Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Bleeding remains a frequent complication after transcatheter aortic valve implantation (TAVI). Recently, the Valve Academic Research Consortium High Bleeding Risk (VARC-HBR) criteria were introduced to identify patients at (very) high risk of bleeding.
Aims: This study aimed to evaluate the validity of the VARC-HBR criteria for predicting bleeding risk in TAVI patients and to compare its performance with other existing criteria.
Methods: Data were obtained from the POPular PAUSE TAVI trial, a randomised clinical trial that evaluated the safety and efficacy of continuation versus interruption of oral anticoagulation during TAVI. Major and minor bleeding risk criteria were identified at baseline, and bleeding events were recorded up to 30 days after TAVI. Patients were classified into three groups: those with ≤1 minor criterion (moderate risk), those with 1 major or 2 minor criteria (high risk), and those with ≥2 major or ≥3 minor criteria (very high risk).
Results: A total of 856 patients were included: 332 (39%) were classified at moderate bleeding risk, 337 (39%) at high bleeding risk, and 187 (22%) at very high bleeding risk. Major bleeding occurred in 4.2% of moderate-risk patients, 9.5% in the high-risk group, and 15.0% in the very high-risk group (p<0.001). Receiver operating characteristic analysis showed moderate discriminative performance (area under the curve=0.64, 95% confidence interval: 0.58-0.70). Despite higher-than-expected event rates, the VARC-HBR criteria demonstrated good calibration with observed outcomes.
Conclusions: The VARC-HBR criteria effectively identified distinct subgroups with a stepwise increase in major bleeding post-TAVI. However, their predictive performance for individual risk was moderate.
Transcatheter aortic valve implantation (TAVI) is a well-established treatment for patients with symptomatic severe aortic stenosis1. Despite numerous technical advancements in recent years, procedure-related bleeding complications remain frequent2. This is particularly true in patients with a concomitant indication for oral anticoagulation, who represent about 35% of the current TAVI population3. Major bleeding occurs in 3-10% of patients and has been associated with up to a threefold increase in mortality24. It is also associated with reduced mental and physical quality of life, longer hospitalisation and higher healthcare costs5. To anticipate and potentially avoid these events, preprocedural bleeding risk assessment has been recommended to guide preventive strategies46. As standardised bleeding risk criteria for patients with valvular heart disease were limited, the Valve Academic Research Consortium High Bleeding Risk (VARC-HBR) criteria were recently introduced7. Twenty-one clinical, anatomical, and procedural factors were combined, weighted as 15 major and 6 minor criteria. These criteria were developed based on expert consensus; hence, they require empirical validation to substantiate their use in clinical practice. Therefore, we evaluated the VARC-HBR criteria for risk stratification and prediction of 30-day major bleeding risk in patients undergoing TAVI with a concomitant indication for oral anticoagulation.
Methods
Study design
This study is a subanalysis of the POPular PAUSE TAVI (ClinicalTrials.gov: NCT04437303) trial, a randomised clinical trial, conducted at 22 European sites, that evaluated the safety and efficacy of continuing versus interrupting oral anticoagulation during TAVI. Details of the design of the study have been described previously8. Briefly, patients were eligible if they were on any oral anticoagulant and scheduled to undergo transfemoral or transsubclavian TAVI. Patients randomised to the continuation strategy maintained oral anticoagulation throughout the periprocedural period, including on the day of the TAVI procedure. Patients randomised to the interruption strategy interrupted oral anticoagulation at least 48 hours before TAVI. Bridging with low-molecular-weight heparin was not recommended. Oral anticoagulation was restarted after TAVI, as soon as deemed safe by the operator and/or treating physician. The TAVI procedures were performed according to the local protocol of each participating study site, including the choice of valve type, whether cerebral embolic protection was used, the amount of periprocedural heparin, the amount of protamine (when administered), and the type of vascular closure device used. Follow-up visits were performed at discharge and 30 days after TAVI. If necessary, the patient’s primary care physician and/or treating specialist was contacted for additional information. The trial was approved by the national authorities and ethics committees and by the institutional review board at each participating site.
Patients
Patients planned for transfemoral or transsubclavian TAVI, who were using long-term oral anticoagulation and provided written informed consent, were included. The exclusion criteria were the presence of a mechanical heart valve prosthesis, intracardiac thrombus, venous thromboembolism within 3 months before TAVI or transient ischaemic attack or stroke in patients with atrial fibrillation within 6 months before TAVI.
Bleeding risk criteria
Baseline and procedural characteristics, including the VARC-HBR criteria, were registered in standardised electronic case report forms. Slightly modified definitions of severe hepatic disease, prior ischaemic stroke and active malignancy were used. A full list of the criteria and their respective definitions is provided in Supplementary Table 1. Patients were classified at moderate risk if no more than one minor criterion was met, at high risk if one major or two minor criteria were met, and at very high risk if at least two major or three minor criteria were met7. To compare the VARC-HBR criteria with existing bleeding risk scores, the criteria of the HAS-BLED, ORBIT, DOAC and PREDICT-TAVR bleeding risk scores were also assessed9101112. Full lists of these criteria and their respective definitions, adapted to the current study, are provided in Supplementary Table 2.
Bleeding definitions
Bleeding events were collected until 30 days after TAVI and adjudicated by a blinded clinical events committee. Adjudication was based on the Bleeding Academic Research Consortium (BARC) criteria and the Valve Academic Research Consortium (VARC)-3 criteria1314. For this analysis, major bleeding was defined as BARC Type 3-5 bleeding occurring within 30 days after TAVI7. The VARC-3-based major bleeding definition (Type 2-4) was used as a sensitivity analysis14. BARC and VARC-3 bleeding definitions are detailed in Supplementary Table 3.
Statistical analysis
The analysis population included all patients who had undergone randomisation and subsequent TAVI. Continuous variables are summarised as mean±standard deviation (SD) or as median and interquartile range, as appropriate. Categorical variables are presented as numbers and percentages. Proportions of major bleeding were compared between risk groups using the chi-square test. The discriminative ability of the VARC-HBR criteria, as well as the other bleeding risk scores, was assessed based on the area under the receiver operating characteristic curve (ROC-AUC) with corresponding 95% confidence intervals (CIs). The VARC-HBR criteria were assessed as a three-class risk score (moderate, high, very high risk) and as a point-based score, where minor criteria were given one point and major criteria two points. Calibration was evaluated by comparing predicted probabilities with observed frequencies of major bleeding per risk group. Multivariate logistic regression analysis was performed to assess the relative contribution of each criterion, which is expressed as odds ratios (ORs) with corresponding 95% CIs. Since the main trial did not show non-inferiority of the continued oral anticoagulation strategy, the impact of continuation of oral anticoagulation for the different VARC-HBR risk groups was evaluated. Additional logistic regression analyses were conducted, considering continuation of oral anticoagulation as a major criterion, to evaluate its impact both independently and in combination with other variables. There were no missing data in the evaluated criteria or bleeding outcomes. Statistical analyses were performed using R software, version 4.1 (R Foundation for Statistical Computing).
Results
Baseline characteristics
Between November 2020 and December 2023, a total of 869 patients were enrolled. Thirteen patients were excluded because TAVI was not initiated or they withdrew informed consent before the procedure. The mean±SD age of the patients was 81.1±5.9 years, and 34.5% were female. The indication for long-term oral anticoagulation was atrial fibrillation in 94.9% of the patients. The majority (81.6%) of patients used a direct oral anticoagulant, of whom 30.4% were on a reduced dose. Out of 856 patients included, 332 (39%) were classified at moderate bleeding risk, 337 (39%) at high bleeding risk, and 187 (22%) at very high bleeding risk. Patients in the higher bleeding risk categories had a greater prevalence of cardiovascular risk factors and comorbidities, consistent with the VARC-HBR criteria. Randomisation to a continued oral anticoagulation strategy was not significantly different between the groups (p=0.43). Baseline and procedural characteristics are detailed in Table 1 and Supplementary Table 4, respectively.
Table 1. Baseline characteristics.
| Moderate bleeding risk (n=332) | High bleeding risk (n=337) | Very high bleeding risk (n=187) | |
|---|---|---|---|
| Age, years | 80.1±5.6 | 81.9±5.7 | 79.2±6.6 |
| Female sex | 114 (34.3) | 128 (38.0) | 53 (28.3) |
| Body mass index, kg/m² | 27.9±4.6 | 27.3±4.8 | 26.6±4.6 |
| EuroSCORE II, % | 3.4±3.5 | 3.9±4.0 | 4.6±5.0 |
| NYHA Class III or IV | 191 (57.5) | 209 (62.0) | 130 (70.6) |
| Atrial fibrillation | 319 (96.1) | 323 (95.8) | 176 (94.1) |
| Paroxysmal | 154 (48.7) | 135 (41.9) | 87 (49.4) |
| CHA2DS2-VASc | 4.07±1.3 | 4.6±1.4 | 4.9±1.4 |
| Hypertension | 256 (77.1) | 253 (75.1) | 150 (80.2) |
| Diabetes | |||
| None | 243 (73.2) | 249 (73.9) | 115 (61.5) |
| Non-insulin dependent | 68 (20.5) | 60 (17.8) | 48 (25.7) |
| Insulin dependent | 21 (6.3) | 28 (8.3) | 24 (12.8) |
| Coronary artery disease | 128 (38.6) | 171 (50.7) | 113 (60.4) |
| History of myocardial infarction | 40 (12.0) | 56 (16.6) | 40 (21.4) |
| Previous cerebrovascular event | 33 (9.9) | 79 (23.4) | 55 (29.4) |
| Peripheral artery disease | 32 (9.6) | 63 (18.7) | 68 (36.4) |
| Chronic obstructive pulmonary disease | 51 (15.4) | 43 (12.8) | 22 (11.8) |
| Chronic renal insufficiency | 151 (45.5) | 173 (51.3) | 108 (57.8) |
| Previous heart valve surgery | 23 (6.9) | 26 (7.7) | 15 (8.0) |
| Previous spontaneous bleeding$ | 15 (4.5) | 31 (9.2) | 38 (20.3) |
| Active malignancy# | 0 (0) | 21 (6.2) | 26 (13.9) |
| Type of oral anticoagulation | |||
| Vitamin K antagonist | 48 (13.0) | 66 (19.6) | 45 (24.1) |
| Direct oral anticoagulant | 289 (87.0) | 271 (80.4) | 142 (75.9) |
| Randomised to continuation of OAC | 168 (50.6) | 162 (48.1) | 101 (54.0) |
| Data are presented as mean±SD or n (%). #Excluding non-melanoma skin cancer. $Requiring hospitalisation or transfusion. EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; OAC: oral anticoagulation; SD: standard deviation | |||
Prevalence of VARC-HBR criteria
The prevalence of VARC-HBR criteria, when present in at least 1% of the patients, is summarised in Central illustration A. The most common criterion was severe femoral artery calcification and tortuosity, which was present in 26.8% of the patients. Other prevalent criteria were dual antithrombotic therapy (oral anticoagulation+antiplatelet therapy; 12.5%), history of ischaemic stroke (10.5%), and anaemia (haemoglobin <11 g/dL) at hospital admission (11.6%). The following criteria were rarely observed: non-deferrable major surgery (0.4%), severe hepatic disease (0.7%), history of haemorrhagic stroke (0.9%), dual antiplatelet therapy (meaning triple therapy in this population; 0.5%), conversion to open-heart surgery (0.4%), and spontaneous bleeding >6 and <12 months before TAVI (0.4%). Severe thrombocytopaenia (platelet count <50x109/L) at baseline was not observed.
Risk stratification
Major bleeding occurred in 4.2% of patients classified at moderate risk, in 9.5% classified at high risk, and 15.0% at very high risk (p<0.001), as shown in Central illustration B. Fatal bleeding (BARC Type 5) occurred in 6 patients: 3 (0.9%) in the high-risk group and 3 (1.6%) in the very high-risk group (Table 2). Access site bleeding was the most common bleeding phenotype, which occurred in 4.5% of the moderate-risk group, in 7.1% of the high-risk group and in 10.2% of the very high-risk group. Further details regarding the sites of bleeding across the VARC-HBR subgroups are provided in Supplementary Table 5. Major bleeding according to the VARC-3 definition occurred in 6.3% of patients classified at moderate risk, in 10.4% classified at high risk, and 15.5% at very high risk. Bleeding events adjudicated by the VARC-3 criteria are displayed in Supplementary Table 6.
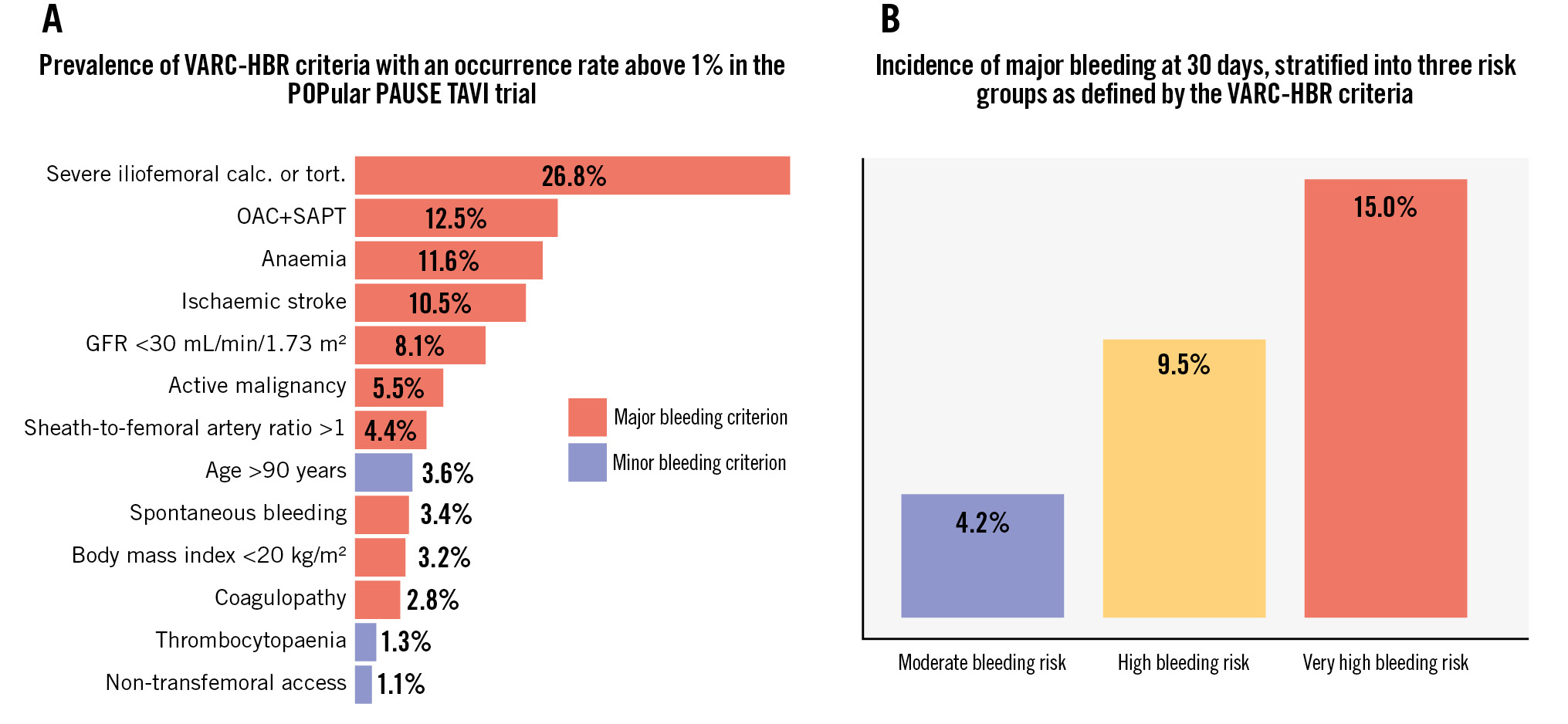
Central illustration. A) Prevalence of VARC-HBR criteria (occurrence >1%). B) 30-day major bleeding incidence stratified by VARC-HBR risk. calc.: calcification; GFR: glomerular filtration rate; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation; tort.: tortuosity; VARC-HBR: Valve Academic Research Consortium High Bleeding Risk
Table 2. BARC bleeding types stratified according to VARC-HBR risk groups.
| Bleeding type | Moderate (n=332) | High (n=337) | Very high (n=187) |
|---|---|---|---|
| Minor bleeding (Type 2) | 63 (19.0) | 53 (15.7) | 40 (21.4) |
| Major bleeding (Type 3-5) | 14 (4.2) | 32 (9.5) | 28 (15.0) |
| Type 3a | 7 (2.1) | 16 (4.8) | 19 (10.2) |
| Type 3b | 6 (1.8) | 13 (3.9) | 6 (3.2) |
| Type 3c | 1 (0.3) | - | - |
| Type 5a | - | - | - |
| Type 5b | - | 3 (0.9) | 3 (1.6) |
| Data are n (%). BARC: Bleeding Academic Research Consortium; VARC-HBR: Valve Academic Research Consortium High Bleeding Risk | |||
Risk prediction
The ROC-AUC of the VARC-HBR criteria was 0.64 (95% CI: 0.58-0.70) when assessed as a three-class risk score and 0.65 (95% CI: 0.58-0.71) when assessed as a point-based score (Figure 1). The ROC-AUC of the HAS-BLED score was 0.52 (95% CI: 0.45-0.60), the ORBIT score 0.54 (95% CI: 0.48-0.60), the DOAC score 0.55 (95% CI: 0.48-0.62), and the PREDICT-TAVR score 0.54 (95% CI: 0.47-0.61) (Figure 2). Although the observed event rates were slightly higher than predicted, the VARC-HBR criteria showed overall good calibration with observed outcomes (Central illustration B). Based on logistic regression analysis, severe femoral artery calcification and tortuosity (OR 2.5, 95% CI: 1.5-4.3), anaemia at baseline (OR 2.2, 95% CI: 1.1-4.2), and conversion to open-heart surgery (OR 21.2, 95% CI: 1.8-491.5) appeared to be the most influential predictors. The VARC-HBR model, showing the univariate and multivariate associations of the individual criteria with the occurrence of major bleeding, is presented in Table 3. Additionally, in Supplementary Table 7, the randomised strategy was evaluated as a major criterion and showed no significant interaction in either univariate or multivariate analyses (OR 1.1, 95% CI: 0.7-1.9). Accordingly, multivariate logistic regression models of the other bleeding scores are reported in Supplementary Table 8-Supplementary Table 9-Supplementary Table 10-Supplementary Table 11. The sensitivity analysis, in which the VARC-HBR criteria were applied to predict major bleeding based on the VARC-3 definition, yielded similar results (ROC-AUC of 0.64 [95% CI: 0.58-0.70]).
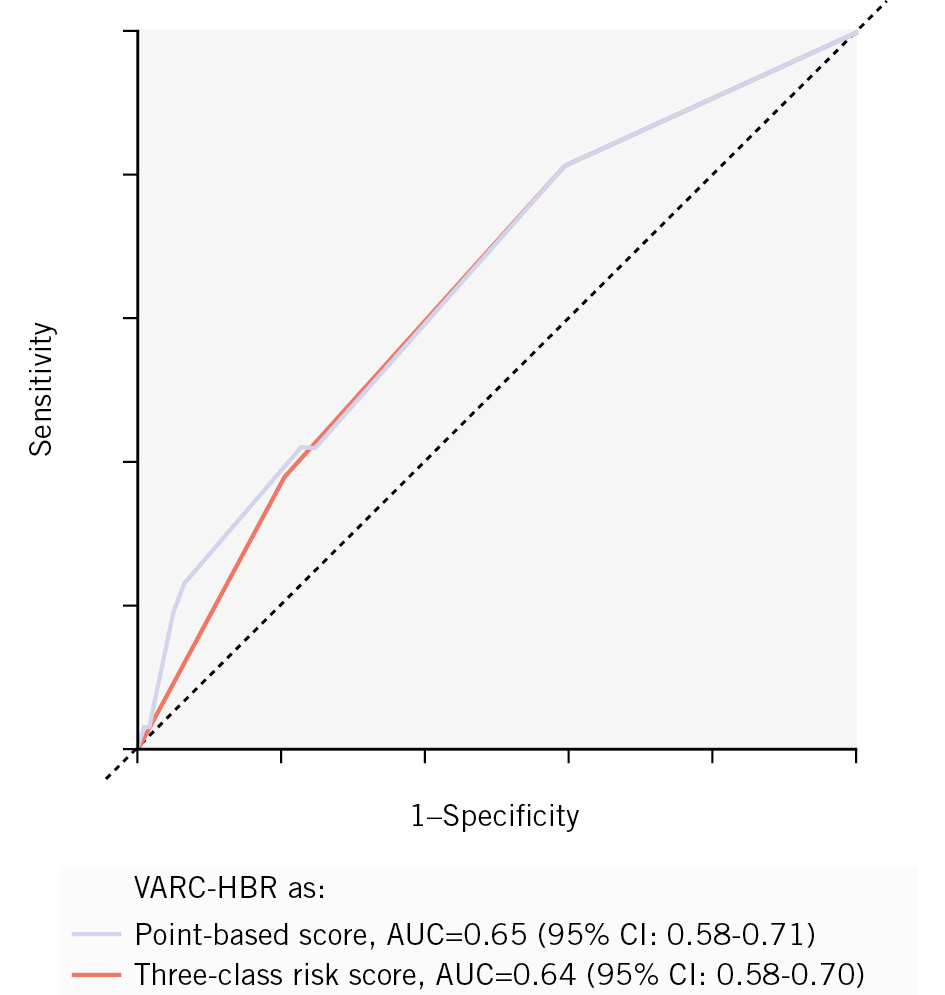
Figure 1. ROC curves of VARC-HBR criteria. AUC: area under the curve; CI: confidence interval; ROC: receiver operating characteristic; VARC-HBR: Valve Academic Research Consortium High Bleeding Risk
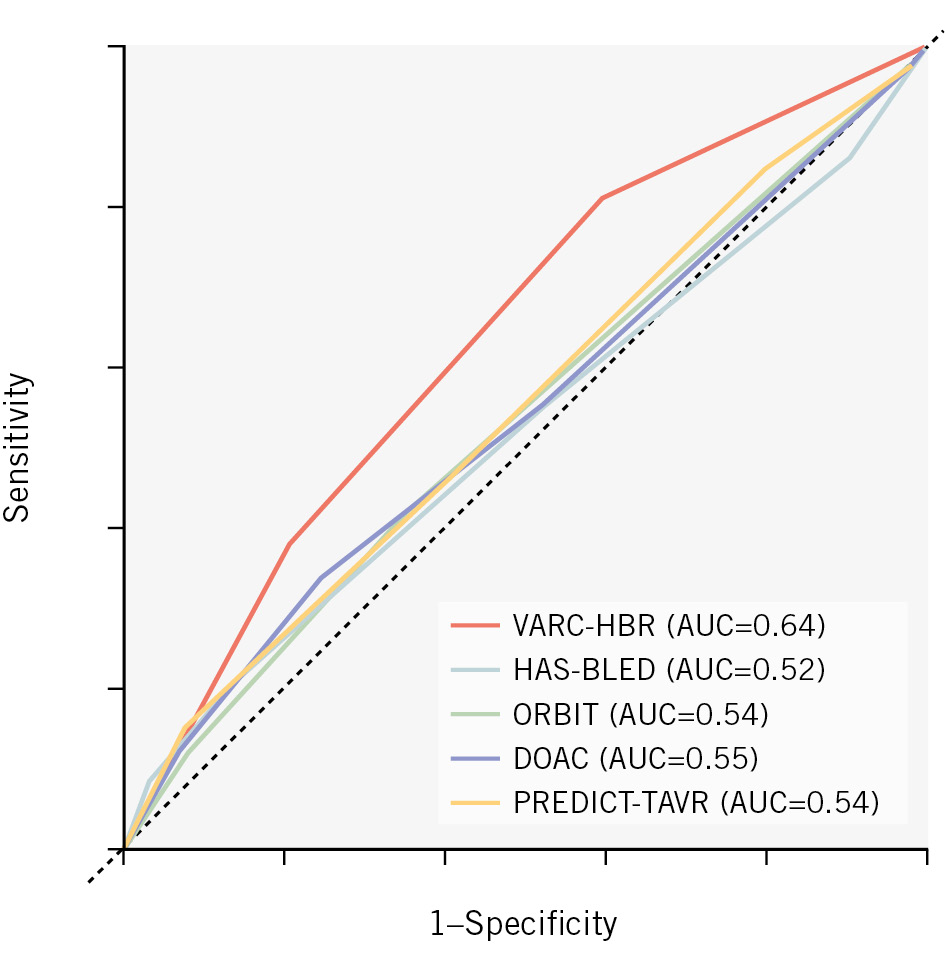
Figure 2. Performance of the VARC-HBR criteria compared to other bleeding risk scores. AUC: area under the curve; VARC-HBR: Valve Academic Research Consortium High Bleeding Risk
Table 3. Logistic regression analysis.
| VARC-HBR criteria* | Univariate OR (95% CI) | p-value | Multivariate OR (95% CI) | p-value |
|---|---|---|---|---|
| Minor criteria | ||||
| Age >90 years | 1.14 (0.27-3.31) | 0.84 | 1.40 (0.32-4.27) | 0.60 |
| Dual antiplatelet therapy (besides OAC) | 3.56 (0.17-28.18) | 0.27 | 3.79 (0.17-34.62) | 0.28 |
| Non-transfemoral access | 1.33 (0.07-7.37) | 0.79 | 0.79 (0.04-4.72) | 0.83 |
| Major criteria | ||||
| BMI <20 kg/m² | 1.33 (0.31-3.94) | 0.64 | 1.37 (0.31-4.32) | 0.63 |
| Chronic kidney disease (eGFR <30 mL/min/1.73 m²) | 1.43 (0.61-2.96) | 0.37 | 1.05 (0.40-2.40) | 0.91 |
| Active malignancy | 1.94 (0.77-4.24) | 0.12 | 1.80 (0.67-4.23) | 0.20 |
| Anaemia (Hb <11 g/dL) | 2.11 (1.11-3.80) | 0.02 | 2.16 (1.06-4.21) | 0.03 |
| Previous ischaemic stroke | 1.37 (0.64-2.66) | 0.38 | 1.37 (0.61-2.80) | 0.42 |
| Chronic bleeding diathesis | 1.53 (0.36-4.58) | 0.50 | 1.60 (0.34-5.48) | 0.49 |
| Spontaneous bleeding# | 1.73 (0.50-4.62) | 0.32 | 1.19 (0.29-3.83) | 0.79 |
| Dual antithrombotic therapy (OAC+SAPT) | 1.91 (1.01-3.42) | 0.04 | 1.53 (0.74-2.96) | 0.22 |
| Non-deferrable major surgery | 5.34 (0.25-56.40) | 0.17 | 3.60 (0.15-44.46) | 0.33 |
| SFAR >1 | 1.64 (0.55-4.00) | 0.32 | 1.21 (0.38-3.19) | 0.72 |
| Severely calcified and tortuous iliofemoral arteries | 2.26 (1.38-3.68) | 0.001 | 2.50 (1.46-4.29) | 0.001 |
| Immediate conversion to open-heart surgery | 21.69 (2.05-470.18) | 0.012 | 21.20 (1.77-491.47) | 0.02 |
| *Due to limited occurrence, associations for the following variables could not be estimated: moderate thrombocytopaenia, first spontaneous bleeding >6 and <12 months before TAVI, severe hepatic disease, severe thrombocytopaenia, previous intracranial haemorrhage, and oral anticoagulation (applied to everyone). #Defined as spontaneous (non-intracranial) bleeding requiring hospitalisation or transfusion in the previous 6 months (or at any time if recurrent). BMI: body mass index; CI: confidence interval; eGFR: estimated glomerular filtration rate; Hb: haemoglobin; OAC: oral anticoagulation; OR: odds ratio; SAPT: single antiplatelet therapy; SFAR: sheath-to-femoral artery ratio; VARC-HBR: Valve Academic Research Consortium High Bleeding Risk | ||||
Discussion
In this subanalysis of the POPular PAUSE TAVI trial, we evaluated the VARC-HBR criteria for risk stratification and prediction of 30-day major bleeding risk in patients undergoing TAVI with a concomitant indication for oral anticoagulation. The main findings were as follows: (1) the VARC-HBR criteria effectively identified three well-distributed subgroups, with a stepwise increase in major bleeding risk across the risk categories; (2) for individual risk prediction, the discriminative performance of the VARC-HBR criteria was moderate, yet, it appeared to outperform existing bleeding risk scores in this population; (3) severe femoral artery calcification and tortuosity, anaemia, and conversion to open-heart surgery were identified as the most contributory criteria.
In contemporary studies, major bleeding has been reported in 3-10% of patients within 30 days after TAVI415161718. The observed bleeding rate in our study was slightly higher, which could be attributed to the fact that we evaluated a subgroup of patients receiving oral anticoagulation, half of whom continued their therapy throughout the periprocedural period8. These high rates of bleeding emphasise the need for adequate risk assessment7. Based on the current findings, the VARC-HBR criteria seem to be a valuable tool for this purpose. The clinical implications of identifying patients at (very) high risk of bleeding may lie in adopting precautionary measures for access site management, since access site bleeding appeared to be the most common bleeding phenotype early after TAVI. For example, the use of the radial artery for secondary vascular access, protamine administration for heparin reversal at the end of the procedure, and the use of an additional vascular closure device may mitigate the bleeding risk in these patients171819. Recently, a dedicated stepwise vascular closure algorithm was shown to be associated with a major vascular complication rate (including major bleeding) of less than 1%20. This systematic approach may particularly be useful in patients at (very) high risk of bleeding.
Regarding the choice of antithrombotic therapy, the additional value of the VARC-HBR criteria may be limited, particularly in this subpopulation using oral anticoagulation, since interrupting oral anticoagulation before TAVI and restarting oral anticoagulation monotherapy after TAVI seems to be the appropriate strategy in general815. Dual antiplatelet therapy in addition to oral anticoagulation (triple therapy) is discouraged based on current literature. Our dataset showed that it was potentially an important predictor (OR 3.8, 95% CI: 0.17-34.62). Switching from a vitamin K antagonist to a direct oral anticoagulant after TAVI may be best avoided in (very) high-risk patients, as this has been associated with an increased risk of major bleeding21. Interestingly, in patients without a concomitant indication for oral anticoagulation or antiplatelet therapy, the need for lifelong single antiplatelet therapy has recently been questioned in high bleeding risk patients22. However, randomised controlled trials are needed before omitting antiplatelet therapy can be recommended. The ongoing Non-antithrombotic Therapy After TAVI Trial (NAPT; ClinicalTrials.gov: NCT06007222) and the Personalized, CT-guided Antithrombotic Therapy Versus Lifelong Single Antiplatelet Therapy to Reduce Thromboembolic and Bleeding Events in Non-atrial Fibrillation Patients After TAVI trial (POPular ATLANTIS; ClinicalTrials.gov: NCT06168370) are expected to provide further evidence on this topic23.
To the best of our knowledge, PREDICT-TAVR is the only other bleeding risk score specifically developed for patients undergoing TAVI12. Previous external validation showed a much better predictive performance than our data. This may be due to our evaluation of the version of the model without serum iron and our assessment of the common femoral artery diameter as a binary variable (<6 mm or not) instead of the original per-millimetre variable. The HAS-BLED, ORBIT and DOAC scores were specifically designed for patients on oral anticoagulation, but independent of the need for TAVI91011. Their limited predictive performance in this setting is likely due to the fact that these scores were developed to predict spontaneous bleeding rather than procedure-related bleeding, which involves different risk factors. The VARC-HBR criteria provide a more comprehensive approach, distinguishing factors that impact periprocedural and non-periprocedural bleeding, or both. Still, the discriminative performance observed in our data was only moderate, quite similar to what has been reported in studies evaluating the ARC-HBR criteria in patients undergoing percutaneous coronary intervention2425. In a large-scale observational study, the ROC-AUC of the ARC-HBR criteria was 0.64 (95% CI: 0.61-0.66) when assessed as a two-class variable, which increased to 0.68 (95% CI: 0.65-0.71) when assessed as a point-based variable25. Such an improvement was not observed in our analysis. This may be related to the fact that the VARC-HBR definition was designed as a three-class instead of a two-class system, thus providing a more granular approach. Upon exploration of our data, we observed that the point-based scores were clustered in three groups (1 point, 3 points, and 5 points), indicating that the three-class risk score appropriately described the degree of variation in our data.
Severe iliofemoral calcification and tortuosity are widely recognised risk factors for major bleeding2627. However, the VARC-HBR document provides no specific guidance on how this criterion should be determined. Considering its prevalence and contributory value, a more specific definition may improve the predictive value of the VARC-HBR criteria. Previous studies have shown that ventral (or anterior) common femoral artery calcification seems to be more relevant than overall iliofemoral calcification28. Also, the degree of longitudinal and especially circumferential extent of calcification appears to be associated with major bleeding risk29. Finally, considering severe femoral tortuosity as an independent criterion, given its distinct aetiology, may further enhance predictive performance2627.
Limitations
Our findings should be interpreted considering the following limitations. Although all 21 VARC-HBR criteria were included in the dataset, three variables had to be slightly modified because of data availability. Second, due to the limited sample size, the predictive value of uncommon criteria could not be assessed. Additionally, for a few variables, this resulted in wide confidence intervals, which should be interpreted with caution. Third, follow-up was limited to 30 days after TAVI, which resulted in access-related bleeding being more prominent compared to the 1-year major bleeding defined by VARC-HBR. Finally, almost all patients were treated using the transfemoral approach, so the results should not be generalised to other access site approaches for TAVI. The same applies to patients not using oral anticoagulation.
Conclusions
Among patients with a concomitant indication for oral anticoagulation, the VARC-HBR criteria identified three well-distributed subgroups, with a stepwise increase in major bleeding risk within 30 days after TAVI. However, for individual risk prediction, the discriminative performance of the VARC-HBR criteria were moderate but appeared to outperform existing bleeding risk scores in this population. Severe femoral artery calcification and tortuosity, anaemia, and conversion to open-heart surgery were identified as the most contributory criteria.
Impact on daily practice
The Valve Academic Research Consortium High Bleeding Risk (VARC-HBR) criteria effectively identify three distinct subgroups of patients with a stepwise increase in major bleeding risk after transcatheter aortic valve implantation. Applying these criteria in clinical practice may help select subgroups of patients who could benefit most from precautionary measures for access site management (e.g., radial secondary access, heparin reversal with protamine, and the use of an additional closure device). Given the significant association with bleeding, alternative approaches could be considered for patients with severe calcification or tortuosity of the iliofemoral arteries. For individual risk prediction, the discriminative performance observed in our data was moderate but outperformed other bleeding scores. While moderate, the VARC-HBR performs comparably to other bleeding scores, for example in studies evaluating the Academic Research Consortium High Bleeding Risk criteria in patients undergoing percutaneous coronary intervention.
Funding
The POPular PAUSE TAVI trial (ClinicalTrials.gov: NCT04437303) was supported by grants from the Netherlands Organization for Health Research and Development and the St. Antonius Research Fund. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Conflict of interest statement
Y. Kobari has received consulting fees from Boston Scientific. O. De Backer has received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. F. van der Kley has received consulting/lecturer fees from Edwards Lifesciences, Boston Scientific, and Abbott. N.M. Van Mieghem has received grants or contracts from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Daiichi Sankyo, AstraZeneca, and Teleflex; and consulting/lecturer fees from Abbott, Boston Scientific, Medtronic, Daiichi Sankyo, PulseCath BV, Siemens, Teleflex, JenaValve, Anteris, and Amgen. M. Voskuil has received lecturer fees from Edwards Lifesciences, Medtronic, and Abbott. A.J.J. IJsselmuiden has received consulting/lecturer fees from Angiocare, Meril Life Sciences, and Medtronic. R.S. Hermanides has received lecturer fees from Abbott, Amgen, Edwards Lifesciences, and Novartis. E. Barbato has received lecturer fees from Abbott, Insight Lifetech, and Boston Scientific. D. Mylotte has received consulting fees from Boston Scientific, Medtronic, and MicroPort. M.J. Swaans has received consulting/lecturer fees from Abbott, Bioventrix Inc., Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, GE HealthCare, Medtronic, Philips Healthcare, and Siemens Healthineers. T. Adriaenssens has received consulting fees from Abbott. J.M. Montero-Cabezas has received lecturer fees from Penumbra, Inc.; and a research grant from Shockwave Medical. J.J. Wykrzykowska has received institutional research grants from Medtronic; lecturer fees from Boston Scientific, Meril Life Sciences, Abbott, SMT, Cordis, and Medis Medical Imaging; and participates on the advisory board of Medtronic and Novo Nordisk. A.W.J. van ’t Hof has received institutional research grants from Medtronic, AstraZeneca, and Boehringer Ingelheim; consulting fees from CeleCor Therapeutics; and participates in the Data Safety Monitoring Board for Diagram Research. N. van Royen has received institutional research grants from Abbott, Biotronik, Medtronic, and Philips; and lecturer fees from Abbott, Bayer, MicroPort, and Rainmed. R. Delewi has received institutional research grants and consulting fees from Abiomed, Amgen, Boston Scientific, Edwards Lifesciences, and Novartis. J.M. ten Berg has received institutional research grants from ZonMw, AstraZeneca, and Daiichi Sankyo; and consulting/lecturer fees from AstraZeneca, Daiichi Sankyo, CeleCor Therapeutics, and Boehringer Ingelheim. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

