Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Patients with tricuspid regurgitation (TR) are at high risk for morbidity and mortality, with poorer outcomes associated with increasing TR severity. Tricuspid transcatheter edge-to-edge repair (T-TEER) has emerged as a promising treatment option.
Aims: TriCLASP is a prospective, single-arm, European post-market study evaluating the safety and effectiveness of T-TEER with the PASCAL system to treat patients with ≥severe TR.
Methods: The TriCLASP study enrolled 300 patients to evaluate the safety and performance of T-TEER. Major adverse events (MAE), reduction in TR grade, and clinical, functional, and quality-of-life outcomes were assessed at 1 year.
Results: Enrolled patients had a mean age of 80.1 years, 52.0% were female, and 75.8% had ≥severe TR. Tricuspid regurgitation was reduced to ≤moderate in 87.7% of patients (p<0.001). The composite MAE rate was 1.7% at 30 days and 12.7% at 1 year. Kaplan-Meier estimates for survival and freedom from heart failure hospitalisation (HFH) were 88.3±1.9% and 83.2±2.3%, respectively. Annualised HFH rates decreased by 72.2% in the 12 months pre- versus post-procedure (p<0.001). Significant functional and quality-of-life improvements were observed from baseline to 1 year, including 74.5% of patients in New York Heart Association Class I/II, a 29.4-metre increase in the 6-minute walk distance, and an 8.3-point increase in the Kansas City Cardiomyopathy Questionnaire score (p<0.001).
Conclusions: The 1-year results of the TriCLASP study confirm the safety and effectiveness of T-TEER with the PASCAL system in patients with ≥severe TR. Patients experienced significant TR reduction, low mortality, high freedom from HFH, and significant improvements in symptoms, functional capacity, and quality of life.
Tricuspid regurgitation (TR) is a progressive valvular disease independently associated with increased morbidity and mortality, with poorer outcomes and worse quality of life associated with increasing TR severity12. Each year, an estimated 300,000 patients in Europe and 200,000 in the United States are affected by TR34, many presenting with signs or symptoms of heart failure (HF) and right ventricular (RV) congestion and experiencing recurrent hospitalisations.
Despite the prevalence of TR and its insensitivity to pharmacotherapy for left-sided heart failure, the majority of patients remain undertreated36. Although isolated tricuspid valve (TV) surgery has been shown to improve functional status, the procedure is rarely performed and has a high perioperative mortality rate of 8-10%, as most patients are referred late in the course of the disease789. More recently, minimally invasive tricuspid transcatheter edge-to-edge repair (T-TEER) was added to the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines10, with studies showing TR reduction and other clinical benefits11112131415. Outcomes from post-market and real-world use of T-TEER therapy will help further elucidate its long-term benefits and risks.
The Transcatheter Repair of Tricuspid Regurgitation with Edwards PASCAL Transcatheter Valve Repair System (TriCLASP) study is a European post-market clinical follow-up study evaluating the safety and effectiveness of the PASCAL and PASCAL Precision transcatheter valve repair systems (both Edwards Lifesciences) in patients with ≥severe TR who were deemed eligible for treatment with T-TEER. The primary safety endpoint (a composite of major adverse events [MAE] at 30 days) and primary effectiveness endpoint (TR severity at discharge relative to baseline) were met and have been previously described16. In this report, we present the 1-year follow-up of the full cohort of TriCLASP study patients.
Methods
Study design and patient selection
The TriCLASP study is a prospective, single-arm, post-market clinical follow-up study with centres in Germany, Switzerland, Greece, and Italy. Enrolled patients had ≥severe TR and were deemed eligible for T-TEER by the local Heart Team and according to the PASCAL system indications for use.
Patients were excluded from the study if the screening echocardiogram showed evidence of severe leaflet tethering or leaflet immobility or a septolateral coaptation gap >10 mm in the grasping area. Additional key exclusion criteria included previous TV replacement; severe stenosis and/or regurgitation of the aortic, mitral, and/or pulmonic valves; femoral artery access not feasible with a 22 Fr guide; echocardiographic evidence of intracardiac mass, thrombus, or vegetation; severe renal insufficiency with an estimated glomerular filtration rate (eGFR) ≤25 mL/min/1.73 m² or requiring chronic renal replacement therapy; history of bleeding diathesis or coagulopathy, or a patient who refuses blood transfusions; and concurrent medical condition with a life expectancy <12 months.
The TriCLASP study was conducted in accordance with the ethical principles set forth by the 1964 Declaration of Helsinki and its amendments and the International Conference on Harmonization Good Clinical Practice principles. The research protocol was approved by the locally appointed ethics committees. Patients provided written informed consent. An independent clinical events committee (CEC) adjudicated MAE up to 1-year follow-up, and an independent echocardiographic core laboratory (ECL; Cardialysis, Rotterdam, the Netherlands) assessed all baseline and follow-up echocardiograms. The TriCLASP study is sponsored by Edwards Lifesciences and registered on ClinicalTrials.gov: NCT04614402.
Study endpoints
The primary safety endpoint was a composite of the following MAE at 30 days: cardiovascular mortality, stroke, myocardial infarction, non-elective TV reintervention (percutaneous or surgical), major access site and vascular complications, major cardiac structural complications, device embolisation, renal complications requiring unplanned dialysis or renal replacement therapy, and severe bleeding (defined as fatal, life-threatening, extensive, or major bleeding by the Mitral Valve Academic Research Consortium17). The primary effectiveness endpoint was TR severity at discharge relative to baseline, as evaluated by the ECL.
Secondary effectiveness endpoints were evaluated at baseline, 30 days, 6 months, and 1 year, and will be assessed annually up to 5 years. These include changes in New York Heart Association (NYHA) Functional Class, 6-minute walk distance (6MWD), and Kansas City Cardiomyopathy Questionnaire overall summary (KCCQ-OS) score.
Echocardiographic assessment
Patients were enrolled in the study according to site qualification of TR severity, as assessed by transthoracic or transoesophageal echocardiography (TTE or TOE). After patient enrolment, echocardiographic endpoints were evaluated by the ECL at baseline, discharge, 30 days, 6 months, and annually. Study sites used a standardised protocol following published guidelines18 to conduct echocardiographic examinations. TR severity was assessed on a 5-grade scale19 with 2- and 3-dimensional and colour Doppler echocardiography, using a multiparametric approach that integrated quantitative, semiquantitative, and qualitative parameters.
The PASCAL system
The PASCAL system comprises an implant, a delivery system containing a 22 Fr guide sheath, a steerable guide catheter, and an implant catheter. There are two delivery system models (PASCAL and the newer PASCAL Precision) and two implant models (PASCAL and PASCAL Ace with a narrower profile [both Edwards Lifesciences]). The PASCAL system is differentiated by implant design and ease of use. Both implants have a central spacer that bridges the coaptation gap and is secured between the native valve leaflets by contoured paddles and clasps. Each clasp features a single row of retention elements to clasp, reclasp, and preserve leaflets. During the implantation procedure, which has been previously described1620, the implant can elongate into a slim profile to enable navigation and repositioning within dense chordae that are common to tricuspid subvalvular anatomy.
Statistical analysis
Procedural and safety outcomes are reported for the intention-to-treat population, and effectiveness endpoints are analysed in the as-treated population. The sample size of 300 patients was based upon clinical considerations to estimate the primary endpoint with sufficient precision. Significance for continuous variables was assessed using the paired Student’s t-test or Wilcoxon signed-rank test, as appropriate, and ranked categorical variables were compared using the Wilcoxon signed-rank test. Two-sided significance tests had an alpha level of 0.05. Survival and time-to-first-event curves were constructed using Kaplan-Meier analysis. Statistical analysis was performed using SAS, version 9.4 (SAS Institute).
Results
Baseline characteristics
Three hundred patients were enrolled at 17 sites between February 2021 and March 2023, with all available patients followed up to 1 year (Figure 1). The mean age was 80.1 years, 52% were female, 78.0% of patients were in NYHA Functional Class III/IV, and 75.8% had TR ≥severe, as retrospectively graded by the ECL. Comorbidities included hypertension (85.3%), atrial fibrillation (91.3%), dyslipidaemia or hyperlipidaemia (37.0%), HF hospitalisation in the past year (63.4%), and renal insufficiency or failure (61.7%). TR aetiology was functional in 75.3% of patients, degenerative in 15.7%, mixed in 5.7%, pacemaker-related in 2.0%, and indeterminate in 1.3%. Baseline characteristics are described in Table 1.
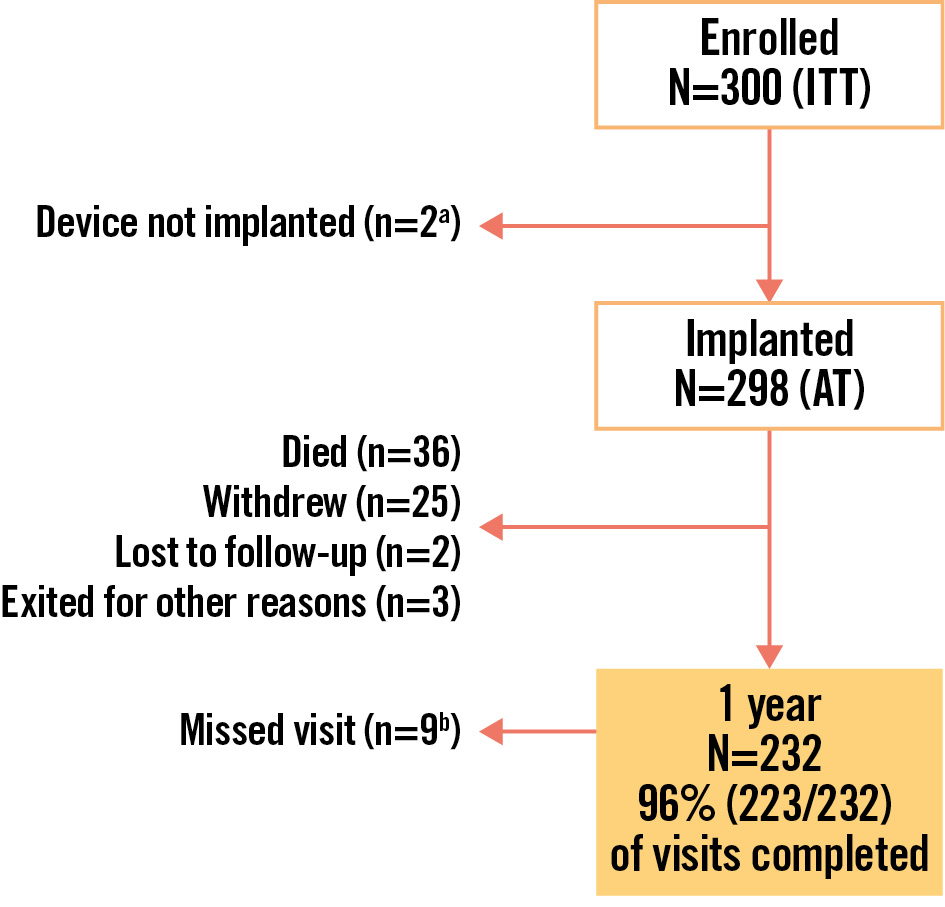
Figure 1. Patient disposition and study flow. a1 procedure aborted due to complex anatomy; 1 device not implanted after patient had a cardiac arrest during the procedure; b2 patients lost to follow-up with the date of declaration after the 1-year visit window; 1 died after the 1-year visit window; 3 completed the 2-year visit but missed the 1-year visit; 3 missed the 1-year visit but are not in the 2-year window. AT: as-treated; ITT: intention-to-treat
Table 1. Baseline characteristics.
| Characteristic | N=300 |
|---|---|
|
Age, years |
80.1±6.0 |
|
Female |
52.0 |
|
TR aetiologya |
|
|
Secondary (functional or non-structural) |
75.3 |
|
Primary (degenerative, organic or structural) |
15.7 |
|
Mixed |
5.7 |
|
Pacemaker-related |
2.0 |
|
Indeterminate |
1.3 |
|
EuroSCORE II, % |
6.2±5.7 |
|
STS-MV repair, % |
8.3±6.0 |
|
NYHA Functional Class III/IV |
78.0a |
|
KCCQ-OS score (points) |
54.5±18.7 |
|
TR ≥severe |
75.8b,c |
|
RV function moderately-severely depressed |
41.9 |
|
Systemic hypertension |
85.3 |
|
Pulmonary hypertension (within the past year)d |
48.0 |
|
Cardiomyopathy |
14.0 |
|
Myocardial infarction |
10.7 |
|
Prior stroke |
6.7 |
|
Dyslipidaemia or hyperlipidaemia |
37.0 |
|
Heart failure hospitalisation within the last year |
63.4e |
|
Atrial fibrillation |
91.3 |
|
Pacemaker or ICD |
20.0 |
|
CABG surgery |
8.3 |
|
Prior surgery/intervention, any valve |
30.3 |
|
Renal insufficiency or failure |
61.7 |
|
Diabetes |
26.0 |
|
History of ascites |
6.0 |
|
Values are mean±SD or %. aN=299. bAssessed by the echocardiographic core laboratory: Cardialysis, Rotterdam, the Netherlands. cN=281. dEvaluated by echocardiography. eN=298. CABG: coronary artery bypass graft; ICD: implantable cardioverter-defibrillator; KCCQ-OS: Kansas City Cardiomyopathy Questionnaire overall summary; MV: mitral valve; NYHA: New York Heart Association; RV: right ventricular; SD: standard deviation; STS-MV: Society of Thoracic Surgeons - Mitral Valve; TR: tricuspid regurgitation |
|
Procedural characteristics
Device implantation was successful in 99.3% of patients, and the mean number of devices implanted per patient was 1.9 (98% with the PASCAL Ace). The PASCAL Precision system, which was introduced later, was used in 19.3% of patients. The median device time (implant system insertion to guide sheath removal) was 72.0 minutes (interquartile range [IQR]: 52.0, 99.0), and the median procedure time (skin incision to access closure) was 100.0 minutes (IQR: 75.0, 140.0). The median hospital stay from the index procedure was 4.0 days (IQR: 3.0, 5.0), with 96.0% of patients discharged home (Table 2).
Table 2. Procedural characteristics and outcomes.
| Characteristic | Value |
|---|---|
|
Successful implantation ratea |
99.3 (298/300) |
|
Procedural successb |
85.7 (233/272) |
|
Number of devices implanted per patient |
1.9±0.7 (298) |
|
Device time (implant system insertion to guide sheath removal), mins |
72.0 (52.0, 99.0) |
|
Procedure time (skin incision to closure), mins |
100.0 (75.0, 140.0) |
|
Fluoroscopy duration, mins |
14.0 (8.0, 26.0) |
|
Length of hospital stay (procedure to discharge), days |
4.0 (3.0, 5.0) |
|
Discharged home |
96.0 (287/299) |
|
Values are % (n/N), mean±SD (n), or median (Q1, Q3). aPercentage of patients who had the study device implanted, deployed as intended, and retrieved successfully. bDevice deployment success with at least one grade reduction in TR at discharge without surgical or percutaneous intervention prior to hospital discharge. TR grade availability was dependent on data entry timing and imaging readability. Q1: first quartile; Q3: third quartile; SD: standard deviation; TR: tricuspid regurgitation |
|
Safety outcomes
Five patients experienced 8 events, resulting in a CEC-adjudicated per-patient composite MAE rate of 1.7% (5/291) at 30 days. Event rates were 0.3% cardiovascular death, 0.3% stroke, 0.3% myocardial infarction, 0.3% renal complications requiring unplanned dialysis, and 1.4% severe bleeding. Four events (3 severe bleeds, 1 myocardial infarction) were adjudicated as related to the device or procedure. At 1 year, the composite MAE rate was 12.7%, composed of 7.3% cardiovascular mortality, 0.8% stroke, 0.8% myocardial infarction, 0.4% non-elective tricuspid valve reintervention, 2.3% renal complications requiring unplanned dialysis, and 5.8% severe bleeding. There were no major cardiac structural complications, major access site and vascular complications, or device embolisations (Table 3). Two events that occurred after 30 days were adjudicated as device related: 1 cardiovascular death caused by cardiac decompensation due to single leaflet device attachment (SLDA) and 1 non-elective TV reintervention with T-TEER attributed to SLDA and worsening TR.
SLDA, as adjudicated by the ECL, occurred in 12 patients (4.0%) (Table 3). Eight did not have a reintervention, two had elective surgical explant and valve replacement, one had T-TEER as described above, and one had an attempted reintervention that was aborted due to unfavourable device positioning.
The 1-year Kaplan-Meier estimate for survival was 88.3±1.9%, and freedom from HF hospitalisation was 83.2±2.3% (Central illustration). Heart failure hospitalisations decreased by 72.2% (p<0.001) between the 12 months pre- and post-procedure (Figure 2).
Table 3. CEC-adjudicated events at 30 days and 1 year.
| CEC-adjudicated MAE | 30 days N=291a |
1 year N=260a |
|---|---|---|
| Composite MAE rate | 1.7 (5) | 12.7 (33) |
|
Cardiovascular mortality |
0.3 (1) |
7.3 (19) |
|
Myocardial infarction |
0.3 (1) |
0.8 (2) |
|
Stroke |
0.3 (1) |
0.8 (2) |
|
Renal complications requiring unplanned dialysis or renal replacement therapy |
0.3 (1) |
2.3 (6) |
|
Major cardiac structural complications |
0 (0) |
0 (0) |
|
Non-elective tricuspid valve reintervention |
0 (0) |
0.4 (1) |
|
Device embolisationb |
0 (0) |
0 (0) |
|
Severe bleedingc |
1.4 (4) |
5.8 (15) |
|
Major access site and vascular complications |
0 (0) |
0 (0) |
| Other events | N=300 | N=300 |
|
All-cause mortality |
1.3 (4) |
11.0 (33) |
|
Heart failure rehospitalisation |
3.3 (10) |
15.3 (46) |
|
SLDAb |
2.3 (7) |
4.0 (12) |
|
Values are % (n). Composite MAE rates are per patient; patients may have had more than 1 event. aThe denominator for the % calculation includes patients who had an event or did not have an event but were followed up to 30 days and 1 year, respectively. bAdjudicated by the echocardiographic core laboratory: Cardialysis, Rotterdam, the Netherlands. cSevere bleeding is major, extensive, life-threatening, or fatal bleeding, as defined by Mitral Valve Academic Research Consortium. CEC: clinical events committee; MAE: major adverse events; SLDA: single leaflet device attachment; TV: tricuspid valve |
||
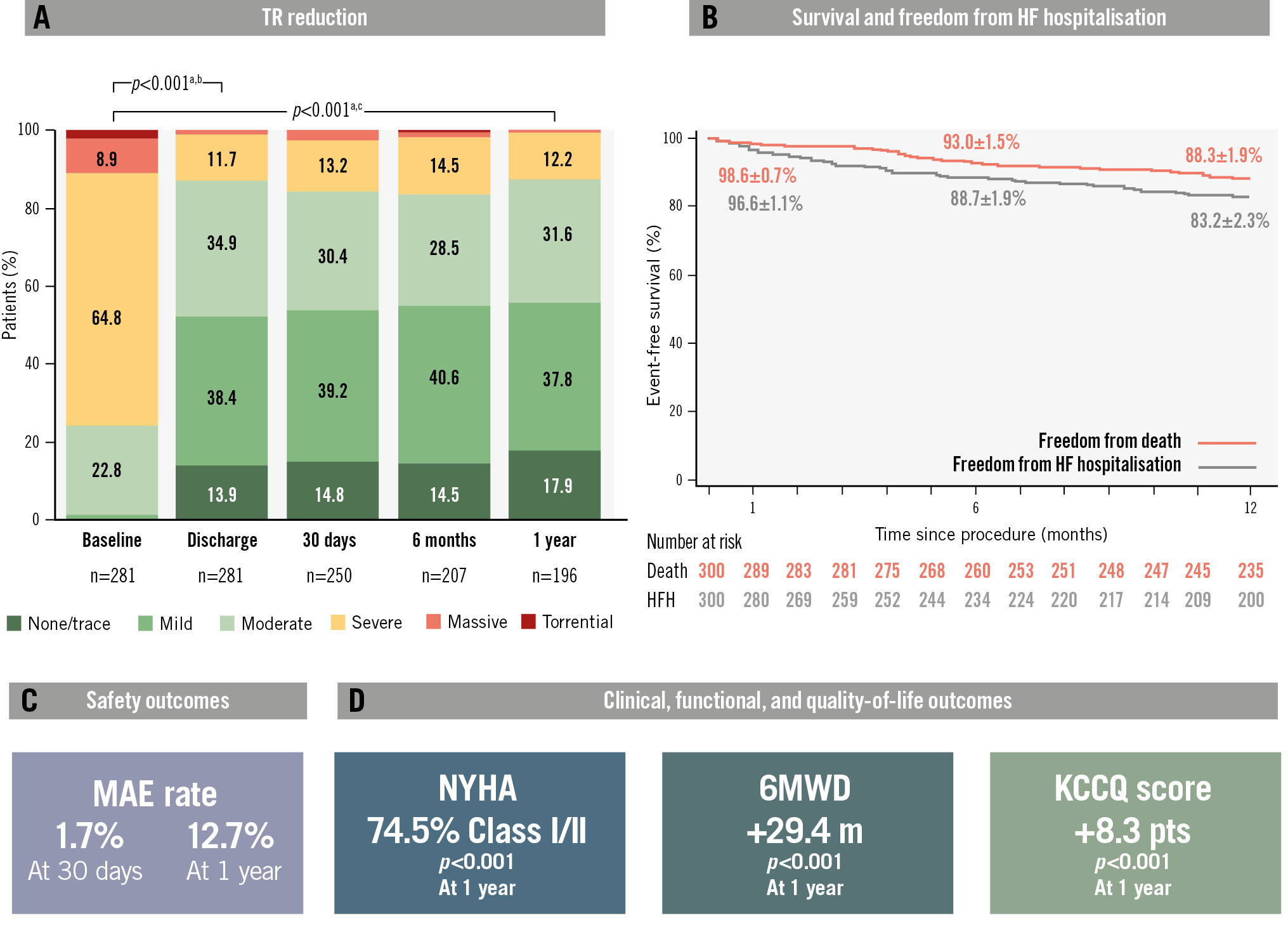
Central illustration. One-year outcomes of patients treated with the PASCAL transcatheter valve repair system in the TriCLASP European, single-arm, post-market clinical follow-up study. A) The TR graph shows the unpaired analysis of the as-treated population. In the paired analysis, 87.7% of patients achieved significant TR reduction to grade moderate at 1 year. B) Survival was 88.3%, and freedom from heart failure hospitalisation was 83.2%. C) A low composite MAE rate of 1.7% at 30 days was sustained up to 1 year with a composite MAE rate of 12.7%. D) Significant improvements in functional and quality-of-life outcomes were observed, including an 8.3-point increase in KCCQ score, a 29.4 m increase in the 6MWD, and 74.5% of patients in NYHA Class I/II, compared to 27.4% at baseline. aWilcoxon signed-rank test, paired analysis of TR grade ≤moderate. bN=269; baseline=23.8%; discharge=87.4%. cN=187; baseline=28.3%; 1 year=87.7%. HF: heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; MAE: major adverse events; NYHA: New York Heart Association; TR: tricuspid regurgitation; 6MWD: six-minute walk distance
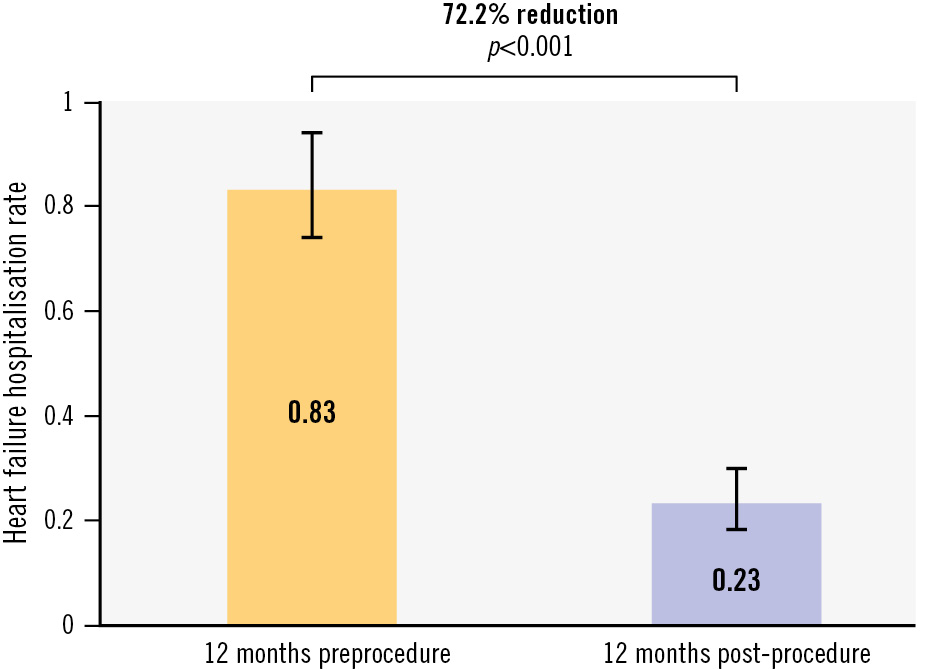
Figure 2. Survival and freedom from heart failure hospitalisation. The annualised rate of heart failure hospitalisation decreased by 72.2% (p<0.001) between the 12 months pre- and post-intervention.
Echocardiographic outcomes
Patients experienced significant reductions in TR severity, with 87.4% of patients achieving ≤moderate TR at discharge compared to baseline (p<0.001). This result was sustained up to 1 year, at which point 87.7% had ≤moderate residual TR (p<0.001 from baseline) (Central illustration). At 1 year, 85.0% of patients experienced a reduction in TR of at least 1 grade, and 44.4% had at least a 2 grade reduction. Of the 24.2% of patients who had moderate or less TR at baseline, as assessed by the ECL, 75.5% improved, 20.8% stayed the same, and 3.8% worsened by 1 grade at 1 year.
Paired analyses of echocardiographic variables showed significant remodelling from baseline to 1 year (Table 4). Structural changes were observed, with a reduction in the RV dimension at both the 4-chamber basal (−2.6±6.0 mm; p=0.004) and mid-location (−2.3±7.4 mm; p=0.033) and in the inferior vena cava diameter at end-expiration (−1.9±6.3; p=0.001). The left ventricular (LV) outflow tract stroke volume significantly increased (8.6±16.2 mL; p<0.001), as did cardiac output (0.65±1.2 L/min; p<0.001). Right atrial (RA) volume decreased (−18.2±41.2 mL; p<0.001), and the TV mean pressure gradient increased from baseline and remained stable up to 1 year (1.0±1.2 mmHg; p<0.001). There were no significant changes in tricuspid annular plane systolic excursion (TAPSE) or mean pulmonary artery pressure.

Central illustration. One-year outcomes of patients treated with the PASCAL transcatheter valve repair system in the TriCLASP European, single-arm, post-market clinical follow-up study. A) The TR graph shows the unpaired analysis of the as-treated population. In the paired analysis, 87.7% of patients achieved significant TR reduction to grade moderate at 1 year. B) Survival was 88.3%, and freedom from heart failure hospitalisation was 83.2%. C) A low composite MAE rate of 1.7% at 30 days was sustained up to 1 year with a composite MAE rate of 12.7%. D) Significant improvements in functional and quality-of-life outcomes were observed, including an 8.3-point increase in KCCQ score, a 29.4 m increase in the 6MWD, and 74.5% of patients in NYHA Class I/II, compared to 27.4% at baseline. aWilcoxon signed-rank test, paired analysis of TR grade ≤moderate. bN=269; baseline=23.8%; discharge=87.4%. cN=187; baseline=28.3%; 1 year=87.7%. HF: heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; MAE: major adverse events; NYHA: New York Heart Association; TR: tricuspid regurgitation; 6MWD: six-minute walk distance
Table 4. Paired echocardiographic parameters at baseline and 1 year.
| Parameter | N | Baseline | 1 year | Change | p-valuea |
|---|---|---|---|---|---|
|
Tricuspid annular A-P diameter (RV inflow) |
87 |
40.3±4.7 |
40.1±5.4 |
−0.23±4.3 |
0.617 |
|
Tricuspid annular S-L (transverse) diameter (4CH view) mid-diastole, mm |
99 |
43.6±6.1 |
40.4±7.0 |
−3.2±5.0 |
<0.001* |
|
LVOT SV, mL |
116 |
48.7±16.0 |
57.3±18.2 |
8.6±16.2 |
<0.001* |
|
LVOT CO, L/min |
115 |
3.3±1.2 |
4.0±1.3 |
0.65±1.2 |
<0.001* |
|
RV dimension (4CH view, basal), mm |
50 |
50.0±7.9 |
47.4±7.6 |
−2.6±6.0 |
0.004* |
|
RV dimension (4CH view, mid-cavity), mm |
49 |
36.9±8.7 |
34.6±7.2 |
−2.3±7.4 |
0.033* |
|
RA volume (Simpson) (4CH view), mL |
68 |
159.3±83.5 |
141.1±79.4 |
−18.2±41.2 |
<0.001* |
|
IVC expiration diameter, mm |
115 |
23.8±6.9 |
21.9±6.5 |
−1.9±6.3 |
0.001* |
|
Estimated mPAP, mmHg |
75 |
30.1±9.2 |
28.6±8.7 |
−1.5±9.3 |
0.174 |
|
TAPSE, mm |
167 |
16.9±4.8 |
16.4±4.4 |
−0.46±4.5 |
0.185 |
|
TAPSE/PASP ratio |
63 |
0.44±0.16 |
0.46±0.20 |
0.02±0.18 |
0.387 |
|
TV mean pressure gradient, mmHg |
86 |
1.4±0.7 |
2.5±1.1 |
1.0±1.2 |
<0.001* |
|
Values are mean±SD. aP-values calculated by t-test for paired analyses. *Values are statistically significant. A-P: anterior-posterior; CH: chamber; CO: cardiac output; IVC: inferior vena cava; LVOT: left ventricular outflow tract; mPAP: mean pulmonary artery pressure; PASP: pulmonary artery systolic pressure; RA: right atrium; RV: right ventricle; S-L: septal-lateral; SV: stroke volume; TAPSE: tricuspid annular plane systolic excursion; TV: tricuspid valve |
|||||
Clinical, quality-of-life, and functional outcomes
Patients experienced significant and sustained improvements in clinical, quality-of-life, and functional outcomes at 1 year compared with baseline. The proportion of patients in NYHA Functional Class I or II improved from 27.4% to 74.5% (p<0.001) in the paired analysis, and the mean KCCQ-OS score increased by 8.3±18.8 points (p<0.001). KCCQ-OS score improvements by TR reduction showed that patients with a 1 grade reduction improved KCCQ-OS scores by 7.0 points and patients with a ≥3 grade change improved by 17.6 points. The mean 6MWD increased by 29.4 metres (p<0.001) (Figure 3).
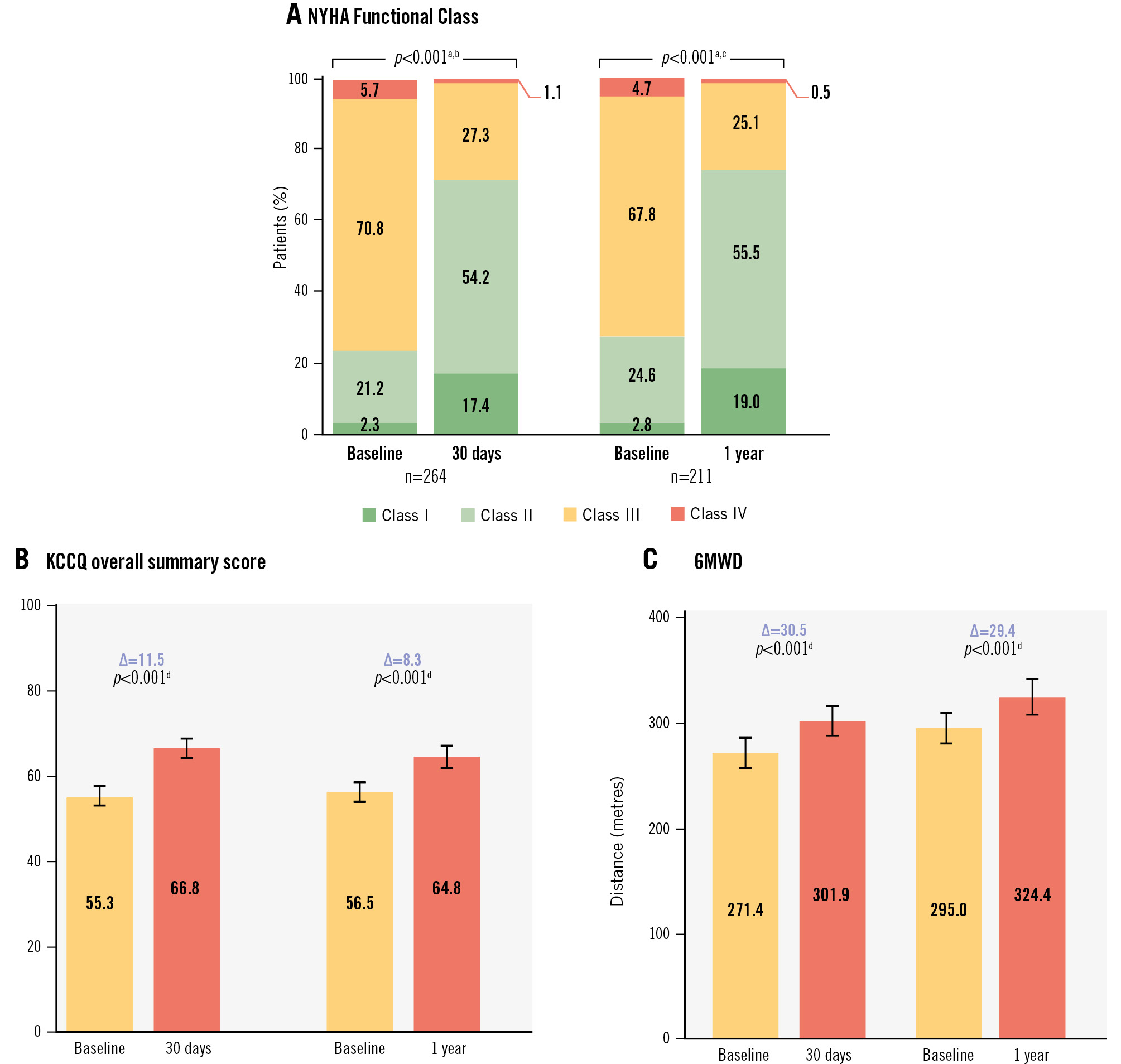
Figure 3. NYHA Functional Class, KCCQ score, and 6MWD. A) The NYHA Functional Class improved significantly between baseline and 1 year. At 1 year, 74.5% of patients achieved Class I/II. B) The KCCQ overall summary score significantly improved by 8.3 points, with 5 points considered a minimal clinically important difference. C) The 6MWD significantly increased by 29.4 metres at 1 year. aWilcoxon signed-rank test, paired analysis of the proportion of patients in NYHA Class I or II. bN=264; baseline=23.5%; 30 days=71.6%. cN=211; baseline=27.4%; 1 year=74.5%. dStudent’s t-test, paired analysis. KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; 6MWD: 6-minute walk distance
Discussion
TriCLASP is the first prospective European post-market study evaluating the safety and effectiveness of T-TEER with the PASCAL system for patients with symptomatic TR. The 1-year results demonstrated significant and sustained efficacy with 87% of patients achieving ≤moderate TR and 85.0% having ≥1 grade reduction, which corresponded with favourable functional RV and RA remodelling and significant improvements in NYHA Class, 6MWD, and KCCQ scores.
Transcatheter TV repair has evolved from an experimental procedure available only to patients in their final stage of disease to a routine procedure offered to patients with symptomatic severe TR in a growing number of heart valve centres11. Results from the TriCLASP study reinforce the safety and efficacy of T-TEER with the PASCAL system in reducing TR2021. TR improvement was associated with significant reverse RV remodelling from baseline to 1 year. Structural changes included significant reduction in RV dimensions, consistent with a previous observational echocardiographic study showing slow but continuous RV reverse remodelling accompanying TR reduction22. In addition, TriCLASP patients had a significant increase in LV filling and a consecutive increase in LV stroke volume, parameters linked to improved long-term prognosis22.
Of note, patients in TriCLASP also experienced a reduction in HF-related hospitalisations in the first year post-procedure: the annualised HF hospitalisation rate decreased by 72.2%, which compares well to the bRIGHT registry that reported a 50% reduction within 1 year12. Given TR severity may vary over time due to natural disease progression/regression and that echocardiographic assessment is inherently subject to variability, reduction of HF hospitalisation is a more tangible benefit to patients. The 1-year annualised rate of HF hospitalisation in TriCLASP (0.23 events/patient-year) is comparable to the T-TEER arm of the TRILUMINATE randomised trial (0.21 events/patient-year), which also showed similar rates of annualised HF hospitalisation in the medical therapy group (0.17 events/patient-year)15. Longer-term follow-up and further research are needed to better understand factors contributing to HF hospitalisation to improve patient management over time.
This study confirms that T-TEER is a fundamentally safe procedure. Cardiovascular mortality was 0.3% within 30 days of the procedure, and there were no urgent reinterventions, device embolisations, or relevant access site complications. Patients were discharged after 4 days, which compares well to other reports on transcatheter edge-to-edge repair therapy in both the tricuspid and mitral positions23. The 1-year rates of SLDA in the TriCLASP study were low (4.0%) and comparable to previously reported data for T-TEER therapies131415. Continued echocardiographic follow-up is important, and future advances in tricuspid imaging and devices may further improve leaflet capture and reduce occurrence.
Although patient populations and study designs differ, both large registries (e.g., the bRIGHT study) and randomised controlled trials, such as TRILUMINATE and TRI.FR13, show meaningful improvements in symptoms and quality of life after T-TEER, which are reinforced by the results from TriCLASP. Compared with another real-world study, bRIGHT, quality-of-life outcomes were very similar. Both studies had a similar baseline symptomatic profile with a high proportion of patients in NYHA Class III/IV (78.0% in TriCLASP vs 80% in bRIGHT), improving to 74.5% in NYHA Class I/II in TriCLASP and 75% in bRIGHT. Although bRIGHT patients started with a lower mean KCCQ-OS score, patients in both studies reached nearly the same scores after 1 year (64.3 points in TriCLASP vs 65 points in bRIGHT)12. Conversely, T-TEER patients in the TRILUMINATE and TRI.FR randomised controlled trials were less symptomatic in terms of NYHA Class at baseline (59.4% and 38.9%, respectively, in Class III/IV) but had baseline KCCQ-OS scores within 2 points of TriCLASP patients. All three studies showed a large shift from NYHA Class III/IV to I/II. Regarding improvement in 6MWD, TriCLASP patients had a greater increase (29.4 m vs 11.5 m in TRILUMINATE and 10 m in TRI.FR). However, KCCQ-OS score improvement was more modest in the TriCLASP study (8.3 points vs 12.3 points in TRILUMINATE and 15.9 points in TRI.FR)1315. Of note, in the TriCLASP study, the increase in the KCCQ-OS score was dependent on the extent of TR reduction, with improvements between 7.0 points and 17.6 points for patients who improved from 1 to ≥3 grades. These results, similar to those seen in the TRISCEND II trial, show greater symptomatic benefits with greater degrees of TR reduction2425. The improvements in the KCCQ-OS score observed in the TriCLASP study compared with the other T-TEER studies may be related to baseline TR severity differences between the studies (≤moderate TR in 24.2% of patients in the TriCLASP study vs 2.3% of patients in the TriClip arm of TRILUMINATE, 1.5% in the TriClip arm of TRI.FR, and 2% in bRIGHT)11213152425.
Patients were qualified for study enrolment by site echocardiography (TTE or TOE), and the ECL subsequently evaluated baseline echocardiography (TTE). Patients with TR graded ≤moderate at baseline by the ECL were treated if they had been initially assessed as having TR ≥severe by their respective sites. The discrepancy between site and core lab assessments has been well described and is noted most frequently as an exclusion in trials that require core lab grading prior to trial inclusion2627. Other trials have shown that almost a third of subjects deemed eligible by highly experienced sites are assessed to have lesser regurgitation by the ECL28. Some of these patients may have had clearly severe or greater TR on prior TOE, or on TTE studies that were not submitted to the ECL. Among TriCLASP patients whose TR at baseline was evaluated by the site as ≥severe and subsequently adjudicated by the ECL as moderate or less, 75.5% showed a decrease in TR from baseline to 1 year. Moreover, these patients were symptomatic and derived benefit with respect to increases in the KCCQ-OS score and 6MWD at 1 year, thus re-emphasising the notion that early therapy may have not only symptomatic but also long-term prognostic implications for patients even at lower grades of TR.
Limitations
The current trial has important limitations. As a non-randomised trial, the improvements in NYHA Class, 6MWD, KCCQ-OS score, and the substantial reduction in annualised hospitalisation needs to be interpreted with caution. However, the improvements in these metrics observed in the TriCLASP study are directionally consistent with TRILUMINATE, TRI FR, and bRIGHT, thus adding to the body of evidence reflecting symptomatic improvement from treatment with T-TEER. A main limitation is the variability in echocardiographic assessment, highlighted by a discordance between site and ECL evaluations, which impacts the comparison of observed treatment effects with other studies. Limitations of imaging technology (e.g., acoustic shadowing, far-field imaging, etc.)26 impacted image quality and contributed to missing echocardiographic data. Moreover, as a single-arm post-approval registry, analyses were performed post hoc, and data reflect limitations in follow-up or missing data. Finally, data on any medical therapy used were not systemically collected in TriCLASP, and therefore any potential confounding effect could not be analysed.
Conclusions
The TriCLASP study confirms the safety and effectiveness of T-TEER by means of the PASCAL system at 1 year in patients with symptomatic TR. Patients experienced sustained TR reduction, low mortality rates, high rates of freedom from HF hospitalisation, and significant improvements in symptoms, functional capacity, and quality of life. The randomised CLASP II TR Pivotal Clinical Trial (ClinicalTrials.gov: NCT04097145) is underway comparing T-TEER with the PASCAL system to optimal medical therapy alone in patients with TR.
Impact on daily practice
The 1-year outcomes of patients in the prospective, single-arm, post-market TriCLASP study demonstrate the safety and marked efficacy of the PASCAL system in treating patients with symptomatic tricuspid regurgitation (TR). At 1 year, patients demonstrated high survival, reduced heart failure hospitalisation, and sustained TR reduction. Patients also experienced favourable right heart remodelling and significant and sustained clinical improvements in New York Heart Association Class, Kansas City Cardiomyopathy Questionnaire score, and 6-minute walk distance.
Acknowledgements
The authors thank the staff and patients of the study centres that participated in the TriCLASP study. In addition, they thank the following from Edwards Lifesciences for their support of this publication: Ted Feldman, MD; Suzanne Y. Gilmore, MPIA; Ann Krzmarzick, MBC; Laura Gerik, MS; Hillary Alberta, PhD; Zhaoxun Hou, PhD; and Lakshmi Teja Chamala.
Funding
This study is funded by Edwards Lifesciences.
Conflict of interest statement
S. Baldus has received a research grant from Abbott; and lecture fees from Abbott and Edwards Lifesciences. N. Schofer is a consultant for Edwards Lifesciences; and has received travel support from Abbott. T. Geisler has received personal fees (lecture honoraria) and institutional research grants from Edwards Lifesciences. P. Lurz has been a consultant to Abbott, Edwards Lifesciences, and Recor Medical. P. Lüdike has received research grants and honoraria for consulting and lectures from Edwards Lifesciences. T. Rassaf receives research funding from the Deutsche Forschungsgemeinschaft and Abbott; and receives honoraria for lectures or advisory boards from AstraZeneca, Bayer, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, Novartis, and Pfizer. J. Hausleiter received personal fees and institutional grant support from Edwards Lifesciences. K. Friedrichs is a consultant for Edwards Lifesciences; and has received honoraria from Abbott. C. Frerker has received honoraria and travel support from Edwards Lifesciences and Abbott. E. Lubos has received lecture fees and research grants from Abbott and Edwards Lifesciences; honoraria for advisory board activities from New Valve Technology and Cardiovalve; and travel expenses from Cardiovalve, Abbott, and Edwards Lifesciences. K. Spargias has received honoraria or consultation fees from Edwards Lifesciences. T. Schmitz receives speaker and proctor honoraria from Edwards Lifesciences and Abbott. G. Nickenig has received honoraria for lectures or advisory boards from Abbott, Boston Scientific, Cardiovalve, Edwards Lifesciences, and Medtronic. F. Praz received consulting fees from Edwards Lifesciences during the conduct of the study. S. Berti receives honoraria and is a consultant for Procter & Gamble, Abbott, and Boston Scientific. M. Chrissoheris has received honoraria, lecture fees, and grant support from Edwards Lifesciences and Abbott. M. Eißmann receives honoraria or consultation fees from Edwards Lifesciences and Abbott. C.B. Ren reports institutional contracts for echocardiography core laboratory analyses with Boston Scientific, Edwards Lifesciences, NVT GmbH/Biosensors, and Shenqi Medical, for which she has received no personal compensation; and she has received honoraria from Abbott. The other authors have no conflicts of interest in relation to the contents of this paper to declare. H. Lapp, T. Kister, N. Schofer, T. Geisler, P. Lüdike, T. Rassaf, J. Hausleiter, M. Kessler, P. Lurz, and F. Praz report some overlap with PASTE registry enrolment.

