Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Transcatheter edge-to-edge repair (TEER) using the TriClip tricuspid valve repair system has emerged as a therapy for tricuspid regurgitation (TR). Patients with TR undergoing TEER commonly present with an endocardial lead across the tricuspid valve (TV).
Aims: We sought to examine the effectiveness and safety of tricuspid TEER (T-TEER) in subjects with endocardial leads in the bRIGHT EU Post-Approval Study (PAS).
Methods: The bRIGHT EU PAS is a prospective, single-arm, open-label, multicentre, post-market registry conducted at 26 sites in Europe. Echocardiographic assessments of endocardial lead placement, interaction, and TR grade were performed at a core laboratory.
Results: Of the 511 enrolled subjects, a total of 110 had an endocardial lead, and in 80.7% of these subjects, TR was at least partially related to the lead. At 30 days, 71% of subjects with endocardial leads had TR of moderate or less. The percentage of subjects with endocardial leads categorised as New York Heart Association Functional Class I-II increased from 17% at baseline to 75% at 30 days (p<0.0001), and quality of life with the Kansas City Cardiomyopathy Questionnaire showed a mean improvement of 20±24 points from baseline to 30 days (p<0.0001). T-TEER was safe in subjects with endocardial leads, with similar rates of events, including TV reintervention/reoperation and TV surgery, to those in subjects without leads. No reports of lead malfunction were reported.
Conclusions: In the bRIGHT EU PAS, T-TEER using the TriClip system was safe and effective in severe TR subjects with an endocardial lead across the TV.
Clinically significant tricuspid regurgitation (TR) affects approximately 1% of the population. If left untreated, TR can lead to increased morbidity and mortality1. When specifically looking at the impact of TR on survival in patients with cardiac implantable electronic devices (CIEDs), there was >50% excess mortality attributed to severe TR after multivariate adjustment compared with subjects without CIEDs2. To emphasise the complexity of this TR pathomechanism, the Tricuspid Valve Academic Research Consortium has recently defined a separate category for patients with lead-related TR3.
In recent years, several catheter-based techniques have emerged to manage TR, providing an optimistic outlook for a previously neglected patient population4. However, catheter-based repair or replacement therapies may face technical difficulties in patients with CIEDs due to the lead crossing the tricuspid valve (TV). In light of increasing treatment options for patients with lead-induced TR, the latest recommendation for treatment advocates a Heart Team approach involving electrophysiologists, heart surgeons with experience in lead extraction, imaging specialists and interventional cardiologists5. Currently, tricuspid transcatheter edge-to-edge repair (T-TEER) is the most implemented procedure worldwide, owing to its safety, availability, and user-friendliness. Most recently, the TRILUMINATE Pivotal Trial − a randomised trial comparing T-TEER with medical treatment alone in symptomatic subjects with TR − showed that the TriClip system (Abbott) has a high safety profile, efficiently reduces TR, and significantly improves quality of life post-procedure6. The bRIGHT EU Post-Approval Study (PAS) is a prospective, single-arm, open-label, multicentre, post-market registry assessing the safety and efficacy of this transcatheter tricuspid valve repair system in an unselected real-world cohort7. While the outcomes of the complete cohort have been reported previously7, this subanalysis of bRIGHT EU PAS assesses T-TEER procedural safety and performance in subjects with CIEDs.
Methods
Study design and patient population
The bRIGHT EU PAS is a prospective, single-arm, open-label, multicentre, post-market registry designed to confirm the safety and performance of the TriClip TEER system in a contemporary real-world setting (ClinicalTrials.gov: NCT04483089). A total of 511 consecutive subjects were enrolled at 26 sites in Europe, where eligibility for treatment with the TEER system was determined through site-specific, standard-of-care procedures, in addition to evaluating the patients according to the protocol-specific inclusion and exclusion criteria. Briefly, subjects were required to have severe, symptomatic TR despite medical therapy, be at least 18 years of age, eligible to receive T-TEER per the currently approved intended use and target patient population, and not to be a participant in another clinical study that could affect the follow-up or results of the bRIGHT EU PAS. Local ethics committees and the respective health authorities of the participating countries approved the study. All subjects provided written informed consent.
Echocardiographic assessment
All echocardiograms were analysed by an independent core laboratory that followed the European Association of Cardiovascular Imaging and American Society of Echocardiography standards8910. Tricuspid regurgitation was assessed using standard two-dimensional (2D) colour Doppler methods and was graded using a class-grading scheme of none, mild, moderate, severe, massive, and torrential, thereby enabling a broader and yet differentiated assessment311. The presence of a pacemaker lead across the TV was verified by the echocardiography core laboratory.
To delineate the lead position and to examine possible lead-leaflet interference, the echo core lab reviewed both screening and procedural echocardiographic data. In a first step, 2D transthoracic echocardiogram (TTE) images (right ventricle [RV]-focused apical 4-chamber, parasternal RV long-axis and RV inflow-outflow views) were used to obtain an overview of the course of the lead through the TV into the RV. In addition, these views helped to identify lead adherence to the subvalvular apparatus. To detect an interference between the lead and the valve on a leaflet level, the following transoesophageal views were systematically analysed: transgastric TV short- and long-axis, midoesophageal-focused TV, and RV inflow-outflow views. A thorough comparison of the lead position in the transgastric short-axis view before and after device implantation was performed. Not all studies included optimal three-dimensional (3D) zoom images of the TV, but these data were analysed using direct “en face” and multiplanar reconstruction views using a dedicated software tool (4D CARDIO-VIEW [TOMTEC]). Leaflet impingement was defined as blocking of the leaflet systolic excursion towards the valve centre, which is associated with an eccentric colour Doppler jet originating from the lead. The motion of the leaflets was looked at carefully. TR induced by a lead was frequently associated with an asymmetric restriction of the leaflets, mostly involving the septal leaflet. Lead-leaflet adhesion was suspected when a lead was visually attached to the leaflet with thickening and tethering at the point of contact, resulting in leaflet malcoaptation. Subvalvular entanglement was considered when the lead was anchored in a constrained TV apparatus, resulting in a colour jet coming from the lead below the leaflet coaptation line and a concordant movement of both structures. Perforation was determined from a conjunction of elements, including the absence of the usual characteristics of a TV regurgitant jet, a colour jet coming from the body of the leaflet, an eccentric or unusual jet orientation, and jet location. Based on TTE examination at discharge and 30-day follow-up echocardiography, the core laboratory graded the impact of T-TEER on TR severity and detected the occurrence of single leaflet device attachment (SLDA) when present.
Clinical outcomes
Implant success was defined as the successful delivery and deployment of the T-TEER device. Acute procedural success was defined as successful device implantation resulting in TR reduction of at least 1 grade at discharge (or 30-day follow-up if discharge TR was unavailable), as assessed by the echocardiography core laboratory. Major adverse events − including cardiovascular mortality, myocardial infarction, stroke, new-onset renal failure, endocarditis requiring surgery, and non-elective cardiovascular surgery for tricuspid valve repair system-related adverse events − were adjudicated by an independent events committee. Additional safety endpoints (e.g., major bleeding, new-onset liver failure) and heart failure hospitalisations were assessed at each site according to definitions provided in the clinical investigation plan. Clinical status was assessed using New York Heart Association (NYHA) Functional Class and the Kansas City Cardiomyopathy Questionnaire (KCCQ).
Statistical analysis
All subjects who signed and dated an informed consent form and had an attempted procedure, upon femoral vein puncture, with the TriClip system were included in the analysis population. Data are presented as mean±standard deviation (SD) for continuous variables and are presented as counts and percentages for categorical variables. A paired t-test was used to compare the mean of paired continuous variables, and McNemar’s test was used to compare paired categorical data. All statistical analyses were performed using SAS, version 9 (SAS Institute).
Results
Baseline characteristics
The baseline characteristics of enrolled subjects with pacing leads are compared with those of subjects without leads in Table 1. A total of 110 subjects with pacing leads (44% female, mean age of 79±8 years) and 401 subjects without a pacing lead (59% female, mean age of 79±7 years) were included in this analysis. Baseline TR severity was massive or torrential in most subjects, with 87% and 88% for lead and non-lead subjects, respectively. Subjects with and without leads also had similar percentages of baseline NYHA Class III/IV and similar baseline KCCQ scores. All enrolled subjects had significant comorbidities including hypertension (87%), atrial fibrillation (86%), chronic renal disease (40%), diabetes (22%), and prior myocardial infarction (10%), and similar rates were seen for subjects with or without leads. Subjects with leads had higher rates of heart failure hospitalisation in the year preceding the index procedure compared with subjects without leads (49% vs 40%). The location of the pacing lead was commissural (64.6%), central (6.1%), posterior (15.2%), septal (13.1%), or anterior (1.0%) in subjects with leads (Central illustration). Lead interaction was classified as none (12.1%), impingement (65.7%), adhesion (13.1%), subvalvular entanglement (8.1%), or perforation (1.0%). TR was not induced by the lead in 19.3% of subjects, partially induced by the lead in 33.0%, and induced by the lead in 47.7% of subjects.
Table 1. Baseline characteristics.
| Baseline characteristics | Pacing lead (n=110) | No pacing lead (n=401) | Total (N=511) |
|---|---|---|---|
| Age, years | 78.7±7.8 | 79.0±7.0 | 78.9±7.1 |
| Male/female | 56.4/43.6 | 40.9/59.1 | 44.2/55.8 |
| NYHA Class III/IV | 82.6 | 79.1 | 79.8 |
| KCCQ score | 42.32±24.37 | 45.10±22.05 | 44.54±22.55 |
| Baseline TR severity | |||
| Moderate | 0.9 | 2.3 | 2.0 |
| Severe | 12.0 | 9.4 | 10.0 |
| Massive | 60.2 | 61.6 | 61.3 |
| Torrential | 26.9 | 26.7 | 26.7 |
| Hypertension | 87.3 | 86.5 | 86.7 |
| Atrial fibrillation | 86.4 | 86.3 | 86.3 |
| Prior aortic intervention | 6.4 | 10.0 | 9.2 |
| Prior mitral intervention | 31.8 | 25.4 | 26.8 |
| Prior CABG | 14.5 | 10.7 | 11.5 |
| Diabetes | 25.5 | 21.4 | 22.3 |
| Renal disease | 48.2 | 37.2 | 39.5 |
| Chronic obstructive pulmonary disease | 15.5 | 12.5 | 13.1 |
| Peripheral vascular disease | 7.3 | 12.0 | 11.0 |
| Prior stroke | 7.3 | 8.2 | 8.0 |
| Prior myocardial infarction | 13.6 | 9.5 | 10.4 |
| Prior heart failure hospitalisation(1 year pre-index procedure) | 49.1 | 39.9 | 41.9 |
| Data are mean±standard deviation or %. CABG: coronary artery bypass graft; KCCQ: Kansas City Cardiomyopathy Questionnnaire; NYHA: New York Heart Association; TR: tricuspid regurgitation | |||
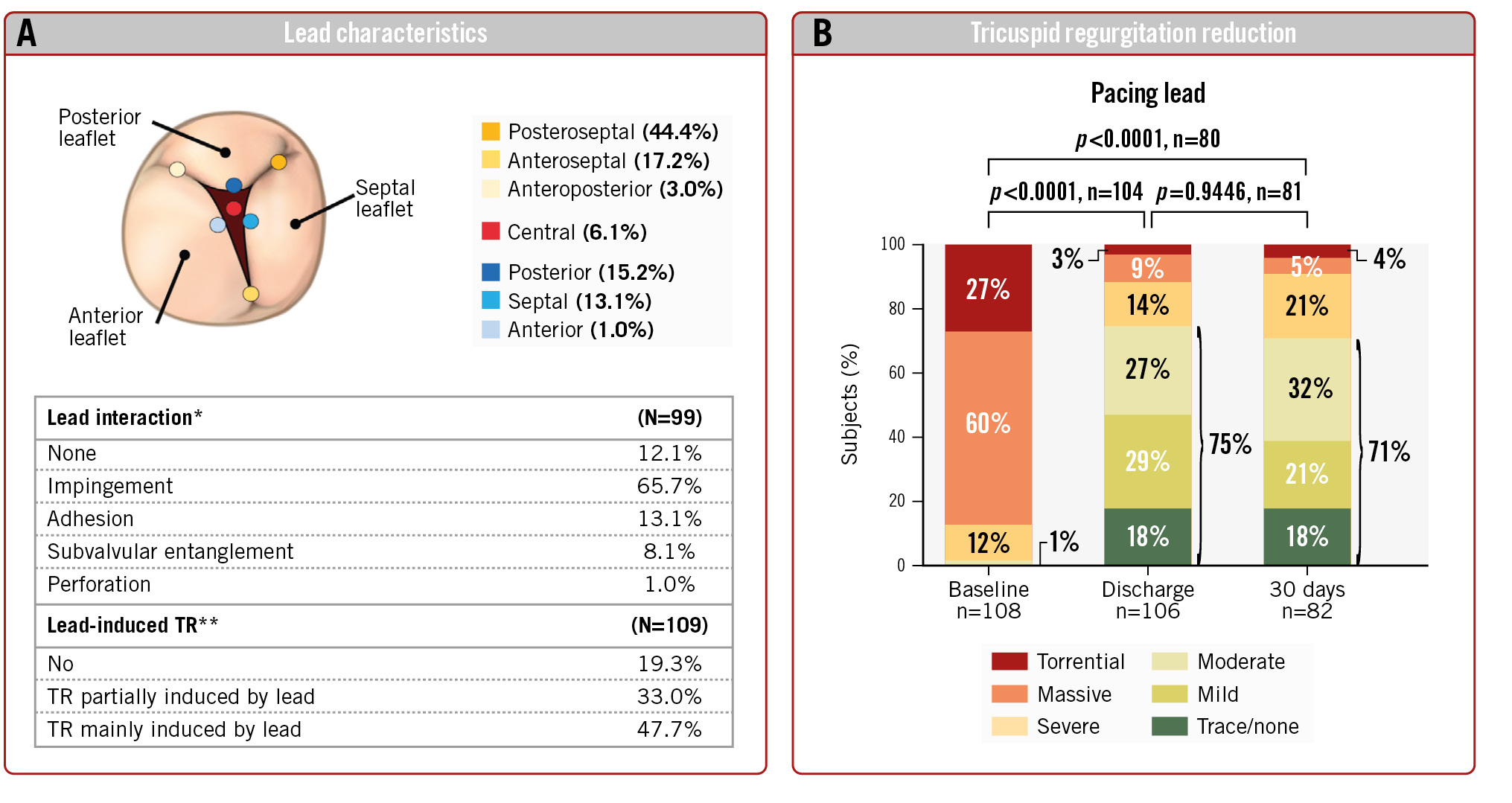
Central illustration. Outcomes of TriClip TEER in subjects with endocardial leads. A) Lead characteristics. The location of the lead, interaction with leaflets, and degree of contribution to the TR. *Most pronounced interaction is reported. **Lead interaction with leaflet was determined to be the mechanism contributing to TR. B) TR reduction. Discharge and 30-day TR grades compared with baseline TR grade for subjects with endocardial leads. TEER: tricuspid edge-to-edge repair; TR: tricuspid regurgitation
Procedural outcomes
Implant success was achieved in 99% of subjects with and without leads. Procedural success was achieved in 92% of the subjects with leads and 91% of the subjects without leads. Acute procedural success also did not differ for subjects with no interaction or impingement as compared with subjects with adhesion, subvalvular entanglement, and perforation (93.1% and 91.3%, respectively). There were minimal differences in device time, fluoroscopy duration, number of clips per subject, clip size, and location of leaflet attachment between subjects with and without leads (Table 2).
Paired analysis showed that TR severity at discharge was moderate or less in 75% of subjects with endocardial leads treated with the TriClip device, compared with only 1% at baseline (p<0.0001) (Central illustration). At 30 days, 71% of subjects remained at moderate or less TR – this difference was not significantly different from discharge. The percentage of subjects with moderate or less TR in subjects without leads was similar at discharge and 30 days (81% and 78%, respectively) (Figure 1).
Significant reductions occurred between baseline and 30-day follow-up for subjects with leads in effective regurgitation orifice area (0.91±0.61 cm2 to 0.49±0.42 cm2; p<0.0001), regurgitant volume (64.83±27.46 mL/beat to 33.96±20.65 mL/beat; p<0.0001), regurgitant jet area (9.79±5.52 cm2 to 6.41±4.76 cm2; p=0.0004), vena contracta width (0.90±0.41 cm to 0.51±0.31 cm; p<0.0001), and proximal isovelocity surface area (PISA) radius (0.87±0.24 cm to 0.59±0.26 cm; p<0.0001) (Table 3).
Table 2. Procedural results.
| Procedural results | Pacing lead (n=110) | No pacing lead (n=401) | Total (N=511) |
|---|---|---|---|
| Implant success | 99.1 | 98.5 | 98.6 |
| Acute procedural success* | 91.5 | 90.8 | 90.9 |
| Device time, min | 80.3±38.5 | 74.7±38.5 | 75.9±38.6 |
| Fluoroscopy duration, min | 17.5±13.9 | 17.3±15.1 | 17.4±14.8 |
| Average number of clips per subject | 2.0±0.7 | 1.9±0.7 | 1.9±0.7 |
| TriClips used | |||
| XT only | 46.4 | 48.4 | 47.9 |
| XTW only | 36.4 | 36.7 | 36.6 |
| XT and XTW | 16.4 | 12.7 | 13.5 |
| Other | 0.9 | 2.1 | 2.0 |
| Location of leaflet attachment | |||
| Anterior-septal leaflets only | 47.2 | 52.8 | 51.6 |
| Anterior-septal and posterior-septal leaflets | 35.2 | 31.6 | 32.3 |
| Posterior-septal leaflets only | 9.3 | 6.1 | 6.7 |
| Anterior-septal and anterior-posterior leaflets | 6.5 | 4.8 | 5.2 |
| Other | 1.9 | 2.8 | 2.6 |
| Data are presented as % or mean±standard deviation. *TR reduced by at least 1 grade. TR: tricuspid regurgitation | |||
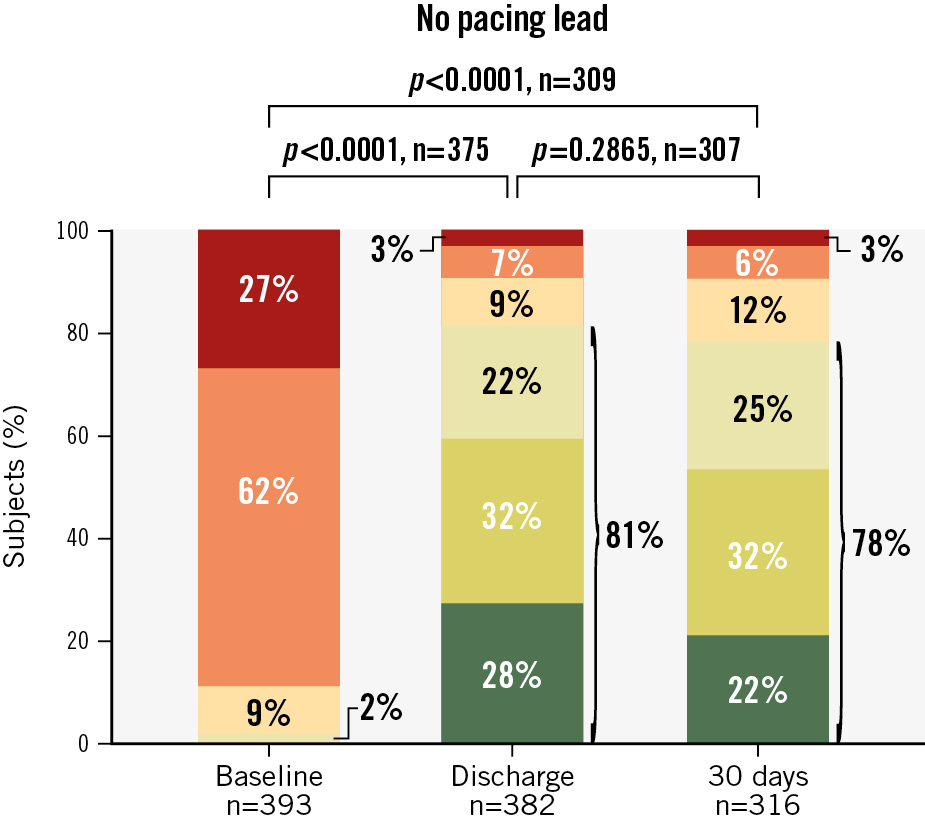
Figure 1. Reduction in tricuspid regurgitation during 30-day follow-up for subjects without pacing leads. Discharge and 30-day TR grades compared with baseline TR grades for subjects without endocardial leads.
Table 3. Summary of echocardiographic endpoints in subjects with a CIED.
| Summary of echocardiographic endpoints | Baseline | 30 days | p-value |
|---|---|---|---|
| Tricuspid regurgitation | |||
| Effective regurgitant orifice area, cm2 | 0.91±0.61 | 0.49±0.42 | <0.0001* |
| Regurgitant volume, mL/beat | 64.83±27.46 | 33.96±20.65 | <0.0001* |
| Regurgitant jet area, cm2 | 9.79±5.52 | 6.41±4.76 | 0.0004* |
| Vena contracta width, cm | 0.90±0.41 | 0.51±0.31 | <0.0001* |
| PISA radius, cm | 0.87±0.24 | 0.59±0.26 | <0.0001* |
| IVC diameter, cm | 2.41±0.71 | 2.33±0.83 | 0.5755 |
| Data are given as mean±standard deviation. *Indicates statistical significance. CIED: cardiac implantable electronic device; IVC: inferior vena cava; PISA: proximal isovelocity surface area | |||
Clinical outcomes
Significant improvements in functional capacity and quality of life were seen at 30-day follow-up for subjects with endocardial leads. The percentage of subjects categorised as NYHA Functional Class I or II increased from 17% at baseline to 75% at 30 days (p<0.0001) (Figure 2A). For subjects without leads, NYHA Functional Class I or II increased from 21% to 80% (Figure 2B). Assessment of quality of life with the KCCQ showed a mean improvement of 20±24 points from baseline to 30-day follow-up (p<0.0001) for subjects with a lead (Figure 3) compared with an 18±22 point increase from baseline to 30-day follow-up (p<0.0001) for subjects without an endocardial lead.
At 30-day follow-up, cardiovascular mortality was 0% for subjects with leads and 1% for subjects without leads (Table 4). Two (1.8%) of the 110 subjects with leads experienced a major adverse event, including one new-onset renal failure and one non-elective cardiovascular surgery for a device-related adverse event. The subject with non-elective cardiovascular surgery was scheduled to undergo an additional tricuspid repair procedure on postoperative day 2, during which it was decided that the valve anatomy was no longer suitable for repair, and thus TV replacement with an Epic valve (Abbott) was performed. Comparatively, the major adverse event rate for subjects without leads was 2.7% (11/401), including cardiovascular mortality (n=4), stroke (n=2), and new-onset renal failure (n=6). Tricuspid valve and cardiac interventions that occurred in subjects with endocardial leads up to 30 days included one TV reintervention/reoperation and one TV surgery; these values were similar to the number of subjects without leads that experienced these events (TV reintervention/operation [n=2], TV surgery [n=1]). There were no cases of myocardial infarction or embolisation in any subjects.
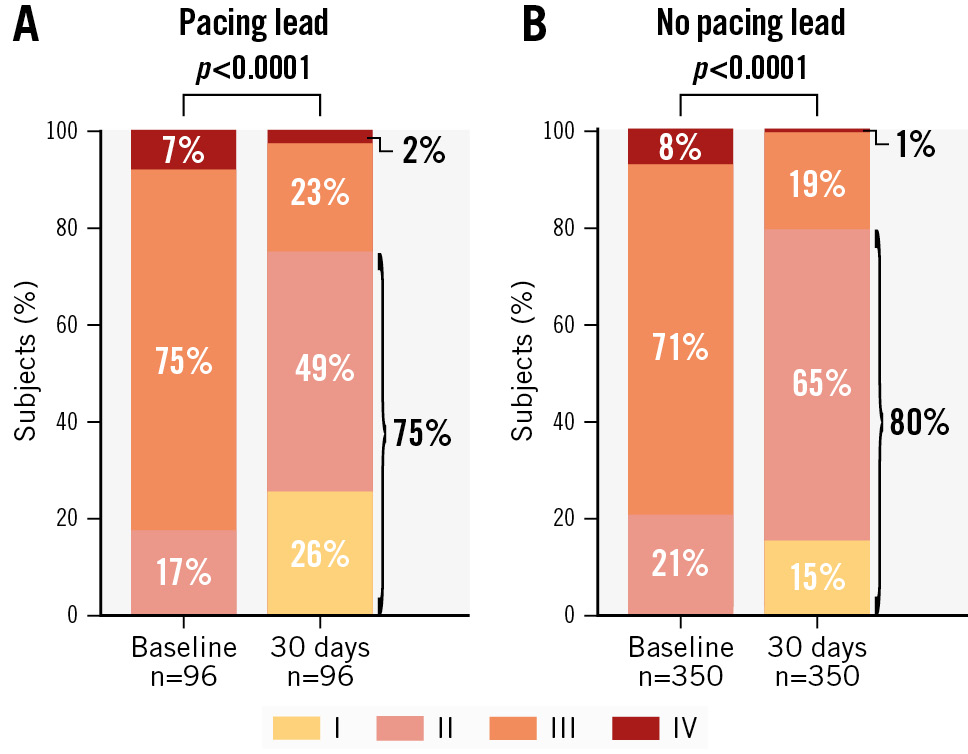
Figure 2. NYHA Class during 30-day follow-up for subjects with and without pacing leads. Thirty-day NYHA Class compared with baseline NYHA Class for subjects with (A) and without (B) endocardial leads.
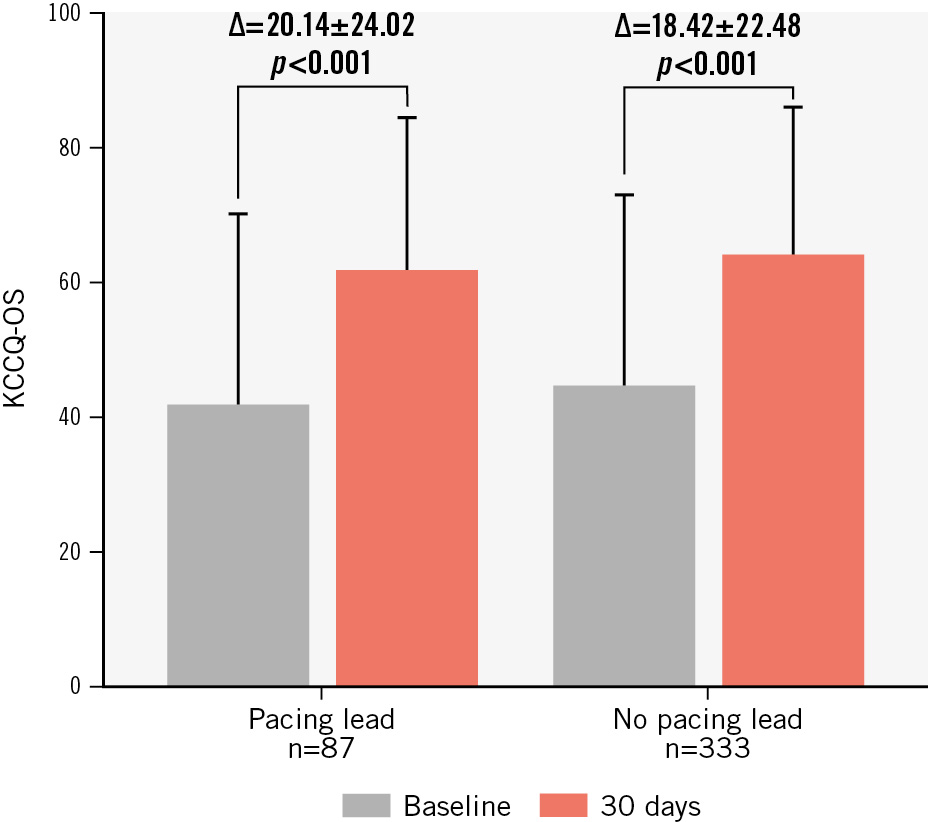
Figure 3. Quality of life (KCCQ-OS) improvement over 30 days of follow-up for subjects with and without pacing leads. Thirty-day KCCQ overall summary scores compared with baseline scores for subjects with and without endocardial leads. KCCQ: Kansas City Cardiomyopathy Questionnaire; KCCQ-OS: KCCQ overall summary
Table 4. Adverse events.
| Key events up to 30 days | Pacing lead (n=110) | No pacing lead (n=401) | Total (N=511) |
|---|---|---|---|
| Major adverse events | 1.8 (2) | 2.7 (11) | 2.5 (13) |
| Cardiovascular mortality | 0 (0) | 1.0 (4) | 0.8 (4) |
| MI | 0 (0) | 0 (0) | 0 (0) |
| Stroke | 0 (0) | 0.5 (2) | 0.4 (2) |
| New-onset renal failure | 0.9 (1) | 1.5 (6) | 1.4 (7) |
| Non-elective CV surgery for TVRS-related AE post-procedure | 0.9 (1) | 0 (0) | 0.2 (1) |
| TV and cardiac interventions | |||
| TV reintervention | 0.9 (1) | 0.5 (2) | 0.6 (3) |
| Tricuspid valve surgery | 0.9 (1) | 0.2 (1) | 0.4 (2) |
| Pacemaker surgery | 0 (0) | 0 (0) | 0 (0) |
| Single leaflet device attachment | 1.8 (2)* | 3.7 (15)** | 3.3 (17) |
| Other adverse events | |||
| Major bleeding*** | 6.4 (7) | 7.5 (30) | 7.2 (37) |
| Data are given as % (n). *One at discharge. **Nine at discharge. ***Defined as Bleeding Academic Research Consortium 3a. AE: adverse event; CV: cardiovascular; MI: myocardial infarction; TV: tricuspid valve; TVRS: tricuspid valve repair system | |||
Discussion
The impact of CIED leads on T-TEER outcomes is underreported, and concerns remain on the safety and effectiveness of T-TEER when CIEDs are present. Here, we report the main findings of this observational study:
⢠In routine clinical practice, a substantial portion of subjects eligible for T-TEER have a CIED and, in many cases, right ventricular leads contribute to the development of TR.
⢠In subjects with endocardial leads, T-TEER reduces TR severity with a similar effectiveness to that observed in subjects without leads, and this results in a profound increase in quality of life.
⢠A T-TEER procedure in subjects with CIEDs is safe with no discernible differences in procedural or early safety outcomes when comparing subjects with leads to those without leads.
Since the development of T-TEER, an increasing number of patients with CIEDs are treated with this procedure despite right ventricular leads posing specific technical challenges regarding interventional imaging and clip placement12. Several clinical registries have included patients with CIEDs, in which patients with CIEDs have been reported to represent up to one-third of the study population131415. The bRIGHT EU PAS, with a 22% share of CIED subjects, demonstrates that these subjects are being treated routinely in clinical practice. While it is obvious if a subject has a right ventricular lead, it is much more difficult to determine if the lead is a contributing factor to TR. The reported frequency of developing significant TR following CIED implantation varies, ranging from 7% to 45%16. The bRIGHT EU PAS echocardiography core laboratory judged relevant lead-leaflet interference as the main factor for TR in almost half of all subjects with CIEDs (considering direct or indirect interaction related to the restricted motion of the septal leaflet)17. In contrast, the TriValve Registry found direct lead-induced TR in only 2.5% of their patients with CIEDs18. One explanation for the higher proportion of lead-induced TR in our study may be the participation of some high-volume T-TEER centres with experienced operators capable of treating more challenging TR cases with lead-leaflet interaction and, thus, the inclusion of subjects for enrolment into the trial that had pacemaker-induced motion of the septal leaflet. In addition, the bRIGHT EU PAS population has a very high percentage of baseline TR grade ≥4+ compared with other T-TEER studies, which may reflect the role of endocardial leads in the development of TR.
Judging a lead as relevant to the TR mechanism based on imaging is a subjective decision. Landmark echocardiographic studies using a systematic approach including advanced 3D-imaging techniques have shown that the prevalence of lead-leaflet interference in cross-sectional studies of subjects with CIEDs is over 40%1920. The bRIGHT EU PAS echocardiography core laboratory had several transthoracic and transoesophageal echo studies available for each subject, including imaging during the T-TEER procedure. In particular, the 2D transgastric short-axis and 3D en face midoesophageal views of the TV allowed accurate imaging of the lead passage through the valve, possibly resulting in a higher detection rate of different types of lead-leaflet interactions, with impingement being the most common type. The analysis of the lead position in our patient cohort showed that a central position, which is optimal for TV function, was quite rare. Conversely, a prevalence of commissural lead position in around two-thirds of cases in our study subjects suggests that this position does not necessarily protect against lead-induced TR.
The bRIGHT EU PAS had a higher proportion of subjects with TR grade >3+ than the early TRILUMINATE trial, reflecting the increasing skill of operators and the further technical development of the coaptation device itself to treat anatomically more advanced TR cases715. In subjects with CIEDs, T-TEER resulted in a profound reduction of NYHA Class and an increase in quality of life. When comparing patient groups with and without CIEDs, we found a slightly lower, but not significant, difference in effectiveness (TR grade ≤2+ post-procedure) after T-TEER in CIED subjects. This is in accordance with an early single-centre experience that reported the compassionate use of the MitraClip (Abbott) device for the treatment of TR in patients with right ventricular leads21. These findings suggest that pathoanatomical aspects of the TV, like coaptation gap, leaflet tethering, leaflet-to-annulus index, and TV anatomy, are more relevant to the success of T-TEER than the presence of a right ventricular lead13222324.
When examining our patient cohort with CIEDs, impingement was found to be the most common type of lead-leaflet interference while more advanced forms, such as adhesion, were less frequent. Unfortunately, like other studies before, the bRIGHT EU PAS did not include information on patients with a CIED who were deemed ineligible for T-TEER. The presence of multiple right ventricular leads, severe lead-lead interference (adhesion or subvalvular entanglement), and poor echocardiographic image quality caused by shadowing artefacts are likely the primary reasons for not considering T-TEER in these patients. Early experience with the use of intracardiac echocardiography during T-TEER suggests that this technique might improve leaflet visualisation in cases with right ventricular leads25. Other interventional techniques have emerged for the treatment for TR. The post-market TriBAND Study included 13 subjects with CIEDs who had undergone transcatheter TV annular reduction with the Cardioband device (Edwards Lifesciences)26. It remains to be seen whether the complex Cardioband anchor deployment along the TV annulus is an effective alternative to T-TEER and applicable to a wider range of patients with right ventricular leads. Transfemoral transcatheter tricuspid valve replacement (TTVR) has been advocated as a technically straightforward interventional solution in pathoanatomically more severe TR cases, including patients with lead-induced TR27. However, the largest TTVR registry showed a 10% rate of lead failure after valve placement28. Moreover, in the case of CIED-related infections after valve implantation, it is virtually impossible to extract the entrapped lead through conventional percutaneous techniques. The latest pivotal study on TTVR using the EVOQUE transcatheter tricuspid valve replacement system (Edwards Lifesciences) included 9 subjects with a CIED already implanted before the procedure29. While device implantation practically abolished TR in most of the cases without evidence of dysfunction of the entrapped leads in the first 12 months, it remains to be seen if long-term chronic lead compression results in a risk of dysfunction. Moreover, experienced TTVR centres have reported that certain lead characteristics, like commissural position and sufficient slack, play a role when considering whether a patient is eligible for this procedure29.
Regarding procedural safety, our registry shows a high safety profile, irrespective of the presence of endocardial leads, for T-TEER with the TriClip device. We did not observe significant differences between subjects with and without a CIED concerning partial clip detachment, reintervention, or tricuspid valve surgery. To date, no case of lead damage caused by the T-TEER intervention has been reported in the literature. Only one single-centre study specifically analysed CIED data before and after T-TEER and showed a non-significant increase in pacing threshold in 3 subjects30. The protocol of bRIGHT EU PAS did not demand an interrogation of CIEDs during the index procedure or follow-up, but according to the adverse event reporting system, no CIED malfunction was reported. In several bRIGHT EU PAS cases, the position of the right ventricular lead was altered by the clip implantation during the procedure. It remains to be seen whether placing the clip close to the lead will result in an insulation failure in the long term.
Limitations
Several limitations to this study should be acknowledged. Firstly, the rationale for patients who were deemed ineligible for T-TEER by the recruiting centres was not collected. Although the bRIGHT EU PAS collected general CIED information, no specific device-related information (e.g., type of device, number of leads) was collected prospectively, and CIED interrogation was not mandatory. Regrettably, details of procedural techniques and intraprocedural imaging were also not required. This information may have provided additional data about factors that may argue against the success of interventions in patients with right ventricular leads. Finally, results here are limited to acute and 30-day outcomes, and long-term outcomes are not yet known.
Conclusions
Results from the bRIGHT EU PAS offer a considerable contribution to the steadily increasing evidence that a T-TEER intervention is a safe procedure for patients with CIEDs. The utilisation of the most-recent generation of clip also displayed a remarkably efficient reduction in TR. Considering that T-TEER is currently the most-used catheter technique in TR patients, it is encouraging that the bRIGHT EU PAS documents a substantial improvement in quality of life in these highly symptomatic subjects. The bRIGHT EU PAS is ongoing, and it will be interesting to see if the reduction of TR severity in patients with CIEDs is sustained and if this has a positive effect on longer-term, harder clinical endpoints like the rehospitalisation rate.
Impact on daily practice
In clinical practice, a substantial proportion of patients with tricuspid regurgitation (TR) who are screened for tricuspid transcatheter edge-to-edge repair (T-TEER) have a cardiac implantable electronic device (CIED), and the presence of this lead can contribute to TR. Although a transvalvular lead may be an obstacle to a catheter-based repair technique, our study shows T-TEER to be a safe and effective treatment option in patients with CIEDs.
Funding
The bRIGHT EU PAS was sponsored by Abbott.
Conflict of interest statement
B. Goebel receives consulting fees from Abbott; and payment or honoraria for speaking from Abbott and Edwards Lifesciences. P. Lurz receives honorary grants, consulting fees, and payment or honoraria for speaking from Abbott, Edwards Lifesciences, ReCor Medical, and Innoventric; travel support from Abbott, Edwards Lifesciences, and ReCor Medical; and stock options from Innoventric. R. Bekeredjian receives grant support from Abbott. H. Möllmann receives honorary grants, consulting fees, payment or honoraria for speaking, and travel support from Abbott, Edwards Lifesciences, and Boston Scientific. R.S. von Bardeleben receives payment or honoraria for speaking from Abbott, Edwards Lifesciences, and Medtronic. M. Heitkemper is a salaried employee of Abbott. R. Estevez-Loureiro receives payment or honoraria for speaking from Abbott, Edwards Lifesciences, Boston Scientific, Venus Medtech, and Jenscare. E. Donal has a research contract with Abbott and GE HealthCare. The other authors have no conflicts of interest to declare.

