Cory:
Unlock Your AI Assistant Now!
The two randomised controlled trials (RCTs) of transcatheter tricuspid valve intervention (TTVI) devices12 failed to show improvements in mortality or heart failure hospitalisation (HFH), leading to numerous unanswered questions about the benefits of device therapies for severe, symptomatic tricuspid regurgitation (TR). The outcomes of these trials were driven by symptomatic improvement and patient-reported outcomes measured by the Kansas City Cardiomyopathy Questionnaire overall summary (KCCQ-OS) score, which showed marked improvements that correlated with the degree of TR reduction, thus giving it biological credence34. However, following U.S. Food and Drug Administration approval of the EVOQUE transcatheter tricuspid valve replacement system (Edwards Lifesciences) and TriClip (Abbott), the Centers for Medicare & Medicaid Services called for more evidence of benefit for a wider range of patients.
Given the clinical availability of TTVI devices, another randomised trial seems a pipe dream, although one randomised study is enrolling: the Deutsches Zentrum für Herz-Kreislauf-Forschung (DZHK) TRICI-HF trial (ClinicalTrials.gov: NCT04634266). The trial is a German multicentre, industry-independent strategy trial randomising patients 2:1 to TTVI with optimal medical therapy (OMT) versus OMT alone. The trial hopes to enrol patients with higher mortality risk by requiring an HFH within the year prior to enrolment, or the presence of cardiorenal or cardiohepatic syndrome. Unfortunately, permitted crossover after the 1-year primary endpoint, necessary to encourage patient enrolment, may be a limitation to seeing hard outcome benefits.
In lieu of RCTs, registries may provide additional evidence, as they typically include a broader patient population without the exclusions of the trials567. Table 1 lists the baseline criteria as well as the outcomes from the TRILUMINATE Pivotal RCT1, the TRI.FR RCT6, the bRIGHT post-market registry5, and the largest registry of tricuspid transcatheter edge-to-edge repair (T-TEER), PASCAL for Tricuspid Regurgitation – a European Registry (PASTE), which recently reported their 1-year results of 1,059 patients treated with the PASCAL device (Edwards Lifesciences)7. With no inclusion or exclusion criteria, the PASTE Registry represents an all-comers, consecutively treated patient registry.
In the current issue of EuroIntervention, Baldus et al8 report the 1-year results of the TriCLASP study − a prospective, single-arm, post-market clinical follow-up study with centres in Germany, Switzerland, Greece, and Italy which enrolled 300 patients deemed eligible for T-TEER with the PASCAL system. Importantly, there was overlap in sites enrolling in this study, and participating in the PASTE Registry (10 sites in Germany and 1 in Switzerland)7. Although patients in the TriCLASP study were enrolled based on site-reported ≥severe TR, an independent echocardiography core laboratory was otherwise used throughout the study. Multiple exclusion criteria were imposed including septolateral coaptation gap >10 mm in the grasping area; severe stenosis and/or regurgitation of the aortic, mitral, and/or pulmonic valves; and severe renal insufficiency, with an estimated glomerular filtration rate ≤25 mL/min/1.73 m² or requiring chronic renal replacement therapy. The baseline characteristics compared to other trials and registries are listed in Table 1.
Baseline TR and change in TR at 30 days and 1 year compared to other trials and registries are shown in Table 1 and Figure 1. Of note, the TriCLASP study enrolled 24% of patients with moderate TR, 65% with severe TR, and only 11% with massive/torrential TR (as adjudicated by the echo core lab), suggesting the severity of disease was much less than in the other trials/registries, which enrolled 55-91% of patients with massive/torrential TR (Figure 1). Given the multiple studies that show an association between baseline massive/torrential disease with worse T-TEER efficacy17 we would have expected this study to show excellent reduction of TR. However, despite enrolling fewer patients with massive and torrential disease, only 56% of patients achieved mild residual TR, a rate not significantly different from those observed in the other studies. The relatively low improvement in KCCQ-OS score (8.3±18.8 points at 1 year) and the subgroup analysis, showing a 7-point improvement with a 1-grade TR reduction, indicate that a large number of patients achieved only a 1-grade reduction in TR. A single grade of TR reduction, however, is no longer considered an acceptable goal for T-TEER. The Tricuspid Valve Academic Research Consortium (TVARC) defines intraprocedural success as a reduction of total TR to optimal (≤mild) or acceptable (≤moderate)9. Recent studies suggest the goal should be to achieve ≤mild-moderate residual TR to affect mortality10.
Further evaluation of the reasons for the modest efficacy results for T-TEER would be important to gain insight into the limitations of this technology. Wild et al7 showed a strong association between operator volume and outcomes; centres that performed ≥21 PASCAL T-TEER procedures per year had significantly lower procedure times, lower single leaflet device attachment (SLDA) rates, higher intraprocedural success rates, and improved 1-year clinical success rates (defined by TVARC as survival without significant TR recurrence or HFH, as well as symptomatic improvement)9. The PASTE registry also showed that the PASCAL Precision system (Edwards Lifesciences) was associated with better clinical success; this newer system was used in only 19% of the TriCLASP study population. Other studies have suggested numerous anatomical limitations other than baseline TR (i.e., septolateral coaptation gap, TR jet location, chordal structure density, image quality and en face tricuspid valve morphology) that impact the efficacy of TTVI, and these should also be explored.
Nonetheless, the authors have certainly confirmed that the procedure remains safe, with low rates of major adverse events at 30 days and 1 year (1.7% and 12.7%), low cardiovascular mortality (0.3%), and low SLDA rates (4%). The 72% reduction in HFH compared to the site-reported incidence over the prior year is equivalent to the report from the large PASTE registry with this same device (Table 1). It is likely that right ventricular (RV) remodelling and improvement in cardiac output, without a change in RV function or pulmonary artery pressures, contributed to these excellent clinical outcomes. Given the discordance between TR reduction and clinical benefit, the role of medical management or other contributors to reductions in HFH should be explored to help clinicians comprehensively manage patients with severe, symptomatic TR.
Table 1. Comparison of baseline clinical characteristics in TRILUMINATE Pivotal, bRIGHT, TRI.FR, thePASTE Registry, and TriCLASP
| Baseline characteristics | TRILUMINATE Pivotal T-TEER group1 (N=175) |
bRIGHT5 (N=511) | TRI.FR T-TEER group6 (N=152) |
PASTE Registry7 (N=1,059) | TriCLASP study (N=300) |
|---|---|---|---|---|---|
|
Age, years |
78.0±7.4 |
78.9±7.1 |
78.3±6.4 |
79±9 |
80.1 |
|
Female sex |
56.0 |
56.0 |
65.5 |
53.0 |
52 |
|
NYHA Class III/IV |
59.4 |
80.0 |
38.9 |
84.0 |
75.8 |
|
Hypertension |
81.1 |
86.7 |
69.7 |
- |
85.3 |
|
Diabetes mellitus |
16.0 |
22.3 |
14.5 |
- |
26 |
|
Atrial fibrillation |
87.4 |
86.3 |
94.1 |
91.0 |
91.3 |
|
Prior stroke |
6.3 |
8.0 |
14.5 |
- |
6.7 |
|
Renal dysfunction |
35.4 |
39.5 |
- |
79.0 |
61.7 |
|
COPD |
10.9 |
13.1 |
5.9 |
15.9 |
- |
|
Peripheral vascular disease |
9.1 |
11.0 |
11.8 |
- |
- |
|
Prior CABG |
17.7 |
11.5 |
7.9 |
21.6 (open heart) |
8.3 |
|
LVEF |
59.3±9.3 |
55.8±10.6 |
57.0 (50-64) |
52.0±12.0 |
|
|
Prior AV intervention |
15.4 |
9.2 |
9.21 (any left valve) |
- |
30.3 (any left valve) |
|
Prior MV intervention |
24 |
26.8 |
- |
||
|
Functional TR |
94.8 |
90.0 |
- |
79.0 |
75.3 (degenerative 15.7%) |
|
Baseline TR severity |
|||||
|
Moderate |
2.3 |
2.0 |
- |
4.0 |
24.2 |
|
Severe |
25.4 |
10.0 |
- |
40.0 |
64.8 |
|
Massive |
21.4 |
61.3 |
63.0 |
35.0 |
8.9 |
|
Torrential |
50.9 |
26.7 |
28.0 |
20.0 |
2.1 |
|
CIED |
16.0 |
22.5 |
13.8 |
27.4 (contributing to TR in 64%) |
20 (contributing to TR in 2% of total) |
|
KCCQ-OS score |
56.0±23.4 |
44.5±22.6 |
54.0 |
- |
54.5±18.7 |
|
HFH within 1 year |
25.1 |
40.3 |
36.2 |
57.3 |
63.4 |
|
TRI-SCORE |
- |
- |
23±18 |
- |
|
| One-year outcomes of T-TEER | |||||
|
All-cause mortality |
8.8 |
15.1 |
3.4 |
14.0 |
11.0 |
|
Cardiovascular mortality |
6.5 |
8.8 |
- |
NA |
7.3 |
|
HFH |
14.9 |
15.3 |
- |
16.0 |
15.3 |
|
New-onset renal failure |
2.3 |
5.5 |
- |
- |
2.3 |
|
New pacemaker |
2.9 |
0.8 |
0.7 |
- |
- |
|
TV intervention/reoperation |
4.1 |
4.7 |
- |
2.3 |
0.4 |
|
Major bleeding |
5.2 |
10.8 |
- |
- |
2.8 |
|
SLDA |
7.0 |
3.9 |
- |
3.9 |
4.0 |
|
NYHA Class I/II |
83.9 |
75.0 |
- |
66.0 |
74.4 |
|
Δ KCCQ-OS |
12.3±1.8 |
19±26 |
15.9±30.1 |
- |
8.3±18.8 |
|
Δ 6MWD, m |
−8.1±10.5 |
- |
31.0 ±16.0 |
40.0 ±86.0 |
29.0 |
|
Data are given as mean±standard deviation, %, or median (IQR). AV: aortic valve; bRIGHT: An Observational Real-world Study Evaluating Severe Tricuspid Regurgitation Patients Treated with the Abbott TriClip Device; CABG: coronary artery bypass grafting; CIED: cardiac implantable electronic device; COPD: chronic obstructive pulmonary disease; HFH: heart failure hospitalisation; IQR: interquartile range; KCCQ-OS: Kansas City Cardiomyopathy Questionnaire overall summary; LVEF: left ventricular ejection fraction; MV: mitral valve; NYHA: New York Heart Association; PASTE: PASCAL for Tricuspid Regurgitation – a European Registry; SLDA: single leaflet device attachment; TR: tricuspid regurgitation; TRILUMINATE Pivotal: Clinical Trial to Evaluate Cardiovascular Outcomes In Patients Treated With the Tricuspid Valve Repair System Pivotal; TV: tricuspid valve; T-TEER: tricuspid transcatheter edge-to-edge repair |
|||||
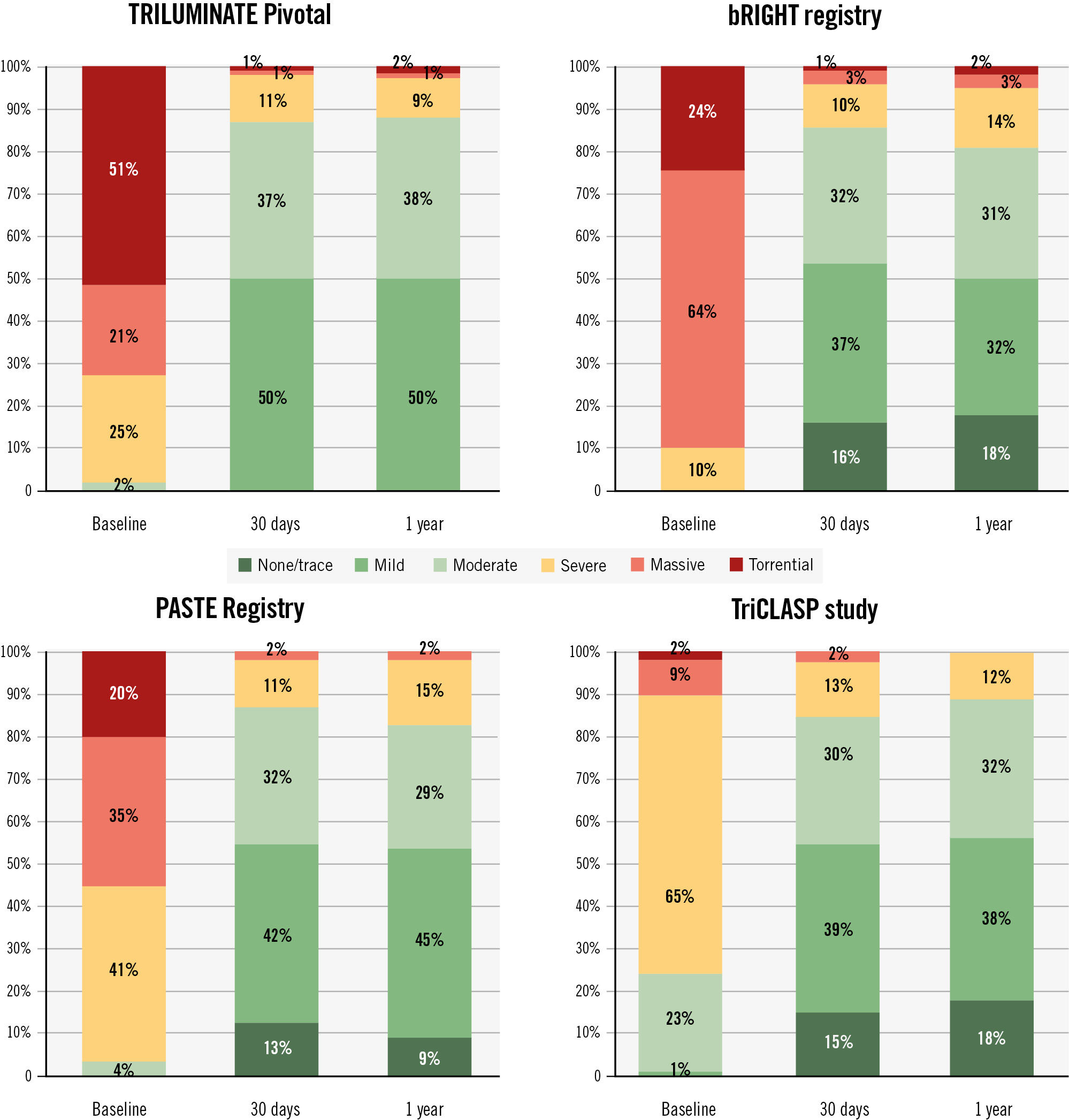
Figure 1. Comparison of change in tricuspid regurgitation in TRILUMINATE Pivotal, bRIGHT, the PASTE Registry and the TriCLASP study based on the 5-grade scale. Note: 3 different core labs were used for the TRILUMINATE Pivotal, bRIGHT registry and TriCLASP. The PASTE Registry used centralised analysis by 1 of 2 readers.
Conflict of interest statement
R.T. Hahn reports speaker fees from Abbott, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; she has institutional consulting contracts for which she receives no direct compensation with Abbott, Anteris, Boston Scientific, Edwards Lifesciences, Medtronic, Novartis, and Philips Healthcare; and she is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored valve trials, for which she receives no direct industry compensation.

