Cory:
Unlock Your AI Assistant Now!
The latest European guidelines recommend intracoronary imaging (ICI) guidance by intravascular ultrasound (IVUS) or optical coherence tomography (OCT) for percutaneous coronary intervention (PCI) on anatomically complex lesions, particularly for left main stem, true bifurcations, and long lesions1. However, large-scale randomised studies comparing the efficacy between ICI-guided and angiography-guided PCI in patients with acute coronary syndrome (ACS) remain limited. In patients with non-ST-segment elevation ACS, OCT-guided PCI has been associated with a higher post-PCI fractional flow reserve compared to angiography guidance, providing detailed information on lesion characteristics and stent expansion2. Performing OCT-guided PCI in acute settings poses significant challenges due to the need for blood clearance, which may lead to complications such as slow flow, dissection, and distal embolisation3. We investigated whether OCT offers comparable clinical efficacy and safety to IVUS for patients with and without ACS in an all-comers PCI population.
In this post hoc analysis of the OCTIVUS trial with extended 2-year follow-up, we evaluated the outcomes of OCT versus IVUS guidance for PCI in ACS and non-ACS patients, building upon the initial 1-year non-inferiority findings4. Although ACS patients were prespecified as a subgroup in the trial protocol and statistical analysis plan, randomisation was not stratified by ACS status. The exclusion criteria of the OCTIVUS trial were ST-segment elevation myocardial infarction, severe renal dysfunction, unstable haemodynamics, decompensated heart failure, lesions preventing ICI catheter delivery, and unsafe assignment to either arm. The protocol mandated a final post-stenting evaluation for PCI optimisation based on predetermined criteria4. The primary outcome of this analysis was 2-year target vessel failure (TVF), defined as a composite of death from cardiac causes, target vessel-related myocardial infarction, or ischaemia-driven target vessel revascularisation. Contrast-induced nephropathy (CIN) was defined as a serum creatinine increase of ≥25% or an absolute increase of 0.5 mg/dL, based on routine measurements obtained within 72 h following the index PCI. Cumulative incidences were analysed using Kaplan-Meier estimates and log-rank tests, with hazard ratios (HR) calculated using Cox proportional hazards models.
Of the 2,008 patients in the OCTIVUS trial, 470 (23.4%) underwent ICI-guided PCI for non-ST-segment elevation ACS, and 1,538 (76.6%) for no ACS. Complex lesions such as left main disease, bifurcations, and diffuse long lesions were less frequent in ACS patients compared to non-ACS patients. Among ACS patients, the mean age was 64±12 years, 79.1% were male, and 29.8% had diabetes. The demographic and clinical characteristics of the OCT- and IVUS-guided PCI groups were similar. Left main disease was less frequent in the OCT group than in the IVUS group among ACS patients (6.5% vs 13.8%; p=0.01). Notably, significantly more contrast media was used in the OCT group than in the IVUS group in both ACS (255±120 mL vs 204±112 mL; p<0.01) and non-ACS patients (233±109 mL vs 200±110 mL; p<0.01). The rates of CIN (percentages) were similar between the OCT and IVUS groups but significantly higher in ACS patients compared to non-ACS patients (3.0% vs 1.0%; p<0.01). During follow-up (median 2.0, range 1-4.8 years), TVF was significantly higher in ACS patients than in non-ACS patients (9.2% vs 5.7%; p=0.03) (Central illustration), with comparable treatment effects between OCT and IVUS (ACS: HR 0.96, 95% confidence interval [CI]: 0.47-1.94; non-ACS: HR 0.82, 95% CI: 0.51-1.30;; p for interaction=0.71). The ICI-guided PCI optimisation rates were similar between the OCT (48.6%) and IVUS (52.3%) groups in ACS patients (p=0.49); however, in non-ACS patients, the IVUS group showed significantly higher optimisation rates than the OCT group (55.5% vs 48.2%; p=0.01).
While OCT offers superior resolution compared to IVUS for detecting plaque rupture, erosion, thrombus formation, and post-stenting complications including edge dissection and malapposition, its clinical adoption remains limited by procedural complexity, higher costs, and contrast-related risks of ischaemia and renal dysfunction. The ILUMIEN IV trial included 1,289 ACS patients (OCT-guided: 659 vs angiography-guided: 630) and demonstrated the feasibility, safety, and reproducibility of OCT-guided PCI in ACS patients5. However, OCT guidance was associated with longer procedure times, increased fluoroscopy durations, higher radiation exposure, and greater contrast use compared with angiography guidance alone. In our study, OCT- and IVUS-guided PCI showed similar 2-year TVF risk in ACS patients, confirming OCT’s clinical efficacy in acute settings. Moreover, although the OCT group required a greater amount of contrast media, the comparable CIN rates reaffirm the safety of OCT guidance in clinical practice. The higher optimisation rates in the IVUS group among non-ACS patients did not translate into improved outcomes, reflecting limited statistical power.
Overall, these findings should be interpreted cautiously, considering the trial’s limitations, including a lower-than-expected number of primary outcome events, its unblinded design, and geographical variations in imaging practices.
In conclusion, OCT-guided PCI yielded comparable safety and effectiveness to IVUS-guided PCI in ACS and non-ACS patients. Despite procedural complexities and increased contrast use, our results support adopting OCT as an effective alternative to IVUS in routine interventional practice, though further research on ICI guidance for ACS patients is needed.
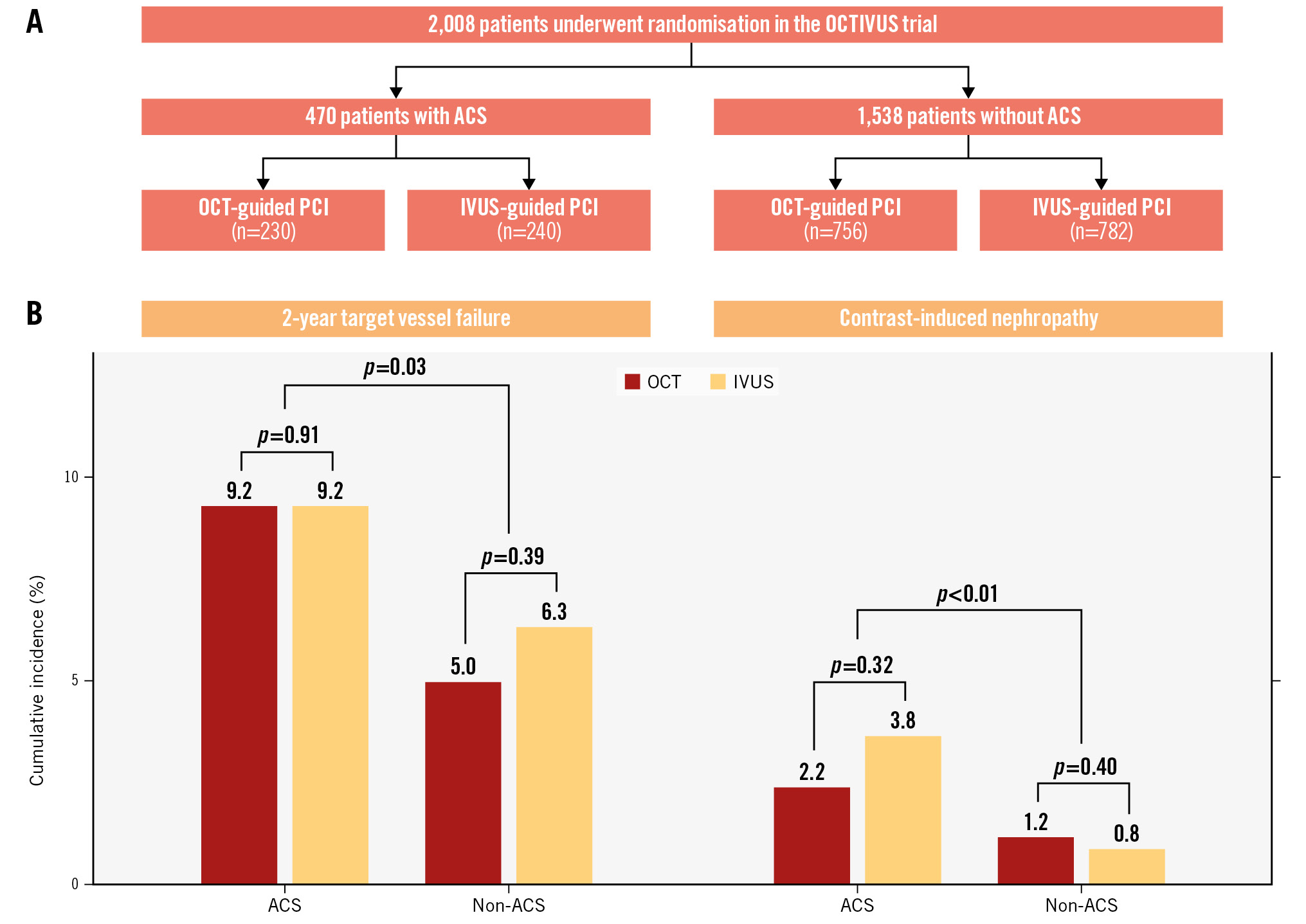
Central illustration. OCT versus IVUS guidance in patients with and without acute coronary syndrome. A) Study flowchart. B) Cumulative incidence analysis demonstrated comparable 2-year target vessel failure risks between the OCT and IVUS groups in ACS and non-ACS patients. The rates (%) of contrast-induced nephropathy were comparable between the OCT and IVUS groups in ACS and non-ACS patients. ACS: acute coronary syndrome; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Funding
This study was an investigator-initiated trial and was funded by the Cardiovascular Research Foundation, Abbott Vascular, and Medtronic. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest statement
The authors have no conflicts of interest relevant to the contents of this study to declare.

