbott and Edwards Lifesciences; and has received educational grants from Edwards Lifesciences. M. Barbanti is a consultant for Boston Scientific, Edwards Lifesciences, and Medtronic. L. Nombela-Franco has been a proctor for Abbott and Edwards Lifesciences. F. Maisano has received grants and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, and Venus MedTech; and has received consulting fees, personal and institutional honoraria from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Mtex, Venus MedTech, Squadra Lifesciences, Valgen, and CroiValve; and also has royalty income/IP rights from Edwards Lifesciences; and is a shareholder (including share options) of Magenta, Transseptal Solutions, and 4Tech. N. Buzzatti served as a proctor for Meril; and a consultant for Biosensors. R. Lorusso has received research grants from Medtronic and LivaNova; and speaker fees from Abiomed; and is a member of the medical advisory board of XENIOS and Eurosets; and is a consultant for Medtronic and LivaNova. F. Bedogni is a consultant and proctor for Abbott, Medtronic, Boston Scientific, Meril, and Terumo. C. Tamburino is a consultant for Medtronic. C. Gandolfo is a proctor for Edwards Lifesciences. A. Latib has served on advisory boards or as a consultant for Medtronic, Boston Scientific, Edwards Lifesciences, Abbott, Philips, Tresquare Technologies, and Anteris. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

