Severe tricuspid regurgitation (TR), leading to a cascade of pathophysiological changes, significantly impacts quality of life, increases hospitalisation rates, and reduces survival if left untreated1. Increased systemic venous congestion from volume overload reduces cardiac output, leading to right-sided heart failure. This also causes right ventricle (RV) dilation and dysfunction, exacerbating ventricular interdependence and resulting in systolic and diastolic dysfunction of the left ventricle (LV) along with pulmonary congestion. These effects can lead to irreversible multiorgan damage, highlighting the importance of timely intervention12.
Current medical therapies primarily focus on alleviating systemic congestion, providing only palliative effects. This highlights the need for more effective interventional therapies that address the underlying structural issues of the tricuspid valve (TV) and right heart chambers. Given the complex anatomy of the TV and the intricate pathophysiology of RV dysfunction, selecting the appropriate patient and device for intervention is challenging23. The ideal transcatheter TV device should not only restore physiological TV function through laminar flow but also minimise interaction with the RV and native TV leaflets, accommodate anatomical variations, and promote positive RV adaptation following TR treatment. Additionally, it should have a low rate of bleeding complications and the ability to be repositioned, recaptured, and retrieved if necessary.
We present the results of the first Italian compassionate experience with the TRicuspid Flow Optimizer (TRiFlO [Triflo Cardiovascular]) in three patients with symptomatic torrential TR and in New York Heart Association (NYHA) Class ≥III, despite optimal medical therapy. The median left ventricular ejection fraction was 50% (range 42-51). All patients were ineligible for surgery or current transcatheter therapies, but they fulfilled the anatomical feasibility criteria for TRiFlO4. All three patients received approval from the regional ethics committee, which carefully assessed the clinical indications, contraindications to currently available devices, and the necessity for compassionate use. Final authorisation was subsequently granted by the Italian Ministry of Health. The median age was 81 years (range 71-86), and all patients presented multiple comorbidities. The TR aetiology was primary in the first two patients, while prevalently atrial secondary in the third (Moving image 1A-Moving image 1B-Moving image 1C).
The device characteristics and the procedure have already been described (Moving image 2)5. Briefly, the device consists of three arms, anchoring the TV commissures, and a central tricuspid flow optimiser (TFO) targeting the effective regurgitant orifice area. The procedures were performed under general anaesthesia, with fluoroscopic and transoesophageal echocardiographic guidance, using a percutaneous approach by the right common femoral vein (Figure 1, Moving image 3-Moving image 4-Moving image 5-Moving image 6-Moving image 7-Moving image 8-Moving image 9). All subjects experienced acute procedural and clinical success, with residual moderate TR and a mean gradient of ≤2 mmHg. The oral anticoagulation therapy, administered for permanent atrial fibrillation (AFib), was maintained after the procedure. The median implantation time (from skin incision to delivery system removal) and in-hospital stay were 140 min (range 80-190) and 9 days (range 4-25), respectively. One patient experienced ProGlide (Abbott) failure, treated with vascular surgery, and postprocedural transient renal failure that was induced by computed tomography (CT) contrast, requiring 10 days of ultrafiltration. The median follow-up time was 10 months (range 6-12). Four weeks after the intervention, the first patient needed an uneventful single-chamber ventricular permanent pacemaker (PM) implantation due to slow-rate AFib. At 10 months of follow-up, he developed PM pocket infection and systemic sepsis. Prosthetic endocarditis was excluded by multiple transoesophageal echocardiograms. Nevertheless, he died at 360 days. No other adverse events were recorded during follow-up. The TR grade remained moderate in all patients. No device thrombosis was observed. Reductions in right atrial volume, TV annulus and inferior vena cava diameters were noted at follow-up, along with improvement of the RV dimension and function and health status (Supplementary Figure 1).
Transcatheter TV therapy with TRiFlO has shown to be feasible and safe in patients with torrential symptomatic TR and impaired RV function, offering promising benefits in a selected population (Figure 1). Its unique design provides several advantages that suggest that the TRiFlO device could have a significant role in the expanding therapeutic options for TR. The device is adaptable to variable TV geometries and can be securely placed in the commissural scallops, maintaining excellent anatomical compliance. Its small footprint and anchoring mechanism ensure that RV contractility is preserved while the integrity of the native leaflets is maintained, allowing for effective coaptation with the implant leaflets during systole. The device avoids creating an atrioventricular gradient, and the polymeric implant leaflets have demonstrated excellent antithrombotic properties. Following implantation, we observed significant echocardiographic and functional improvements for up to 12 months (Supplementary Figure 2). Further clinical validations of our findings are necessary.
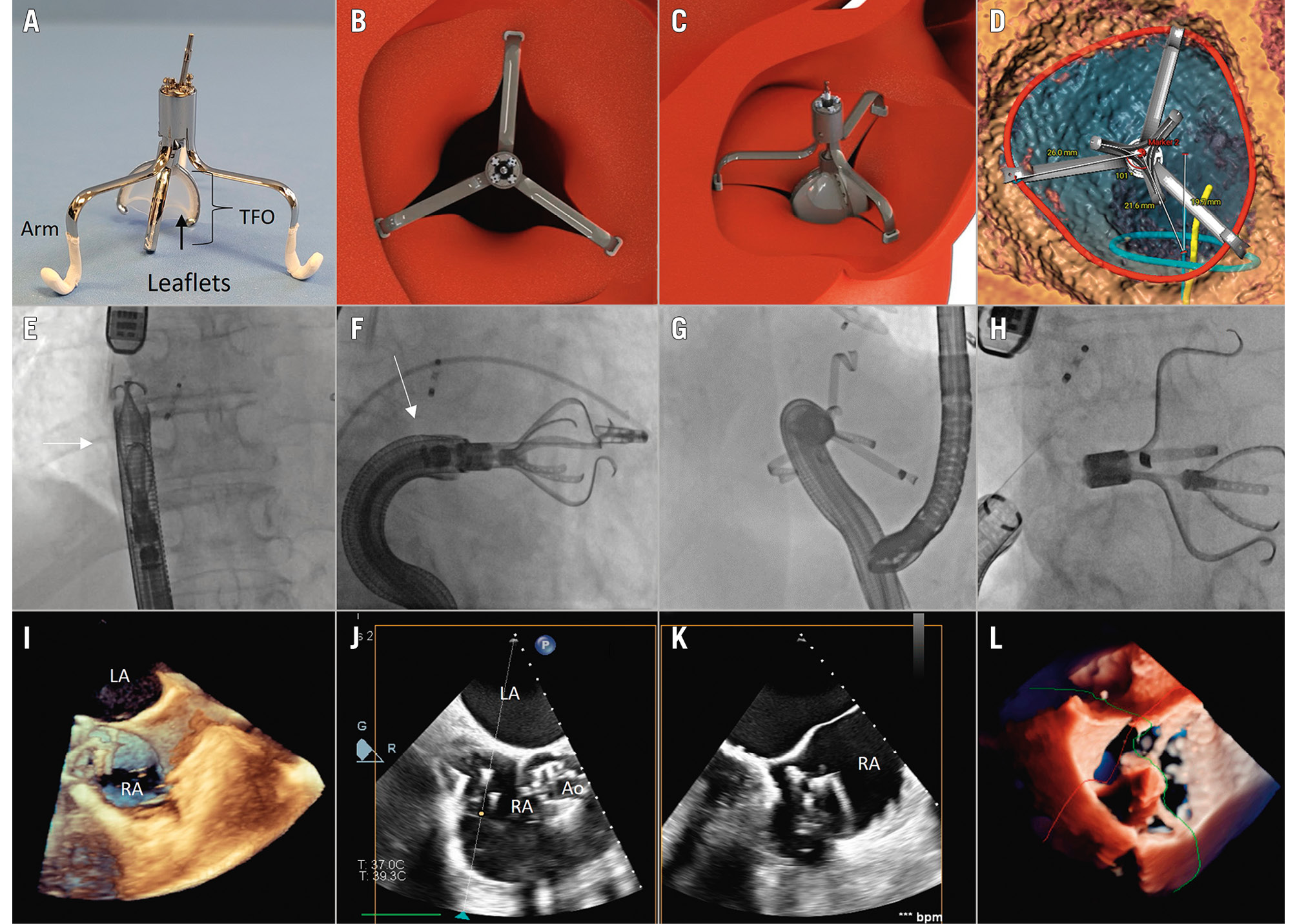
Figure 1. Novel transcatheter tricuspid valve therapy with the Tricuspid Flow Optimizer (TRiFlO) device. The TRiFlO device consists of three arms positioned in the commissural scallops of the tricuspid valve (TV) and a central tricuspid flow optimiser (TFO) targeting the regurgitant orifice (A). The TFO mini-valve polymeric leaflets close in diastole (B), open in systole (C) and coapt with native leaflets, in order to regulate blood flow effectively and reduce tricuspid regurgitation. The procedure requires dedicated CT scan planning and simulation before the intervention (D). For transcatheter implantation, once the 37 Fr steerable catheter (white arrow) enters the right atrium (E, I), the device must be oriented coaxial and coplanar to the tricuspid valve (F, J, K). A lateral deflection of the steerable catheter is necessary to compensate the offset of the inferior vena cava ostium relative to the TV plane (G). Once the anchors are secured to the commissural scallops, the position of the TFO mini-valve is optimised to achieve the best native device leaflet coaptation, and then released (H, L). Dedicated transoesophageal echocardiographic views are required for guiding the procedural steps. Ao: aorta; CT: computed tomography; LA: left atrium; RA: right atrium
Conflict of interest statement
G.P. Ussia is co-founder and scientific advisor for TriFlo Cardiovascular. V. Cammalleri has received speaker honoraria from Abbott. A. Mangieri serves as proctor for P+F Products + Features GmbH, and AliveCor; has received an institutional grant from Boston Scientific; and has received speaker honoraria from Boston Scientific, Concept Medical, Edwards Lifesciences, and Abbott. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Echocardiographic assessment of the tricuspid valve morphology and aetiology of tricuspid regurgitation in patients treated with TRiFlO.
Moving image 2. TRiFlO animation.
Moving image 3. Positioning of the steerable catheter in the right atrium.
Moving image 4. Deflection of the steerable catheter and right ventriculogram.
Moving image 5. Alignment of the TRiFlO device.
Moving image 6. Arm orientation.
Moving image 7. TFO position optimisation under TOE.
Moving image 8. Rotation control following TFO height optimisation.
Moving image 9. Detachment and release of the TRiFlO.

