Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The management of interventricular septal hypertrophy is an area of rapidly increasing interest, spurred by continued challenges with transcatheter mitral valve replacement (TMVR) and the management of obstructive hypertrophic cardiomyopathy (oHCM).
Aims: We sought to evaluate the reproducibility of septal scoring along the midline endocardium (SESAME), a novel transcatheter intervention designed to replicate surgical myotomy.
Methods: This single-centre, retrospective review included all patients who underwent the SESAME procedure at the University of Washington from January 2022 to September 2024.
Results: A total of 54 consecutive patients underwent SESAME at our institution: 47 prior to TMVR, 6 for oHCM, and 1 for a subaortic membrane. Technical success was achieved in 100% of patients. In pre-TMVR patients, the median neo-left ventricular outflow tract (LVOT) and the median skirt neo-LVOT areas gained were 146 (first quartile [Q1]: 76.5, third quartile [Q3]: 286.3) mm2 and 54 (Q1: 32.8, Q3: 100.2) mm2, respectively. In the oHCM population, invasive resting and provocable LVOT gradients immediately decreased from 59 (Q1: 32, Q3: 99) mmHg to 10 (Q1: 5, Q3: 19) mmHg and from 121 (Q1: 53, Q3: 205) mmHg to 34 (Q1: 16, Q3: 56) mmHg, respectively. The median echo gradients decreased from 62 (Q1: 53, Q3: 64) mmHg at baseline to 6 (Q1: 6, Q3: 8) mmHg at 30 days. Among the pre-TMVR population, there were 2 procedural deaths from free-wall rupture early in the experience and 3 restrictive ventricular septal defects that did not require intervention. Three patients (5.5%) required a pacemaker. Procedural complications significantly decreased after the first 10 cases in 2022 (p<0.01).
Conclusions: Our study corroborates the feasibility and efficacy of SESAME for prohibitive surgical risk patients needing septal reduction therapy prior to TMVR or for treatment of oHCM or a subaortic membrane.
Ventricular septal reduction therapy is an area of rapidly increasing interest, spurred by continued challenges with transcatheter mitral valve replacement (TMVR) and therapeutic approaches to obstructive hypertrophic cardiomyopathy (oHCM). Early TMVR experience revealed acute left ventricular outflow tract obstruction (LVOTO) as a potentially fatal complication of the procedure, occurring in at least 9.3% of patients1. This complication is based on the relationship between the displaced subvalvular mitral apparatus or the ventricular aspect of the mitral prosthesis and the basal interventricular septum. Given the morbidity and mortality associated with LVOTO after TMVR, several techniques have evolved to reduce this risk, but each has its limitations. Intentional laceration of the anterior mitral leaflet to prevent outflow obstruction (LAMPOON) allows blood flow across the subvalvular transcatheter mitral valve (MV) stent struts because they are not covered by the anterior mitral leaflet2, but contemporary mitral prostheses have a closed-cell structure which undermines the value of this technique. Alcohol septal ablation (ASA) can reduce basal septal hypertrophy and mitigate LVOTO3. However, the myocardial response to ethanol is unpredictable, a favourable septal perforator anatomy is required, and permanent pacemaker (PPM) rates can exceed 20%, particularly when the septum is not significantly thickened34.
More recently, septal scoring along the midline endocardium (SESAME) has emerged as a novel transcatheter electrosurgical procedure mimicking surgical myotomy5. Greenbaum et al6 reported the first single-centre case series utilising this technique to successfully facilitate transcatheter valve implantation or treat oHCM. The reported outcomes of this case series were very favourable, with a 2.6% procedural mortality rate, 5.3% PPM rate, and a mean left ventricular outflow tract (LVOT) area gain of 141 mm2 despite the ongoing refinement of the technique6. Questions remain, however, about the reproducibility of this technique at different medical centres given the perceived technical challenges of the procedure.
As early adopters of SESAME for our TMVR population and, more recently, our oHCM population, we hereby report the safety and efficacy of this technique in another single-centre, real-world registry to validate its feasibility and reproducibility.
Methods
Cohort
All patients who underwent a SESAME procedure at the University of Washington are included in this report. The majority of these patients had MV disease and were being evaluated for TMVR. Initially, patients deemed poor candidates for ASA were treated with SESAME, although, gradually, SESAME became the default technique for septal reduction therapy prior to TMVR. As comfort with the technique grew, this procedure was introduced for patients with oHCM phenotypes and for 1 patient with subaortic stenosis. The procedure was performed on a compassionate basis with informed consent. The University of Washington Institutional Review Board approved this single-centre retrospective review.
Procedural technique
A detailed description of the technique has been reported elsewhere6. Briefly, percutaneous septal myotomy was performed via femoral vascular access with biplane fluoroscopy and transoesophageal echocardiography (TOE) guidance. Fluoroscopic projections were derived from contrast-enhanced computed tomography (CT) illustrating a long-axis view of the left ventricle (generally right anterior oblique and caudal) and an en face LVOT view (generally left anterior oblique and cranial). A steerable guide (DIREX [Boston Scientific]) was positioned in the ascending aorta, through which a guide catheter was advanced retrogradely across the aortic valve to the basal interventricular septum. The guide catheter − typically a hockey stick catheter − was positioned against the septum at the planned myocardial entry point. Through this, a distally amputated 0.014” CONFIANZA Pro 12 guidewire (Asahi Intecc) within a Turnpike Spiral microcatheter (Teleflex) was used to puncture the septal myocardium encroaching into the LVOT (the “septal knuckle”). In some cases, an Astato XS 40 wire (Asahi Intecc), with a brief pulse of 50 watts of electric current, was used to facilitate myocardial entry. Following septal entry and engagement of the Turnpike Spiral into the muscle, the wire was exchanged for a 300 cm Astato XS 20 wire (Asahi Intecc) with a small (1-2 mm, 20-30 degree) “chronic total occlusion (CTO)-like” tip bend. This wire was then steered and advanced through the myocardium under fluoroscopic and TOE guidance, followed by a microcatheter, to the predetermined cavitary re-entry point.
During the early experience, multiple imaging modalities were utilised to best understand the exact course of the wire trajectory through the septum and ensure appropriate wire position and depth. These included biplane fluoroscopy, TOE, transthoracic echocardiography (TTE), intravascular ultrasound in the myocardium, intracardiac echocardiography (ICE), and ventriculography. Most of these modalities were abandoned due to minimal perceived benefit, with biplane fluoroscopy (rather than the simulated trajectory on CT) and TOE being favoured.
Following confirmation of optimal wire position, the distal end of the lacerating wire was snared. The contemporary technique now includes dilating the intramyocardial tract using a 1.5 mm coronary balloon to facilitate delivery of a larger microcatheter, which double-insulates the wire in the myocardium to minimise the build-up of heat and the potential for steam pops. Following balloon dilation, a “flying V” cutting element was formed in the middle of the exchange-length lacerating wire, and the afferent limb of the wire was sheathed within a mother-daughter microcatheter system (0.014” Finecross and 0.035” NaviCross [both Terumo]) to concentrate the electric current at the cutting element. The efferent limb was typically not insulated with a microcatheter, but continuous flush saline was provided via the snaring catheter during cutting. The “flying V” was positioned across the intramyocardial segment. Gentle retraction of both limbs of the externalised wire was then performed, and the MV was assessed with TOE to ensure the subvalvular mitral apparatus was not entangled. The wire was then electrified using a continuous current delivery of 50 watts. At the same time, gentle traction was applied to both limbs of the wire until fluoroscopy suggested complete muscle laceration.
Analysis
Pre- and post-procedure CT analyses were performed using 3mensio, version 10.3 (Pie Medical Imaging). Predicted neo-LVOT and skirt neo-LVOT area measurements were standardised by obtaining them in end-systole (30-40% cardiac cycle) using a modelled 29 mm wide, 22 mm tall prosthesis positioned 25% atrial (e.g., in a “SAPIEN [Edwards Lifesciences] in mitral annular calcification [MAC]” setting). Although a “SAPIEN in MAC” configuration was used for standardisation in this report, for many patients, the clinical goal was to qualify for a dedicated mitral prosthesis. Consequently, the “SAPIEN in MAC” neo-LVOT and skirt neo-LVOT values were not always germane to the clinical decision-making for proceeding with SESAME. The smallest cross-sectional area measured was recorded from CT scans both pre- and post-procedure, with the post-procedure CT scan typically occurring 1 month after the SESAME procedure. The neo-LVOT was recorded as a negative number when there was no visible neo-LVOT path around the prosthesis and the septal muscle appeared to encroach inside the simulated valve. The cross-sectional muscle area inside the prosthesis at maximal encroachment was then traced to record a negative neo-LVOT. The maximal septal thickness was measured by CT during end-systole, consistent with the neo-LVOT measurements. Right ventricular trabeculations were not included in the septal thickness measurements.
Technical success was defined as a septal laceration visually confirmed by intraprocedural TOE. Consistent with prior reports, we also analysed neo-LVOT failure and skirt neo-LVOT failure, which were defined as minimal post-SESAME LVOT areas below the thresholds of 200 mm2 and 150 mm2, respectively.
All patients who underwent attempted SESAME at our institution were included, irrespective of the intended TMVR plan or prior septal modification with ASA. Data were abstracted retrospectively from the medical records. Serial measurements were analysed as pairs. Data are reported as mean±standard deviation if normally distributed or median (first quartile [Q1], third quartile [Q3] if not. Statistical analysis was performed using Stata, version 15 (StataCorp), and JMP Pro 17 (SAS Institute). Figures were created with Biorender.com. A two-sided p-value<0.05 was considered statistically significant.
Results
In all, 54 consecutive patients who underwent SESAME at our institution from January 2022 to September 2024 were included in the analysis. The majority (87%) were female. The mean patient age was 75.2±9.5 years. A total of 28% of patients had prior stroke or TIA, and 57% had chronic kidney disease (CKD) stage 3 or greater. In all, 53% of patients had prior aortic valve replacement. In patients with MV disease, 91% had moderate or greater MAC, and the mean Society of Thoracic Surgeons (STS)-predicted 30-day mortality for isolated MV replacement was 15±9.5%. Baseline patient characteristics are summarised in Table 1.
Indications for SESAME were septal modification prior to TMVR (n=47), symptomatic LVOT obstruction due to oHCM phenotypes (n=6), or subaortic membrane (n=1). SESAME was performed on an elective basis in 85% of cases, urgently in 13% (for patients admitted with decompensated heart failure symptoms at the time of SESAME), and emergently in 2% (as a post-TMVR rescue). Technical success was 100%. The mean procedural duration was 137±57 minutes. Procedural survival was 96% (52 of 54), and 1-month survival was 90%.
Table 1. Baseline characteristics.
| Clinical and demographic variables | N=54 |
|---|---|
| Age, years | 75.2±9.5 |
| Female | 46 (87) |
| Race | |
| White | 52 (98) |
| Black | 1 (2) |
| Ethnicity | |
| Hispanic or Latino/a or Latinx | 1 (2) |
| Non-Hispanic or Latino/a or Latinx | 52 (98) |
| Body mass index, kg/m2 | 29.2±6.5 |
| NYHA Class | |
| II | 4 (7) |
| III | 39 (74) |
| IV | 10 (19) |
| LVEF, % | 67±9 |
| Known coronary artery disease | 33 (62) |
| Prior stroke or transient ischaemic attack | 15 (28) |
| Prior myocardial infarction | 12 (23) |
| Peripheral artery disease | 19 (36) |
| Hypertension | 54 (100) |
| Diabetes | 27 (51) |
| Chronic kidney disease stage ≥3 | 30 (57) |
| Obstructive lung disease (asthma or COPD) | 24 (45) |
| Obstructive sleep apnoea | 21 (40) |
| Atrial fibrillation | 26 (49) |
| Former or current tobacco use | 27 (51) |
| Cardiac pacemaker, CRT, or defibrillator | 12 (23) |
| Prior alcohol septal ablation | 2 (3) |
| Mitral valve disease | |
| None | 4 (8) |
| Predominant mitral regurgitation | 8 (15) |
| Predominant mitral stenosis | 34 (64) |
| Mixed | 7 (13) |
| Aortic valve disease | |
| None | 25 (47) |
| Prior TAVI | 21 (40) |
| Prior SAVR | 7 (13) |
| STS-predicted 30-day mortality for mitral valve replacement, % | 15±9.5% |
| Data are given as n (%) or mean±standard deviation. COPD: chronic obstructive pulmonary disorder; CRT: cardiac resynchronisation therapy; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SAVR: surgical aortic valve replacement; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |
Mitral valve disease population
Among the pre-TMVR patients, the median baseline predicted neo-LVOT area was 44.5 (Q1: −22.6, Q3: 94.5) mm2, and the median baseline predicted skirt neo-LVOT area was 195.6 (Q1: 157.4, Q3: 272.0) mm2. The mean baseline maximal septal thickness in end-systole by CT was 20.7±4.1 mm. The mean time to postprocedural CT was 34±18 days. Following SESAME, the median neo-LVOT and skirt neo-LVOT areas gained were 146.5 (Q1: 76.5, Q3: 286.3) mm2 and 54.1 (Q1: 32.8, Q3: 100.2) mm2, respectively. Representative analyses of large, medium, and small SESAME slices throughout the full cardiac cycle are included in Supplementary Figure 1. Fifteen (32%) patients had neo-LVOT failure (7 of 12 patients with baseline neo-LVOTs <0 mm2 and 8 of 35 patients with baseline neo-LVOTs>0 mm2). One patient (3%) had skirt neo-LVOT failure. Table 2 summarises the procedural results.
Eleven patients had complications during their index hospitalisations (Table 3). There were two procedural deaths: one from a free-wall perforation caused by an ICE probe in the left ventricle that punctured the lateral wall and one from a free-wall laceration caused by a SESAME slice that was too deep and too apical; both occurred early in our experience (Figure 1). There were 3 additional deaths during the index hospitalisation: a patient with an iatrogenic restrictive ventricular septal defect (VSD) and bradycardia requiring a temporary pacemaker died 4 days post-procedure, and 2 patients died of pre-existing but continued shock without procedural complications.
Two additional patients developed restrictive VSDs that were treated conservatively. Both ultimately required pacemakers. One also underwent a 26 mm Evolut FX (Medtronic) transcatheter aortic valve implantation at the time of SESAME. Both patients ultimately proceeded on to TMVR without complication.
There was one ischaemic stroke without persistent neurological deficits. One patient had MV injury related to SESAME, resulting in a mild increase in already severe mitral regurgitation, and underwent TMVR 67 days later as planned. Three patients developed stage 5 acute kidney injury (AKI) requiring temporary renal replacement therapy. The mean contrast provided to these patients was 6.7±11.5 mL. Two-sample t-testing demonstrated no statistically significant difference between the average contrast use and development of AKI (p=0.21). One patient experienced frequent non-sustained ventricular tachycardia intraprocedurally that did not require additional treatment.
Of 47 patients who underwent SESAME prior to anticipated TMVR, 42 survived to hospital discharge and 34 (81%) have subsequently proceeded to TMVR thus far (5 with trial-based valves and 29 valve-in-MAC procedures). The median time from SESAME to TMVR was 80.5 (Q1: 58, Q3: 125) days. One patient died from her mitral valve disease following SESAME during screening for TMVR options. Of patients receiving a valve-in-MAC TMVR, 23 of 29 (79%) also underwent concomitant LAMPOON. The mean post-SESAME neo-LVOT among those receiving LAMPOON was significantly smaller than that among those who did not (196±40 mm2 vs 386±39 mm2; p<0.01). Figure 2 demonstrates the mean projected baseline neo-LVOT and skirt neo-LVOT, and post-SESAME neo-LVOT and skirt neo-LVOT among the TMVR population. The mean LVOT gradient by invasive measurement following any TMVR was unchanged from baseline (10.6±5.7 mmHg baseline vs 13.4±8.9 mmHg post-TMVR; p=0.11). Thirty-day survival following TMVR was 91%.
Table 2. SESAME area and gradient outcomes.
| Pre-TMVR population | N=47 |
|---|---|
| Baseline CT neo-LVOT, mm2 | 44.5 (−22.6, 94.5) |
| Baseline CT skirt neo-LVOT, mm2 | 195.6 (157.4, 272.0) |
| Baseline CT maximal septal thickness, mm | 20.7±4.1 |
| Neo-LVOT area change, mm2 | 146 (76.5, 286.3) |
| Skirt neo-LVOT area change, mm2 | 54 (32.8, 100.2) |
| Neo-LVOT failure | 15 (32) |
| Skirt neo-LVOT failure | 1 (3) |
| Patients who have undergone TMVR to date | 34 (81) |
| Pre-TMVR inv. LVOT gradient, mmHg | 10.5±5.8 |
| Post-TMVR inv. LVOT gradient, mmHg | 13.0±8.7 |
| Time from SESAME to TMVR, days | 80.5 (58, 125) |
| oHCM population | N=6 |
| Baseline peak LVOT gradient by TTE, mmHg | 62 (53, 64) |
| Baseline inv. resting LVOT gradient, mmHg | 59 (32, 99) |
| Baseline inv. provocable LVOT gradient, mmHg | 121 (53, 205) |
| Post-SESAME inv. resting LVOT gradient, mmHg | 10 (5, 19) |
| Post-SESAME inv. provocable LVOT gradient, mmHg | 34 (16, 56) |
| Post-SESAME peak LVOT gradient at 30 days, mmHg | 6 (6, 8) |
| Data are given as n (%), mean±standard deviation, or median (Q1, Q3). CT: computed tomography; inv.: invasive; LVOT: left ventricular outflow tract; oHCM: obstructive hypertrophic cardiomyopathy; Q1: first quartile; Q3: third quartile; SESAME: septal scoring along the midline endocardium; TMVR: transcatheter mitral valve replacement; TTE: transthoracic echocardiography | |
Table 3. Procedural characteristics and complications.
| Characteristic/complication | Value n=54 |
|---|---|
| Indication | |
| Pre-TMVR | 47 (87) |
| Hypertrophic cardiomyopathy | 6 (11) |
| Subaortic stenosis | 1 (2) |
| Setting | |
| Elective | 45 (85) |
| Urgent | 7 (13) |
| Emergent | 1 (2) |
| Technical success | 54 (100) |
| Case duration, min | 137±57 |
| Contrast, mL | 24 (0, 45) |
| Fluoroscopy time, min | 37.1 (23.5, 57.9) |
| Air kerma, mGy | 1,089 (449, 1,590) |
| Any major complication | 11 (20.8) |
| Death during procedure | 2 (3.8) |
| Acute kidney injury stage 4 | 3 (5.7) |
| Permanent pacemaker placement | 3 (5.7) |
| Ventricular septal defect | 3 (5.7) |
| Mitral leaflet laceration | 2 (3.8) |
| Major vascular complication | 0 (0) |
| Stroke | 1 (1.9) |
| Ventricular arrhythmia | 1 (1.9) |
| Ventricular free-wall perforation | 2 (3.8) |
| Bleeding, major or life-threatening | 0 (0) |
| In-hospital mortality | 5 (9.4) |
| 30-day mortality | 6 (11.3) |
| Data are given as n (%), mean±standard deviation, or median (Q1, Q3). Q1: first quartile; Q3: third quartile; TMVR: transcatheter mitral valve replacement | |
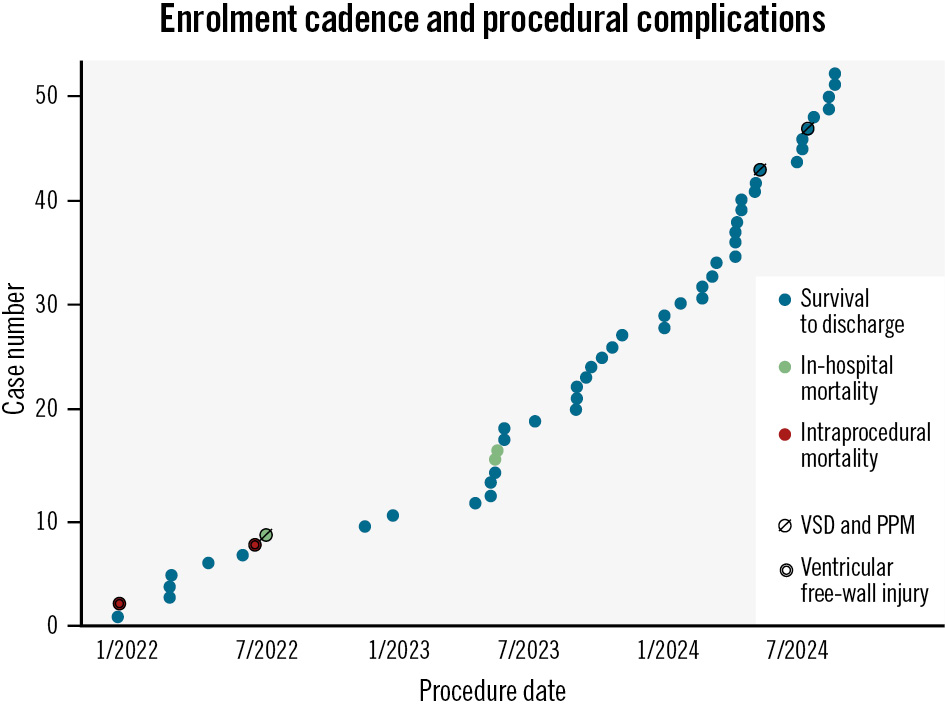
Figure 1. Enrolment cadence and procedural complications. The date of the SESAME procedure is indicated on the x-axis and the case number on the y-axis. Blue dots represent patients with survival to hospital discharge; green dots represent in-hospital mortality; red dots represent intraprocedural mortality. A ventricular septal defect and complete heart block requiring a pacemaker is indicated by a slashed circle. Ventricular free-wall injury is indicated by concentric circles. PPM: permanent pacemaker; SESAME: septal scoring along the midline endocardium; VSD: ventricular septal defect
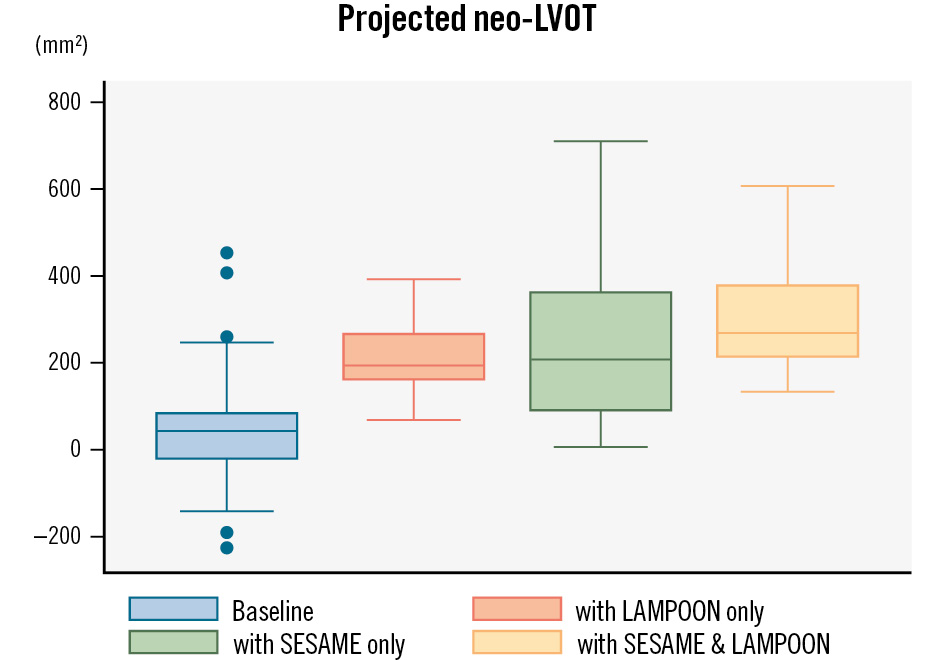
Figure 2. Projected neo-LVOT after TMVR. Box plot demonstrating the anticipated neo-LVOT following TMVR at baseline (blue), with LAMPOON at the time of TMVR (orange), following SESAME without LAMPOON (green) and following SESAME and LAMPOON (yellow). Each box represents the range of results from the 1st to the 3rd quartile with the median value as the horizontal line inside the box. Whiskers are the minimum and maximum values excluding outliers, which are represented as single dots. LAMPOON: laceration of the anterior mitral leaflet to prevent outflow obstruction; LVOT: left ventricular outflow tract; SESAME: septal scoring along the midline endocardium; TMVR: transcatheter mitral valve replacement
LVOT obstruction population
Seven patients underwent SESAME for indications other than MV disease (6 for oHCM and 1 for a subaortic membrane). In the oHCM population, the septal thickness at end-systole as measured by CT scan was 25.2±4.5 mm. Baseline and postprocedural TTE and invasive gradients are summarised in . Invasive measurements demonstrated immediate resolution of resting peak gradients, which was corroborated by echocardiography at 30 days, showing a median peak residual gradient of 6 (Q1: 6, Q3: 8) mmHg.
For the single patient with a subaortic membrane, the invasive gradient was reduced from 51 mmHg to 4 mmHg immediately after SESAME. However, the 30-day TTE results were less dramatic, with a residual peak gradient of 31 mmHg compared to her baseline preprocedural TTE gradient of 70 mmHg. Her procedure was also unusual in that the septal traversal distance was significantly shorter (~10 mm) and less deep (3-4 mm) than is typical. This was specifically intended to split only the limbus of the fibromuscular ridge, rather than to create a splay within the septum itself.
In the oHCM population, there were no deaths, pacemaker requirements, VSDs, or free-wall ruptures. One early patient suffered lacerated mitral chordae requiring transcatheter edge-to-edge repair, which occurred before we routinely checked for chordal entanglement prior to laceration.
Learning curve
Ten patients were treated with SESAME in 2022, 18 in 2023 and 26 in 2024 (up to August). There was a statistically significant decrease in complications following the first 10 cases performed in 2022 as compared to the 44 cases subsequently performed (p<0.01), including reductions in procedural deaths (20% vs 0%), index hospitalisation deaths (30% vs 4%), VSDs (10% vs 4%), need for PPM (10% vs 4%), and damage to the mitral apparatus (10% vs 2%). Simultaneously, the mean resultant SESAME size, measured as a cross-sectional area by follow-up CT, was not statistically different per year and numerically increased each year (92.6 mm2 in 2022, 104.7 mm2 in 2023, and 164.3 mm in 2024; p=0.20).
Discussion
We present the second human cohort of patients undergoing SESAME, a novel transcatheter electrosurgical procedure that mimics surgical myotomy. We initially used SESAME to facilitate septal reduction therapy prior to TMVR in a highly morbid and complex population with significant MAC but evolved our use of the technique to treat high-risk patients with LVOT obstruction from hypertrophic cardiomyopathy or subvalvular aortic stenosis. All patients were considered too high risk to undergo open mitral valve replacement or myectomy, and 15% were already in the hospital for acute decompensated heart failure at the time of their SESAME. We observed the following: (1) SESAME is a highly effective septal reduction technique that enlarges the LVOT and reduces LVOT gradients without tissue excision; (2) SESAME was an acceptably safe procedure in this inoperable and complex patient cohort, with patient risks diminishing over time; (3) as might be expected for a novel procedure under continued refinement during the study period, SESAME was associated with a learning curve where significantly better outcomes were seen following the first 10 cases at our institution (Central illustration).
In the pre-TMVR population, the median augmentation in LVOT area was 146 mm2, which is almost exactly the same as the previously reported neo-LVOT gains following SESAME, and compares very favourably to changes in neo-LVOT reported following ASA37. Despite significant gains in the LVOT area in general, the results following SESAME were heterogeneous, with 32% of pre-TMVR patients not achieving a desired post-SESAME predicted neo-LVOT of 200 mm2. Most of these patients started with predicted neo-LVOTs less than 0 mm2, and as such, it stands to reason that a median result of 150 mm2 gained would not be sufficient. However, 6 patients gained less than 50 mm2 of neo-LVOT following SESAME. This may be due to differences in the myocardial response to SESAME laceration, a procedural wire traversal that was too shallow to result in sufficient splay, or geographical deviation from the intended SESAME laceration, particularly not initiating the laceration sufficiently basally and thereby leaving a ridge of muscle just underneath the aortic valve. Nevertheless, only 1 patient had a resultant skirt neo-LVOT less than 150 mm2. In fact, 81% of our SESAME population has already moved on to TMVR. The remaining patients in the cohort are under evaluation for treatment with a dedicated prosthesis via clinical trial pathways.
Though our oHCM population was small, the immediate and 30-day SESAME results have been quite striking, with statistically significant reductions in immediate resting (59 mmHg vs 10 mmHg) and provocable (121 mmHg vs 34 mmHg) invasive gradients. This finding also carried over to 30-day TTE results (62 mmHg vs 6 mmHg). The immediacy of these results in the oHCM population is particularly notable. Since the splay does not mature immediately and no myocardial mass is removed, the immediate reduction in LVOT gradients may be due to alterations in flow dynamics and abrogation of the Venturi forces that draw the anterior mitral leaflet towards the septum. Further study in a larger population of patients will be required to understand this phenomenon better.
We observed a marked improvement in the safety of this procedure following the initial experience in 2022. This improvement can be attributed to several developments, the most important of which was developing our procedural plans internally using dedicated CT software (3mensio). This software allows curved multiplanar reconstructions along the LVOT centreline to better understand the three-dimensionality of the septum as represented in procedural biplane fluoroscopy. The importance of this evolution in our preprocedural planning cannot be overstated for centres that wish to start a SESAME programme, particularly in the absence of dedicated lacerating equipment. Other evolutionary and local changes to the SESAME technique also played a role in improving the safety of this procedure. These included abandoning ICE assessment from the left ventricle, which was the cause of an early fatal left ventricular free-wall perforation, and double insulating the traversing wire in the myocardium to limit the chance of steam pop. Despite early and frequent collaboration, we also paused our programme at the end of 2022 until our team could attend a “reverse proctoring” opportunity to more directly observe cases and interface with colleagues at another centre. The most notable lesson from that experience was increasing dedication to the aforementioned CT plan as a “source of truth” to be subsequently corroborated by TOE. Specifically, as seen on fluoroscopy, the wire’s trajectory in the myocardium must adhere with high fidelity to the preplanned trajectory described on the CT (Figure 3). TOE is then used to confirm or raise concerns about wire depth and traversal length within the myocardium before considering wire externalisation and laceration (Figure 4). With these changes in place, we saw marked reductions in procedural complications. Nevertheless, the inoperable, pre-TMVR population that makes up the majority of our study participants remains a highly morbid cohort, as reflected in our data and all TMVR datasets8910.
The SESAME procedure is an innovative and distinctive technique that offers new opportunities for patients needing septal reduction therapy. Alternative minimally invasive procedures, such as ASA, are commonly used but come with notable drawbacks. ASA is associated with high rates of PPM placement34, is dependent on specific coronary anatomy, and frequently does not result in an adequate increase in the neo-LVOT area3. Another alternative, radiofrequency ablation, varies in risk depending on the technique used and can also result in near-universal rates of PPM placement1112 and elevated rates of pericardial effusion13. Novel pharmaceutical agents may play a significant role in the management of oHCM specifically, but these medicines are quite expensive, require substantial monitoring that may not be feasible for everyone, and have a non-durable effect if the medication is discontinued. These limitations underscore the need for additional effective solutions. In this context, SESAME emerges as a crucial alternative. It provides a more effective way to manage interventricular septal hypertrophy in a broad range of patients and addresses the limitations of other procedures. Specific to the issues of heart block, our experience with SESAME suggests a PPM rate <6% and no episodes of delayed heart block. This is because we explicitly lacerate anterior to the membranous septum away from the His system. Nevertheless, continued refinement of this nascent technique will be necessary.
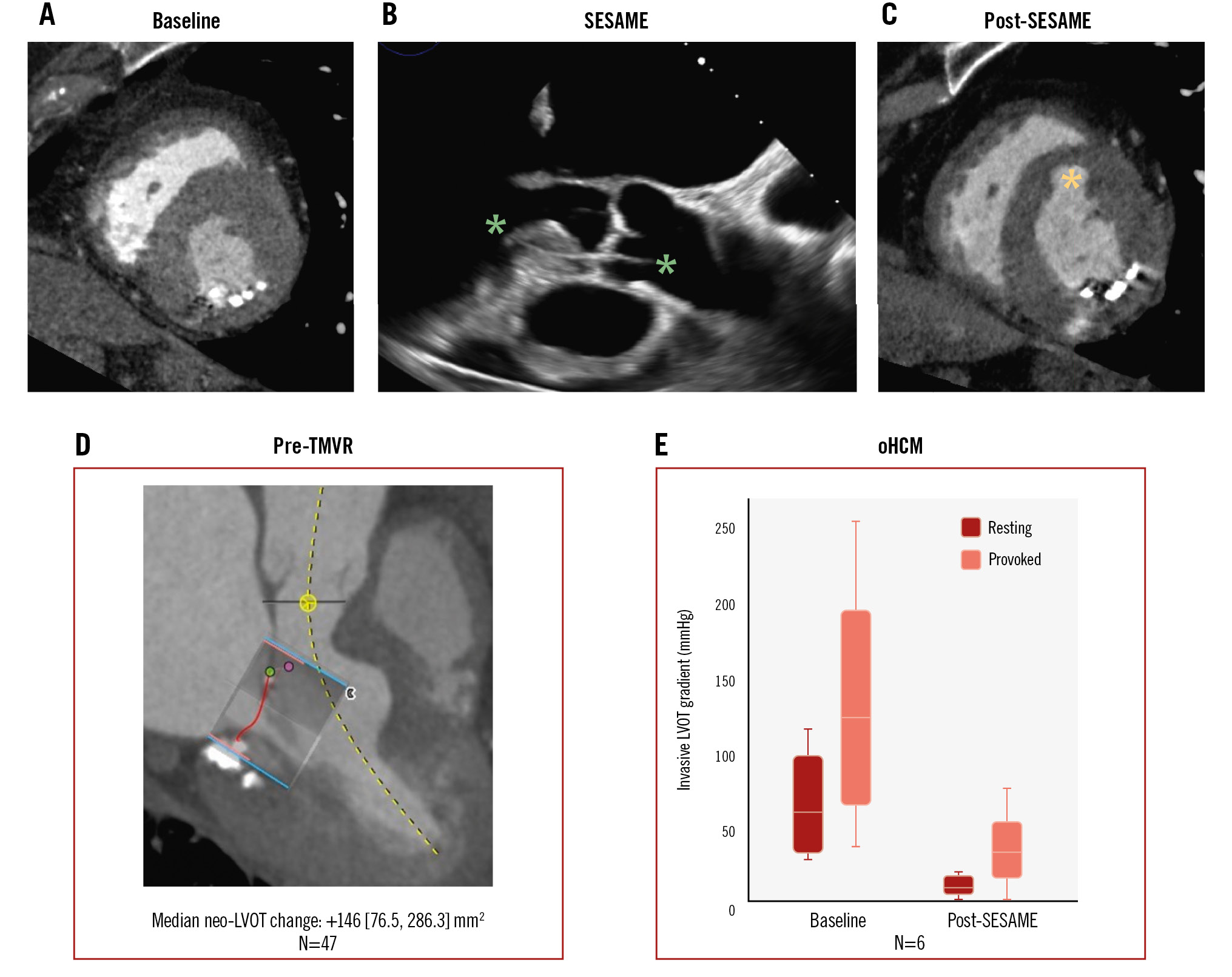
Central illustration. The SESAME technique. Representative CT scan short-axis view of the left ventricle before (A) and after (C) SESAME. B) Trajectory and depth of the SESAME wire traversal in the intraventricular septum as seen on TOE. Green asterisks highlight the wire course. D) SESAME effects in the pre-TMVR population. E) SESAME effects in the obstructive hypertrophic cardiomyopathy (oHCM) population. Ao: aorta; LA: left atrium; LV: left ventricle; LVOT: left ventricular outflow tract; RV: right ventricle; SESAME: septal scoring along the midline endocardium; TMVR: transcatheter mitral valve replacement; TOE: transoesophageal echocardiography
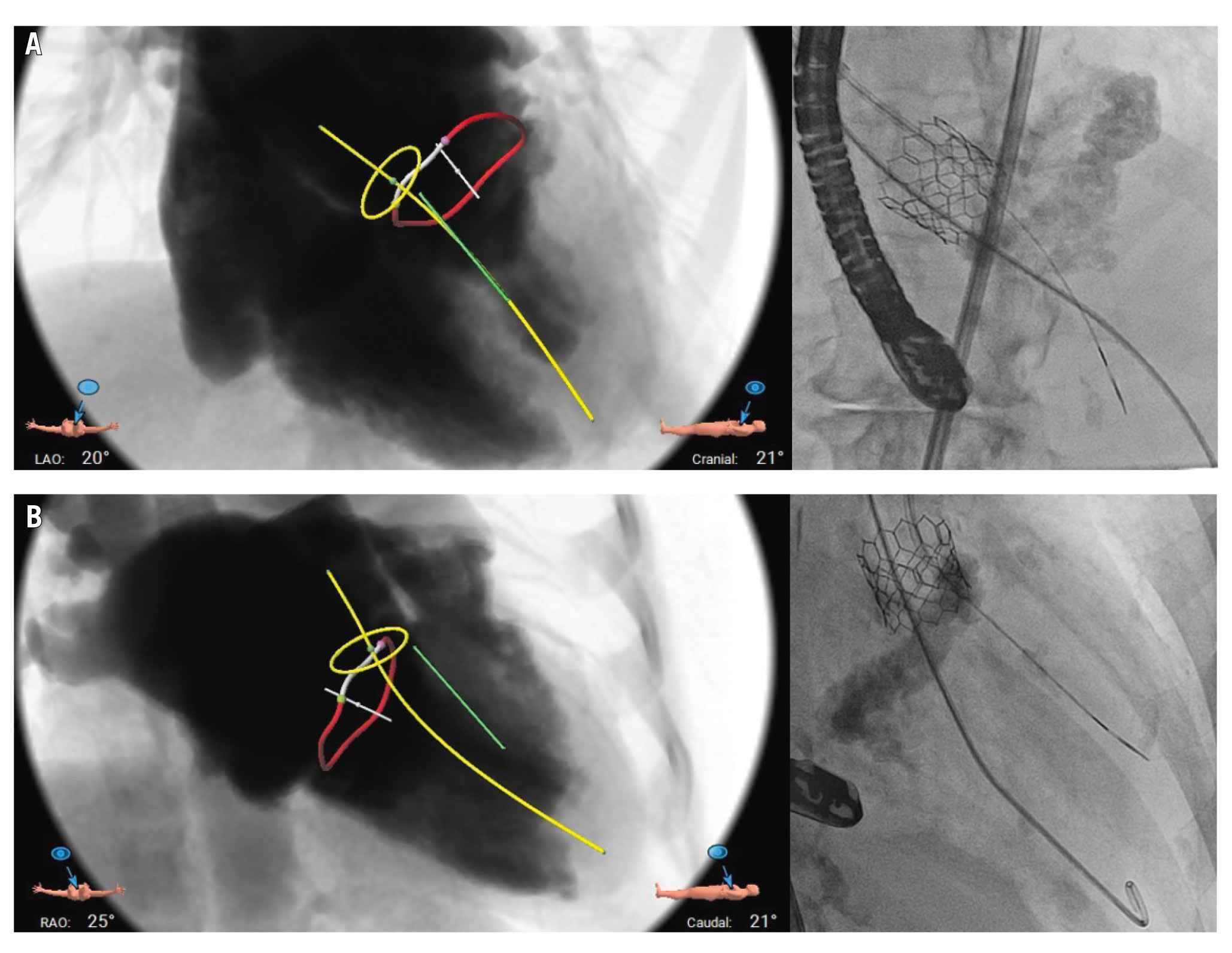
Figure 3. Preprocedural planning using simulated fluoroscopic views by CT scan, shown with corresponding actual procedural fluoroscopy images. Left anterior oblique (LAO) cranial projection (A) and right anterior oblique (RAO) caudal projection (B) demonstrating the relationship between the CT-derived aortic annulus (yellow ring), mitral annulus (red ring), left ventricular outflow tract midline (yellow line), as well as the desired SESAME wire trajectory (green line). Actual wire position is consistent with the intended course. CT: computed tomography; SESAME: septal scoring along the midline endocardium
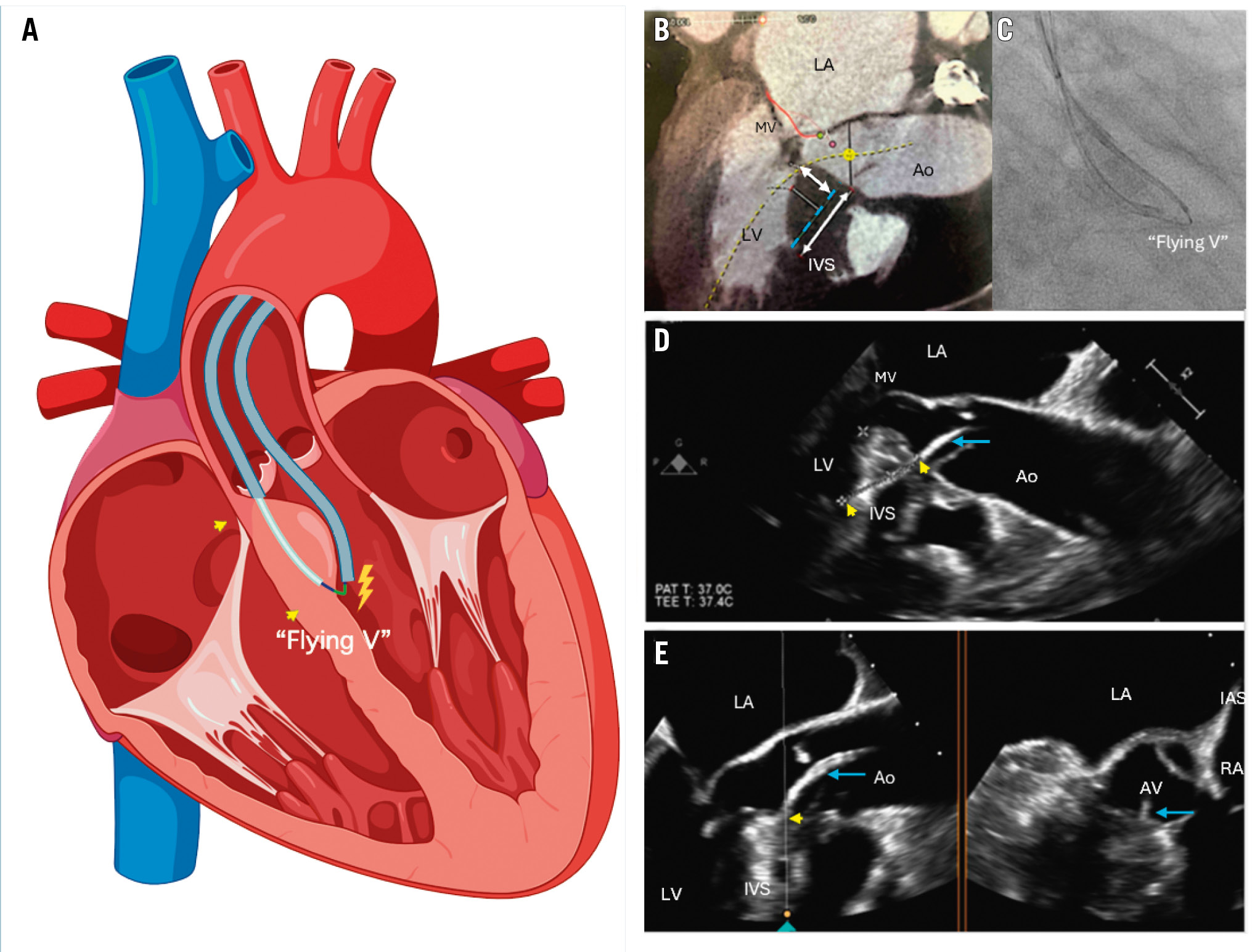
Figure 4. Procedural schematic. A) Schematic illustration of the SESAME technique. The darker blue lines represent the guide catheter, while the light blue line shows the microcatheter navigating through the septum. The “flying V” marks the location where electricity is applied. The yellow arrows indicate the wire’s entrance and exit points. B) Cardiac CT image, with the dashed blue line indicating the desired trajectory of the wire navigating through the septum. The double-headed arrows represent the ideal width and length of the path. C) Fluoroscopic image of the “flying V”. D) TOE showing the guide catheter (blue arrow) and the wire’s entrance and exit points (yellow arrows). E) TOE biplane view, showing the guide catheter (blue arrow) and the wire’s entry point (yellow arrow). Ao: aorta; AV: aortic valve; IAS: interatrial septum; IVS: interventricular septum; LA: left atrium; LV: left ventricle; MV: mitral valve; RA: right atrium; SESAME: septal scoring along the midline endocardium; TOE: transoesophageal echocardiogram
Limitations
This study has significant limitations. The data originate from a single-centre, non-randomised, retrospective study conducted by experienced operators. The SESAME procedure requires biplane fluoroscopy and dedicated procedural echocardiography. The results may not be reproducible in all centres, particularly where operators might have less experience with electrosurgical techniques and the required interventional echocardiographic skills. Dedicated devices for SESAME that require only a single plane of fluoroscopy and less sophisticated TOE imaging are currently in development and should help relieve this limitation. Secondly, the small sample size may limit the generalisability of the findings to a broader population, though it is notable that most of our patients are female. Also, the SESAME procedure was offered as compassionate use to patients with significant comorbidities who were not candidates for surgery, leading to a selection bias towards the most critically ill patients. Additionally, our population is too small to develop technical or patient-related predictors of success at this time. Furthermore, we have not yet tried to treat patients with mid-cavitary or distal cavity obstruction, and it is unclear whether this is a solution for those phenotypes of oHCM. Finally, a retrospective study design lends itself to gaps in data collection, which may affect the reliability of the results.
Conclusions
Our study showcases contemporary experience with SESAME for high or prohibitive surgical risk patients needing septal reduction therapy prior to TMVR, for the treatment of oHCM with LVOT obstruction, or for the treatment of subvalvular aortic stenosis. Our data corroborate previously published data regarding the efficacy and feasibility of this procedure. SESAME is relatively safe and feasible, though technically challenging, and there does appear to be a relevant learning curve. Further research is needed to refine patient selection and procedural techniques.
Impact on daily practice
Septal scoring along the midline endocardium (SESAME) is a novel transcatheter intervention designed to replicate surgical myotomy and holds great promise, but clinical results have only been published from a single medical centre. This second report on the clinical outcomes of SESAME, however, validates the role of this technique as a septal reduction strategy in preparation for transcatheter mitral valve replacement or obstructive hypertrophic cardiomyopathy. We identify a learning curve of 10 cases.
Funding
Our study received indirect funding via the David and Nancy Auth Chair in Cardiovascular Innovation.
Conflict of interest statement
J.M. McCabe: equity: Excision Medical, Transmural Systems, and ConKay Medical; consulting: Edwards Lifesciences, Medtronic, and Abbott. C.J. Chung: consulting: Edwards Lifesciences, Boston Scientific, and Medtronic. A.B. Greenbaum: equity: Excision Medical and Transmural Systems; consulting: Edwards Lifesciences, Medtronic, Abbott, and Polares. D. Elison: equity: Excision Medical. V.C. Babaliaros: equity: Transmural Systems; consulting: Edwards Lifesciences. R.J. Lederman: co-inventor on applicable patents assigned to his employer (NIH). G.B. Mackensen: research grants: Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

