Cory:
Unlock Your AI Assistant Now!
Abstract
Monotherapy with a potent P2Y12 receptor antagonist after 1 month of dual antiplatelet therapy (DAPT) may reduce bleeding in the absence of increased ischaemic events compared to 12-month DAPT in patients with acute coronary syndrome undergoing percutaneous coronary intervention (PCI). PCI guidance with optical coherence tomography (OCT) may enhance stent expansion. COMPARE STEMI ONE is an international, multicentre, open-label, randomised controlled trial. In 1,656 ST-segment elevation myocardial infarction (STEMI) patients, prasugrel monotherapy after 1 month of DAPT, as compared to standard 12-month prasugrel-based DAPT, will be tested for non-inferiority for the primary composite endpoint of net adverse clinical events − defined as all-cause death, myocardial infarction, stroke, or Bleeding Academic Research Consortium Type 3 or 5 bleeding events − at 11 months after randomisation. Furthermore, an ancillary substudy will test the superiority of OCT-guided versus angiography-guided staged complete revascularisation in achieving a larger minimal stent area (MSA) in non-culprit lesions during staged procedures. COMPARE STEMI ONE is the first randomised controlled trial assessing an abbreviated 1-month DAPT regimen followed by prasugrel monotherapy in the context of STEMI. The trial will also study the value of OCT-guided PCI in terms of the MSA of non-culprit lesions and may elucidate potential synergies between intravascular imaging-guided PCI and abbreviated DAPT regimens. (ClinicalTrials.gov: NCT05491200)
Antiplatelet therapy plays a pivotal role in reducing thrombotic complications and systemic ischaemic events among acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI), albeit with an increased risk of bleeding1. In the early phase following PCI for ACS, an aggressive antithrombotic therapy outweighs the risk of bleeding complications. In the maintenance phase (after 30 days), this benefit is counteracted by subsequent higher rates of major bleeding complications, particularly in real-life clinical settings, where patient complexity impacts both haemorrhagic and thrombotic risks2. New-generation drug-eluting stents (DES) and the adoption of intravascular imaging to optimise PCI have reduced the incidence of stent thrombosis and may justify a less intensive antithrombotic therapy34.
Recent randomised controlled trials (RCTs) have explored the hypothesis that abbreviated dual antiplatelet therapy (DAPT) with potent P2Y12 antiplatelet drugs may mitigate bleeding risk without increasing ischaemic events567. In the absence of compelling data supporting the superiority of one agent over the other, the choice between prasugrel and ticagrelor is primarily driven by physician preference, relative contraindications, market availability, and patient characteristics. This has resulted in an increased use of ticagrelor at discharge8. Although the ISAR-REACT 5 Trial demonstrated a lower incidence of the composite endpoint of death, myocardial infarction (MI) or stroke with prasugrel than ticagrelor in 4,018 ACS patients, limited data are available for abbreviated prasugrel-based DAPT in ACS91011.
Up to 50% of patients with ACS present with multivessel disease (MVD)12. Multiple studies have validated the importance of complete revascularisation, demonstrating fewer unplanned revascularisations and reductions in cardiovascular mortality and spontaneous MI13141516. The European Society of Cardiology (ESC) guidelines endorse a Class I recommendation for complete revascularisation in ST-segment elevation MI (STEMI) patients with MVD, either during the index procedure or within 45 days17. Intravascular imaging optical coherence tomography (OCT) may provide valuable insights into plaque characteristics, including fatty content, fibrous cap thickness, lesion severity, lesion length, and stent landing zones before treatment. It may also help assess stent expansion, apposition, lesion coverage and possible complications after PCI1718. However, no data exist on the use of intravascular imaging in the context of complete revascularisation or an abbreviated DAPT regimen in the context of STEMI.
Methods
Objectives and study design
COMPARE STEMI ONE is an international, multicentre, open-label, randomised controlled trial with a 1:1 randomisation comparing prasugrel-based short DAPT (30-45 days) followed by prasugrel monotherapy versus a standard DAPT regimen in STEMI patients in terms of net adverse clinical events (NACE). In the subgroup of STEMI patients with MVD, a subrandomisation compares OCT-guided complete revascularisation versus angiography-guided complete revascularisation in terms of efficacy and safety endpoints.
The primary objective of this study is to demonstrate the non-inferiority of 30-45 days of DAPT followed by prasugrel monotherapy versus standard 12-month DAPT (acetylsalicylic acid [ASA]+prasugrel) in patients admitted for STEMI treated with primary PCI regarding the net composite endpoint of ischaemic and bleeding events.
The ancillary objective of the study is to demonstrate the superiority of OCT-guided revascularisation of non-culprit lesion(s) compared to an angiography-guided approach in terms of minimal stent area (MSA) in patients with MVD who undergo staged complete revascularisation after primary PCI.
The clinical investigation is being conducted in up to 20 centres across Europe. A total of 1,656 STEMI patients, 50% with MVD, will be enrolled and followed up for 3 years.
Patients with MVD who have been randomised to either angio- or OCT-guided PCI and who do not fulfil the eligibility criteria for DAPT randomisation at 30-45 days will be included in the analysis of the co-primary endpoint (post-PCI MSA) and will remain in a parallel registry, receiving clinical follow-up at 1 year.
The study design appears in Figure 1.
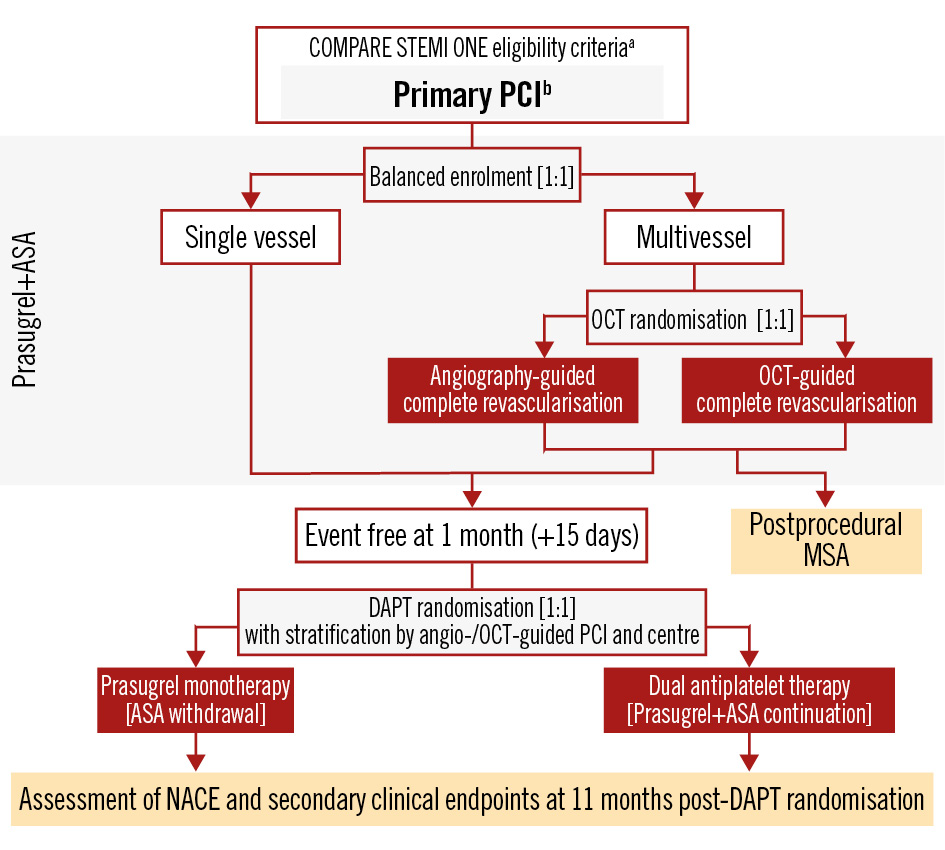
Figure 1. Study design flowchart. aLoading with prasugrel is among the inclusion criteria. bRevascularisation of only the culprit lesion is recommended. ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy; MSA: minimal stent area; NACE: net adverse clinical events; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Randomisation and treatment allocation
Patients are screened for inclusion immediately after STEMI diagnosis. Patients will be screened and enrolled to achieve a 1:1 trial population of single-vessel disease (SVD) and MVD patients. During the index procedure, only the culprit lesion will be treated as per standard of care. Patients with MVD will undergo staged complete revascularisation within 15 days. During the staged procedure, patients will be randomised 1:1 to OCT-guided or angio-guided PCI. All patients will undergo an OCT study at the end of PCI.
At 30-45 days, patients with SVD and MVD will be randomised to prasugrel monotherapy (ASA discontinuation) or standard prasugrel-based DAPT if no ischaemic or major bleeding event and no protocol violation have occurred (Figure 2). Among patients with MVD, randomisation to prasugrel monotherapy or DAPT at 30-45 days will be stratified based on the staged revascularisation strategy (OCT- or angio-guided) to ensure balance in the study treatment algorithms. Prasugrel monotherapy or prasugrel-based standard DAPT will be given in an open-label manner.
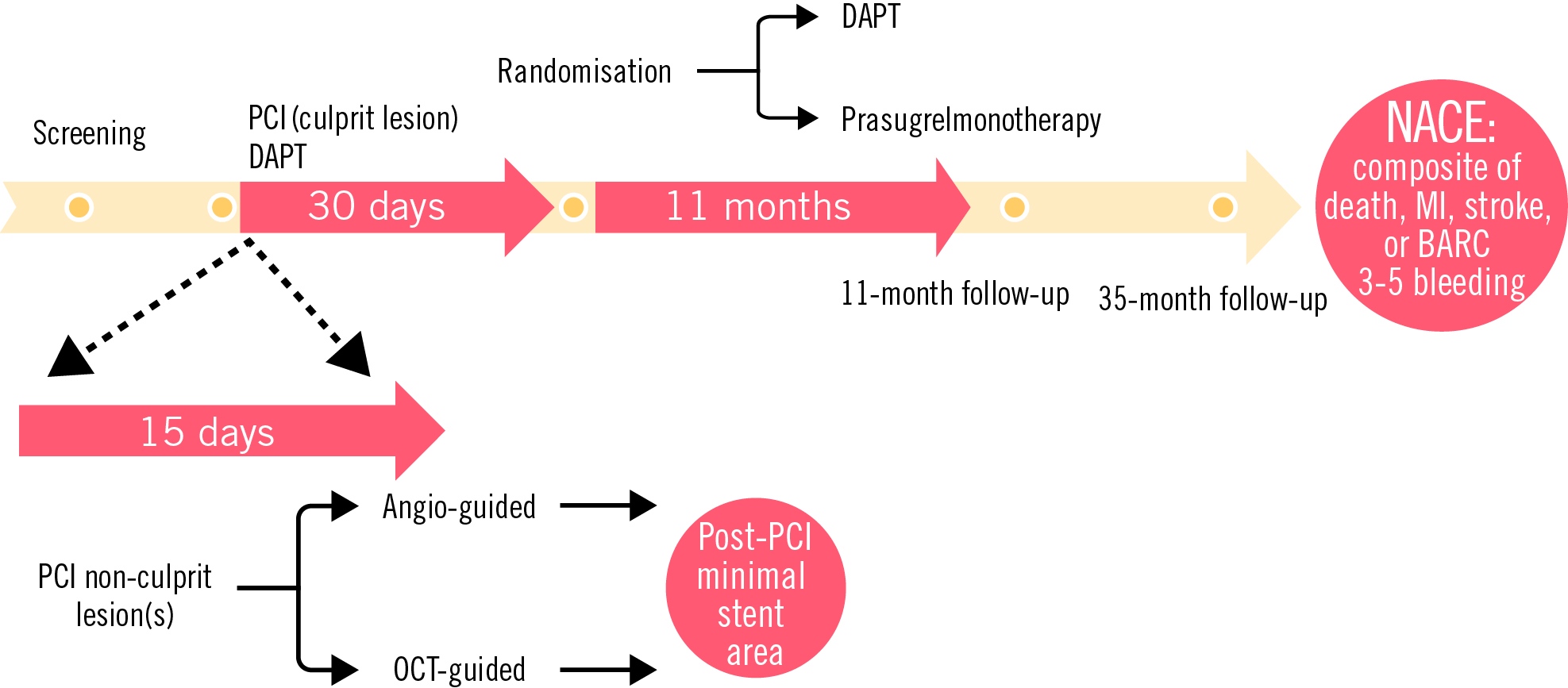
Figure 2. Timeline of COMPARE STEMI ONE. BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; NACE: net adverse clinical events; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Balanced screening and enrolment
Patients will be screened and enrolled to achieve a 1:1 trial population of SVD and MVD patients. Out of every group of 30 patients screened and enrolled per site, 15 patients should present with MVD. After the enrolment of 15 patients with SVD, screening and enrolment of those patients will be temporarily halted until the target number of MVD patients in each group has been reached and vice versa.
Study population, inclusion and exclusion criteria
Consecutive patients admitted for STEMI undergoing primary PCI will be screened and considered for inclusion in the study. Eligible patients will receive ASA as per standard of care and a prasugrel loading dose of 60 mg, followed by 30-45 days of DAPT (ASA+prasugrel 10 mg). In patients with multivessel disease, only primary PCI of the culprit lesion will be allowed during the index procedure. Staged PCI of the non-culprit lesion(s) will be planned within 15 days. During the staged PCI procedure, OCT will be performed at the end of PCI of each vessel, but patients will be randomly assigned 1:1 to OCT- or angio-guided stent optimisation.
The XIENCE everolimus-eluting platform (Abbott) will be the preferred stent for the study. Other DES platforms will be used as bailout options. Details on study devices and study procedures are shown in Supplementary Appendix 1 and Supplementary Appendix 2.
All patients with no ischaemic or major bleeding event at the 1-month office visit following primary PCI will be randomised 1:1 to receive prasugrel monotherapy (ASA discontinuation) for 11 months or DAPT continuation for 11 months (standard 12-month DAPT duration). The randomisation will occur at 1 month, within a 15-day window, to take into account those patients with MVD undergoing staged complete revascularisation.
Figure 1 displays the trial randomisation schemes for STEMI patients with SVD and MVD.
Inclusion and exclusion criteria that are relevant during the index procedure and the 1-month clinical follow-up visit are tabulated in Table 1. Further details on investigational and concomitant treatments are shown in Supplementary Appendix 3 and Supplementary Appendix 4.
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria |
|---|
| Eligibility at index procedure: |
| Patients of 18 years and above |
| Written or witnessed oral consent |
| All STEMI patients who are scheduled to be treated with PCI: |
| Chest discomfort suggestive of cardiac ischaemia ≥20 min at rest with 1 of the following ECG features: |
| ST-segment elevation ≥2 contiguous ECG leads |
| New or presumably new left bundle branch block |
| Eligibility at DAPT randomisation visit (30-45 days): |
| All patients who have provided informed consent |
| Compliance to DAPT with no regimen modifications (non-adherence Academic Research Consortium) |
| No occurrence of significant event (such as MI, unplanned revascularisation, stent thrombosis, stroke, major vascular complication/bleeding BARC Type 3 or greater) |
| Successful revascularisation: successful delivery and deployment of the study device(s), with final residual stenosis of <30% (visually) for all target lesions |
| Complete revascularisation performed when more than 1 significant lesion, in staged procedure(s) occurring within 15 days from the index procedure. Physiological assessment highly recommended for lesions with stenosis between 50% and 90% |
| Exclusion criteria |
| Patients on oral anticoagulation |
| Contraindication to P2Y12 inhibitors and/or to CardioAspirin or to any of the excipients (hypersensitivity, history of any stroke or transient ischaemic attack within the last 12 months, active bleeding or haemorrhagic diathesis, fibrin-specific fibrinolytic therapy less than 24 h before randomisation, severe hepatic dysfunction (Child-Pugh C), history of asthma induced by the administration of salicylates or substances with a similar action, notably non-steroidal anti-inflammatory medicines, history of gastrointestinal perforation or acute gastrointestinal ulcers, severe cardiac failure (NYHA Class III or IV), combination with methotrexate at doses of 15 mg/week or more) |
| Patients who have received P2Y12 inhibitors other than prasugrel in the ambulance (ticagrelor or clopidogrel loading dose) or are already on P2Y12 inhibitors may be enrolled in the protocol, provided that the prasugrel loading dose is administered at admission, according to current guideline recommendations |
| Concomitant oral or intravenous therapy with strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, voriconazole, telithromycin, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, atazanavir, grapefruit juice >1 L/day), CYP3A substrates with narrow therapeutic indices (e.g., cyclosporine, quinidine), or strong CYP3A inducers (e.g., rifampin) |
| Patient use of rifampicin, phenytoin, carbamazepine, dexamethason, phenobarbital |
| Platelet count <100,000/μL at the time of screening |
| Anaemia (haemoglobin <10 g/dL) at the time of screening |
| Comorbidities associated with life expectancy <1 year |
| Pregnancy, giving birth within the last 90 days, or lactation (see Supplementary Appendix 3 for females of childbearing potential) |
| PCI indication for stent thrombosis or previous history of definite stent thrombosis |
| Non-deferrable major surgery on DAPT after PCI |
| Cardiogenic shock |
| Out-of-hospital cardiac arrest unless survivors of ventricular arrythmia with prompt return of spontaneous circulation |
| BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; ECG: electrocardiogram; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction |
Endpoints
Primary endpoints
The study will primarily assess the following endpoints:
- NACE: composite endpoint of all-cause death, myocardial infarction, stroke or Bleeding Academic Research Consortium (BARC) Type 3 or 5 bleeding at 11 months post-DAPT randomisation;
- postprocedural MSA: final post-PCI MSA assessed by OCT in each randomised arm, measured at an independent OCT core laboratory blinded to imaging modality assignment.
Secondary endpoints
The following secondary endpoints will be assessed at 2, 11 and 35 months:
- major adverse cardiac and cerebrovascular events (MACCE): composite endpoint of cardiovascular death, myocardial infarction or stroke;
- BARC Type 3 or 5 bleeding;
- incidence of target vessel failure: composite of cardiac death, target vessel myocardial infarction (per-protocol [PP] MI definition), or ischaemia-driven target vessel revascularisation;
- incidence of stent thrombosis (definite or probable as defined by the Academic Research Consortium [ARC]).
Procedural outcomes and additional clinical endpoints are listed in Table 2. Definitions are provided in Supplementary Appendix 5.
Table 2. Procedural outcomes.
| Outcomes |
|---|
| Stent expansion |
| Stent expansion is defined by the MSA achieved in the proximal and distal stented segments relative to their respective reference lumen areas. The stent length is divided into 2 equal segments (proximal and distal) except for lesions containing a bifurcation (visually estimated side branch ≥2.5 mm). When there is a bifurcation present, rather than splitting the stent into two halves, the division is based upon the midpoint of the most proximal side branch. |
| Acceptable stent expansion (categorical variable): the MSA of the proximal segment is ≥90% of the proximal reference lumen area and the MSA of the distal segment is ≥90% of the distal reference lumen area. |
| Unacceptable stent expansion (categorical variable): the MSA of the proximal segment is <90% of the proximal reference lumen area, and/or the MSA of the distal segment is <90% of the distal reference lumen area. |
| In case either segment (proximal or distal) of the stent meets the criteria for unacceptable stent expansion, the stent is considered to have unacceptable stent expansion. Both segments of the stent must meet the acceptable stent expansion criteria to be considered acceptable. In case a respective reference segment cannot be measured, the determination will be made with only one of the two reference (proximal or distal) segments. |
| Post-PCI stent expansion (%) (continuous variable): the MSA divided by the average of proximal and distal reference lumen areas x 100. |
| Mean stent expansion (%) (continuous variable) |
| The mean stent area (stent volume/analysed stent length) divided by the average of proximal and distal reference lumen areas x 100. |
| Edge dissections |
| Edge dissections will be tabulated as follows: |
| Major (%): ≥60° of the circumference of the vessel at the site of dissection and ≥3 mm in length |
| Minor (%): any visible edge dissection <60° of the circumference of the vessel or <3 mm in length |
| Edge dissections will be further classified as: |
| Intimal: limited to the intima layer, i.e., not extending beyond the internal elastic lamina |
| Medial: extending into the media layer |
| Adventitial: extending through the external elastic membrane/lamina |
| Stent malapposition |
| Defined as the frequency (%) of incompletely apposed stent struts (defined as stent struts clearly separated from the vessel wall [lumen border/plaque surface] without any tissue behind the struts with a distance from the adjacent intima of ≥0.2 mm and not associated with any side branch). |
| Malapposition will be further classified as follows: |
| Major: if associated with unacceptable stent expansion (as defined above) |
| Minor: if associated with acceptable stent expansion (as defined above) |
| Border detection |
| The visibility of the vessel EEL border by OCT will be evaluated at both reference sites (proximal and distal), after intervention, and then classified into 3 grades: |
| Good: ≥75% (270°) of the visible circumference |
| Moderate: ≥50% (180°) to <75% (270°) of the visible circumference |
| Poor: <50% (180°) of the visible circumference |
| Untreated reference segment disease |
| Defined as focal disease with untreated MLA <4.5 mmm2 within 5 mm from the proximal and/or distal stent edges. |
| Subclassified by the amount of untreated lipid plaque, divided into 3 grades: |
| Low: ≤90° of lipid arc |
| Medium: >90° to <180° of lipid arc |
| High: ≥180° of lipid arc |
| Additional procedural and clinical endpoints |
| Device usage endpoints (site reported; assessed per subject) |
| Total stent length |
| Total number of stents |
| Maximal stent size |
| Post-dilatation (yes/no) |
| Total number of post-dilatation balloons |
| Maximal post-dilatation balloon size |
| Maximal device size (stent or post-dilatation balloon) |
| Maximum inflation pressure (atm; stent or post-dilatation balloon) |
| Additional procedural and clinical endpoints |
| Procedure time (first wire insertion to guide catheter removal) |
| Fluoroscopy time |
| Radiation exposure |
| Contrast use |
| Contrast-induced nephropathy (serum creatinine rise >25% or absolute increase >0.5 mg/dL) |
| Need for renal replacement therapy |
| OCT performance success (site reported) (OCT arm only) |
| OCT imaging performed both pre- and post-PCI |
| Additional interventions on the basis of the pre-PCI or post-stent OCT-imaging run that would not have been performed based on angiographic guidance alone (site reported; assessed per subject; OCT arm only) |
| Use of larger balloon |
| Use of higher inflation pressures |
| Use of additional inflation(s) |
| Use of additional stent(s) |
| Thrombus aspiration |
| Performance of atherectomy |
| Other interventions |
| Reason(s) for additional interventions will be documented by the site (e.g., more calcium than anticipated, greater stent underexpansion than appreciated angiographically, greater malapposition than appreciated angiographically, greater tissue protrusion or thrombus burden than appreciated angiographically, more severe edge dissection than appreciated angiographically, residual reference segment disease not appreciated angiographically, other) |
| All procedural outcomes are OCT defined (OCT core laboratory assessed). Subjects in the angiography-guided arm will undergo a post-PCI OCT run. Assessed per target lesion. EEL: external elastic lamina; MLA: minimal lumen area; MSA: minimal stent area; OCT: optical coherence tomography; PCI: percutaneous coronary intervention |
Sample size calculation
With respect to the antiplatelet therapy study component, a sample size of 1,500 subjects was deemed necessary to assess the non-inferiority of short DAPT followed by prasugrel monotherapy compared with standard prasugrel-based DAPT, with 80% power at a 1-sided α of 0.025. This calculation was based on the assumptions of an anticipated event rate of 3.5% in both groups at 11 months following randomisation (i.e., 12 months post-PCI), consistent with previous studies on DAPT duration after contemporary DES implantation; a non-inferiority margin of 2.0%, corresponding to a hazard ratio of 1.59; an accrual period of 42 months; and a 1:1 treatment allocation ratio. The analysis will be primarily conducted in the per-protocol population. Considering an expected rate of dropout and protocol violations of 10%, the final sample size was increased to 1,650561019.
With respect to the intravascular imaging guidance study component, in patients with multivessel disease undergoing early staged complete revascularisation, OCT-guided stent optimisation was assumed to produce a 0.4 mm² mean reduction in MSA after PCI compared with angiography-guided stent optimisation, assuming a common standard deviation of 2.0 mm²20. The analysis will be primarily conducted in the intention-to-treat (ITT) population. Patients will undergo 1:1 stratification in terms of antithrombotic treatment. A sample size of 788 subjects was deemed necessary to test superiority with 80% power at a 2-sided α of 0.05. Considering an expected rate of dropout and protocol violations of 5%, the final sample size was increased to 828.
Considering that the reported prevalence of MVD in STEMI patients is 50%, doubling the intravascular imaging guidance study sample size of 828 subjects led to a total of 1,656. In conclusion, a sample size of 1,656 subjects was determined to comprehensively encompass both components of COMPARE STEMI ONE.
Prespecified subgroup analysis
Analysis of the primary endpoints will be performed in prespecified subgroups defined by the following:
• Age (<75, ≥75 years old)
• Sex (male/female)
• Diabetes mellitus (yes/no)
• High bleeding risk according to the ARC High Bleeding Risk (ARC-HBR) definition (yes/no)
• Bifurcation lesion treated with 2 stents
• Complex high-risk indicated procedures defined as at least one of the following:
a. Angiographic heavy calcification
b. Ostial lesion
c. True bifurcation lesion involving side branch ≥2.5 mm
d. Left main lesion
e. Chronic total occlusion
f. In-stent restenosis
g. Long lesion (estimated stent length >28 mm)
h. Patient with an indication for PCI for any lesion and in need of elective mechanical circulatory support-assisted PCI (staged procedure)
Subgroup analyses will be performed with the Cox proportional hazards model after testing for interaction between the variable defining the subgroup and treatment effect.
Statistical analysis
The following analysis population sets will be defined for the assessment of study endpoints.
The ITT population consists of enrolled subjects who have been randomised. Subjects will be analysed according to the treatment group they were assigned. The co-primary endpoint of MSA will be analysed from the ITT population.
The primary endpoint of NACE will be assessed in the PP population. This population will include all randomised participants who completed the 11-month follow-up without prasugrel or DAPT disruption and remained free of ischaemic and major bleeding. Based on the MASTER DAPT trial results, the anticipated rate of discontinuation of prasugrel will be less than 10%. Here below is the preliminary list of major protocol deviations that will lead to exclusion from the PP population:
• inclusion/exclusion criteria not fulfilled or randomisation criteria not fulfilled;
• non-compliance to the treatment assigned by the randomisation.
Like sensitivity, non-inferiority will be tested in the ITT population.
Details on statistical analysis are displayed in Supplementary Appendix 6.
Data collection and analysis
The co-primary endpoint of post-PCI MSA assessed by OCT in each randomised arm will be measured at an independent OCT core laboratory (CERC, Massy, France) blinded to imaging modality assignment.
An independent clinical events committee (CEC) will adjudicate all primary and secondary endpoints. Definitions of all primary and secondary endpoints are summarised in Table 2. An independent data safety monitoring board (DSMB) will oversee the safety and wellbeing of the participating subjects, ensure the study’s scientific integrity, and recommend actions based on potential safety issues, including study suspension or termination based on prespecified suspension criteria. The organisation and composition of the DSMB and CEC are described in Supplementary Table 1 and Supplementary Table 2. Details on follow-up appear in Supplementary Appendix 7.
Discussion
The conventional approach to STEMI patients undergoing PCI involves aggressive antithrombotic therapy with DAPT, despite the known increased risk of bleeding, especially in the maintenance phase post-PCI2. The COMPARE STEMI ONE trial investigates the non-inferiority of an abbreviated DAPT regimen of 1 month followed by prasugrel monotherapy. The rationale is grounded in the hypothesis that a reduced DAPT duration curtails bleeding risks without compromising its antithrombotic efficacy in reducing ischaemic events7. A few randomised trials focusing on DAPT regimen in ACS (6 vs 12 months in SMART-DATE and DAPT-STEMI and 3 vs 12 months in REDUCE) have attempted to shed light on the optimal timing for DAPT discontinuation, aiming to shorten the duration of P2Y12 inhibitor administration. Results were concordant in reporting the non-inferiority of an abbreviated (3-6 months) DAPT regimen as compared to the standard 12-month DAPT regimen212223. There is a paucity of head-to-head comparison data for ASA versus P2Y12 inhibitor monotherapy following a short period of DAPT24. A recent meta-analysis involving 73,126 patients showed a significantly higher risk of myocardial infarction and a similar risk of bleeding with ASA monotherapy as compared to P2Y12 inhibitor monotherapy following short-term DAPT after PCI25.
Recent trials exploring abbreviated DAPT have yielded favourable results for a single P2Y12 inhibitor strategy. A non-prespecified post hoc analysis of the GLOBAL LEADERS trial in ACS patients investigated the efficacy of ticagrelor monotherapy compared to ASA plus ticagrelor after 1 month of DAPT. Between 1 and 12 months post-PCI, ASA was associated with an increased bleeding risk (1.5% vs 0.8%; p for superiority=0.004) and did not appear to enhance the benefit of ticagrelor on ischaemic events (2.0% vs 1.5%; p for superiority=0.07)26. The findings from the TWILIGHT trial revealed that administering ticagrelor monotherapy following an uneventful 3-month DAPT post-PCI in high-risk patients led to less bleeding without increased ischaemic events5. In the TICO Study (3,056 ACS patients), 3 months of DAPT followed by ticagrelor monotherapy resulted in a significant reduction of major bleeding and cardiovascular events at 1 year compared to 12-month ticagrelor-based DAPT27. In line with previous studies, the ULTIMATE-DAPT RCT showed that ACS patients can benefit from discontinuing ASA and maintaining ticagrelor monotherapy after 1 month of DAPT, resulting in less bleeding and similar major adverse cardiac event rates28. Consistently, in 2,850 ACS patients undergoing PCI, 1-month DAPT followed by ticagrelor monotherapy was non-inferior and superior to 12-month DAPT for the 1-year composite outcome of death, myocardial infarction, stent thrombosis, stroke, or major bleeding29.
However, these results have not been consistent across trials investigating different P2Y12 inhibitors. In patients with ACS and successful PCI, clopidogrel monotherapy after 1 to 2 months of DAPT failed to demonstrate non-inferiority compared to standard 12-month DAPT for the net clinical benefit10. The STOPDAPT-3 trial failed to achieve the primary endpoint of superiority in reducing bleeding events (BARC 3 or 5) and showed a potential increase in the risk of subacute stent thrombosis in patients allocated to prasugrel monotherapy at a dosage of 3.75 mg/day immediately following PCI1011.
Several RCTs are currently investigating either a prasugrel-based or ticagrelor-based single antiplatelet regimen after 0-3 months of DAPT in ACS patients (Figure 3). The design of the COMPARE STEMI ONE trial is unique in focusing exclusively on a full-dose prasugrel-based regimen in the context of STEMI3031. The potentially better ischaemic profile of prasugrel over ticagrelor, which was demonstrated in the ISAR-REACT 5 Trial, adds to the rationale of our trial9.
In addressing the multifaceted nature of STEMI patients, the COMPARE STEMI ONE trial takes a step further by examining the benefit of OCT-guided complete revascularisation in patients with MVD and addressing the growing interest in leveraging intravascular imaging to optimise procedural outcomes4203233. Recently, the Impact of IntraVascular UltraSound Guidance on Outcomes of Xience Prime Stents in Long Lesions trial (IVUS-XPL Study), conducted at 20 centres in the Republic of Korea, revealed a significant interaction between intravascular ultrasound (IVUS) use and DAPT duration, suggesting that IVUS-guided stent optimisation may yield more favourable results with shorter DAPT34.
The COMPARE STEMI ONE RCT will explore synergies between a shortened DAPT duration and OCT-guided revascularisation for STEMI patients with MVD. The potential implications of this dual approach for optimising patient outcomes remain a particular point of interest.
We recognise that the choice of an absolute non-inferiority margin of 2.0 results in a relative non-inferiority margin that is higher than those used in previous trials. This decision, however, was driven by the low, but realistic, expected event rates in our study population. In this context, our approach reflects a pragmatic balance between feasibility and statistical rigour.
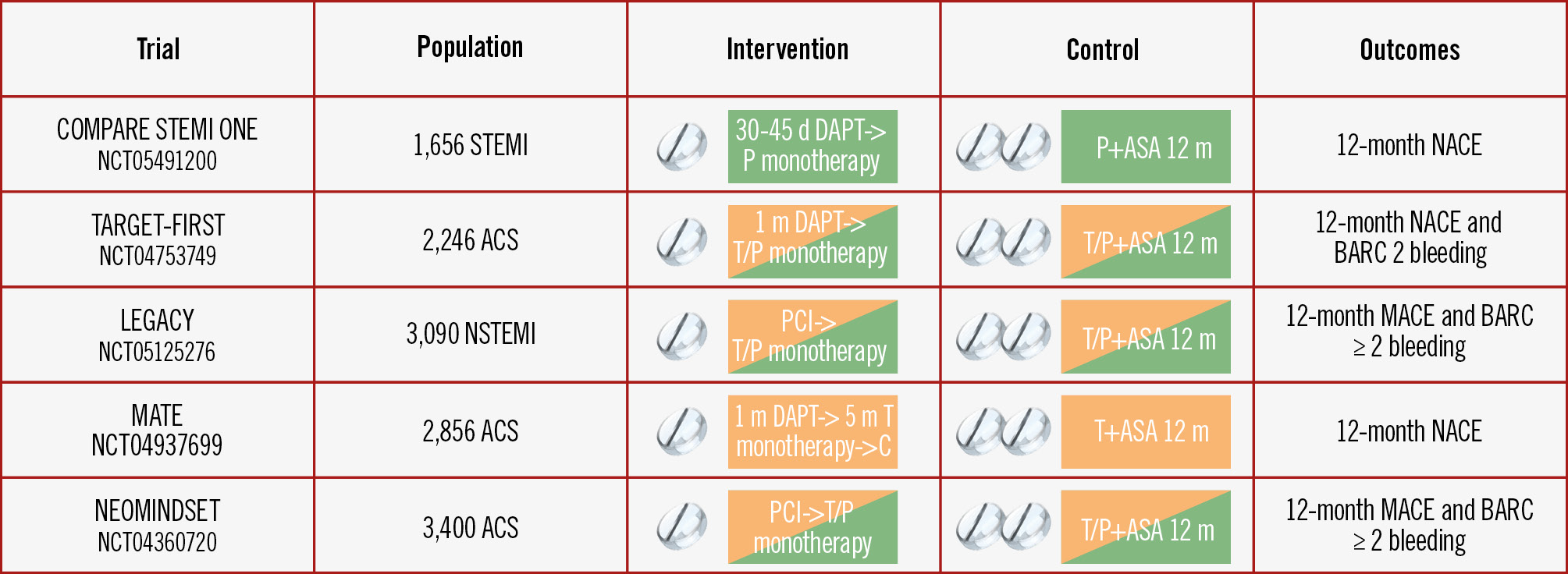
Figure 3. Overview of current RCTs investigating P2Y12 inhibitor monotherapy in ACS patients. ACS: acute coronary syndrome; ASA: acetylsalicylic acid; BARC: Bleeding Academic Research Consortium; C: clopidogrel; DAPT: dual antiplatelet therapy; MACE: major adverse cardiac events; NACE: net adverse clinical events; NSTEMI: non-STEMI; P: prasugrel; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; T: ticagrelor
Conclusions
In summary, the multicentre, randomised, open-label COMPARE STEMI ONE trial addresses critical clinical questions regarding the optimal strategy and choice of antiplatelet therapy in STEMI patients undergoing PCI. By integrating intravascular imaging into the paradigm of complete revascularisation, the trial may also provide meaningful insights into the optimal antithrombotic treatment of STEMI patients with MVD.
Funding
The trial is investigator initiated with an unrestricted grant provided by Abbott, Santa Clara, CA, USA. The authors are solely responsible for the design, conduct and analyses of this study, as well as the drafting and editing of the paper and its final content.
Conflict of interest statement
V. Paradies declares a research grant from Abbott Vascular via the institution; speaker fees from Abbott Vascular, Boston Scientific, and Elixir; and an educational grant from Terumo via the institution. P.C. Smits has received consultancy fees and institutional research grants from Abbott Vascular. R. M. Oemrawsingh declares speaker fees from Abbott Vascular and an educational grant from Terumo. F. Burzotta declares speakers' fees from Abbott, Abiomed, Medtronic, Edwards Lifesciences, and Terumo. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

