Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Vascular access site complications are associated with increased morbidity and mortality after transcatheter aortic valve implantation (TAVI). Current results comparing strategies with plug- (P-VCD; MANTA) and suture-based vascular closure devices (S-VCD; Perclose ProGlide) remain inconsistent.
Aims: It was our aim to assess the incidence of access-related vascular complications after P-VCD or S-VCD strategies after transfemoral TAVI.
Methods: The Plug or sUture based vascuLar cloSurE after TAVI (PULSE) registry retrospectively evaluated 10,120 consecutive patients who had undergone transfemoral TAVI at 10 centres from 2016 to 2021. A propensity score was used to match 900 P-VCD patients with 1,800 S-VCD patients in a 1:2 fashion. The primary outcome measures were major and minor access-related vascular complications at the primary access site, adjudicated according to Valve Academic Research Consortium 3 definitions.
Results: The median age was 81.8 years, 46.4% of patients were female, and the median European System for Cardiac Operative Risk Evaluation II was 3.50%. In matched P-VCD and S-VCD groups, large-bore access-related complications occurred in 14.9% vs 10.3% (p<0.001; major: 3.6% vs 4.6%; p=0.218; minor: 11.3% vs 5.8%; p<0.001) of patients. Bleeding accounted for most of these complications (9.6% vs 7.2%; p=0.028) and was treated with endovascular balloon inflation (5.4% vs 2.6%; p<0.001), stent implantation (4.7% vs 0.7%; p<0.001) or surgical repair (0.7% vs 1.7%; p=0.03).
Conclusions: P-VCD were associated with higher rates of primary access-related vascular complications, driven by minor complications, compared to S-VCD. Endovascular treatment was more common after P-VCD failure.
Transcatheter aortic valve implantation (TAVI) has become the preferred treatment for symptomatic aortic stenosis in elderly patients or those with increased operative risk across all age groups1. The transfemoral access route remains the approach of choice for TAVI procedures, with lower complication rates and the largest evidence base compared to alternative access options. Given the required large-bore access, vascular and bleeding complications remain a relevant concern, as they are associated with impaired outcomes and significantly impact morbidity and mortality2. A relevant number of these vascular access complications are related to insufficient vascular closure at the large-bore access site during the TAVI procedure. Currently, two percutaneous closure techniques are broadly used, including the collagen plug-based vascular closure device (P-VCD; MANTA [Teleflex]) and the suture-based vascular closure device (S-VCD; Perclose ProGlide [Abbott])34. Results remain inconsistent regarding outcomes with both approaches: while the initial results from non-randomised studies demonstrated similar or even lower complication rates with P-VCD compared to S-VCD, two randomised controlled trials found more access-related vascular complications with the P-VCD strategy567891011. Currently, the reasons for these inconsistencies remain unclear, but small sample sizes of single-
centre reports and the application of outdated Valve Academic Research Consortium (VARC) and VARC-2 complication definitions may have had a major impact1213. To overcome these limitations and shed further light on the issue of VCD strategies during TAVI, we assessed vascular access and bleeding complications in a large, multicentre, real-world TAVI registry comparing P-VCD and S-VCD approaches with adjudication of complications according to the latest VARC-3 criteria14.
Methods
Patient population
The Plug or sUture based vascuLar cloSurE after TAVI (PULSE) registry retrospectively evaluated the data of 10,120 consecutive patients who had undergone transfemoral TAVI at 10 German heart centres from 2016 to 2021 (Figure 1). We included all patients undergoing transfemoral TAVI with either P-VCD (n=1,290) or S-VCD (n=8,169) use. Included S-VCD strategies comprised implantation of a single S-VCD, or one S-VCD and one Angio-Seal (Terumo), or two S-VCD. Exclusion criteria included alternative access routes, hostile access requiring intervention to gain access, surgical cutdown or utilisation of other vascular closure devices. All patients provided informed consent for the procedure and data acquisition. Ethics committee approval was obtained according to local requirements. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
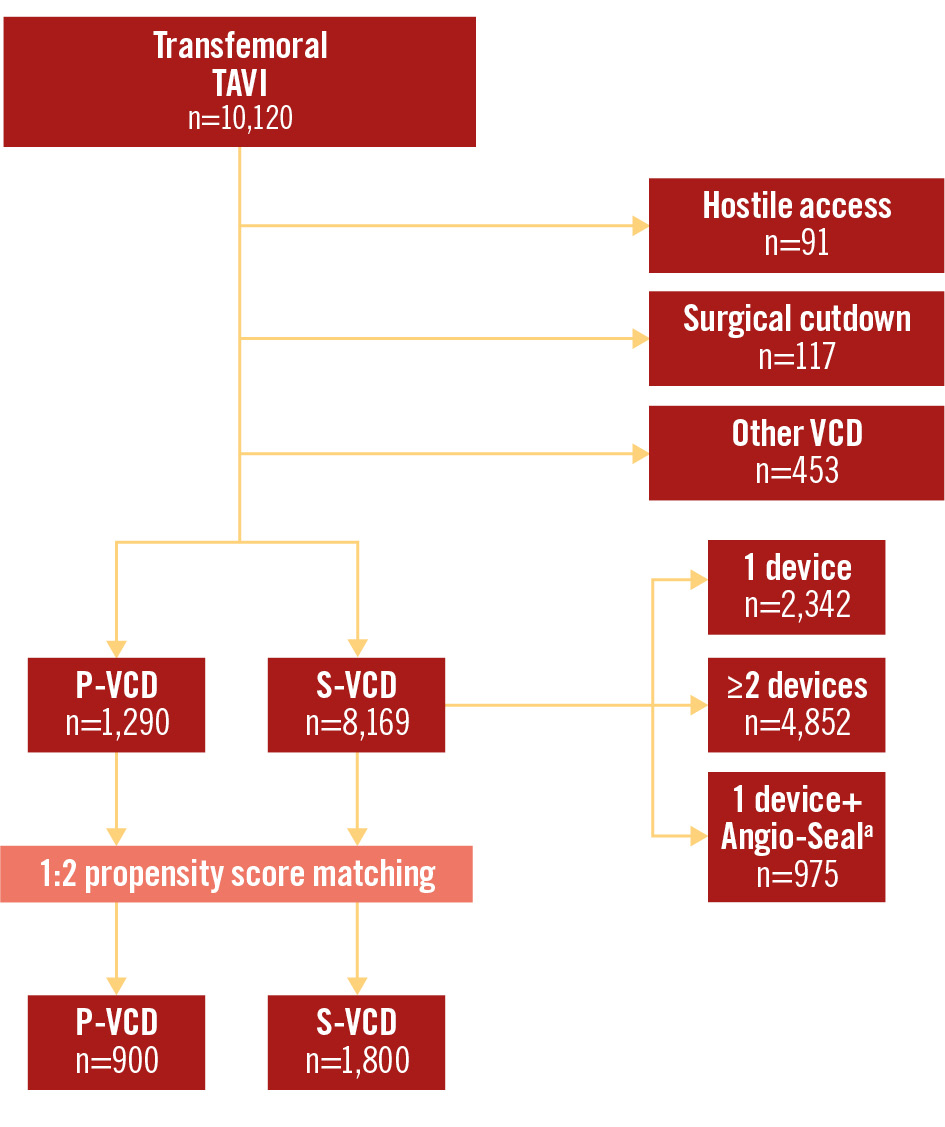
Figure 1. Study flowchart. Overall, 10,120 patients with transfemoral TAVI were evaluated in the PULSE registry. After exclusion of patients with hostile access (peripheral transluminal angioplasty or stent implantation to gain transfemoral access), planned surgical cutdown, or the use of other vascular closure devices, 1:2 propensity score matching was performed. aBy Terumo. PULSE: Plug or sUture based vascuLar cloSurE after TAVI; P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device; VCD: vascular closure device; TAVI: transcatheter aortic valve implantation
Computed tomography assessment
Vascular anatomy was evaluated from contrast-enhanced multidetector computed tomography performed during the workup for TAVI. Evaluation of the primary access (either left or right) included calcification severity − divided into none/mild (spotty), moderate (coalescing), and severe (bulky, protruding, horseshoe, circumferential) − and tortuosity severity from the puncture site to the aortic bifurcation − divided into none/mild (30-60°), moderate (60-90°), and severe (≥90°), as described before1516. The minimal lumen diameter (MLD) was measured in the common femoral artery at the primary access site.
Transcatheter aortic valve implantation and vascular closure
All cases were reviewed by the local Heart Team. TAVI was performed according to local practice and expertise, including the selection of VCD strategies. Both VCD have been described in detail before. In short, the 18 Fr P-VCD, which was exclusively used in this study, consists of a bioabsorbable-polymer toggle which is placed inside the access vessel and a non-resorbable polyester suture attached to a stainless steel lock on top of a haemostatic collagen plug outside the arterial wall (Figure 2A)3. The S-VCD sutures both sides of the arterial wall together by using a retractable foot inside and two deployable needles outside the access vessel while maintaining guidewire access (Figure 2B)4. Procedural variables were documented in a standardised fashion and included the sheath-to-femoral artery ratio (SFAR), calculated from the outer sheath diameter (non-expanded) and minimal vascular lumen diameter2. The SFAR was then evaluated in a dichotomous manner with the cutoff of SFAR=1.0. Time to vascular closure (TTVC) was defined as the time from the final root shot after valve implantation to the final angiography of femoral access. The procedure duration, in minutes, was measured from the first puncture to haemostasis, in order to reflect the entire procedure. Heparin reversal was achieved by using protamine at the operator’s discretion.
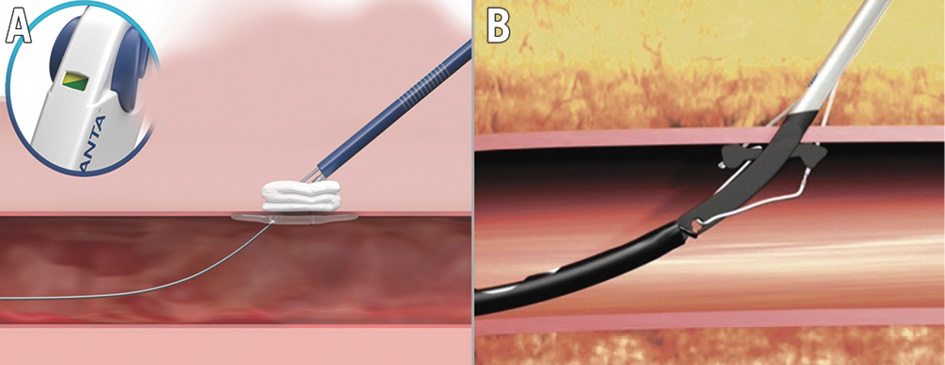
Figure 2. Plug- and suture-based vascular closure. Plug-based vascular closure device (A; MANTA [Teleflex]) and suture-based vascular closure device (B; ProGlide [Abbott]) strategies were evaluated. Reproduced with permission from Teleflex Medical GmbH, Fellbach, Germany (A), and Abbott Vascular International BVBA, Diegem, Belgium (B).
Clinical outcomes and endpoint definitions
Outcomes were evaluated in accordance with VARC-3 definitions at 30 days after the index procedure and were compared between the P-VCD and S-VCD groups14. Primary outcomes were major and minor access-related vascular complications at the primary (large-bore) access site. These were subclassified as bleeding, stenosis/occlusion, femoral dissection, pseudoaneurysm or other for each access site (primary or secondary). Bleeding as a vascular complication was defined as overt bleeding after vessel injury at the location of vessel puncture in order to gain access. Pseudoaneurysms were defined as a locally contained, one-sided, perivascular haematoma including only some or none of the arterial wall layers. Treatment was characterised as surgical repair, endovascular stenting, endovascular balloon inflation, conservative treatment/prolonged manual compression or other. Secondary outcomes included bleeding, stroke, acute kidney injury, myocardial infarction, pacemaker implantation, length of hospitalisation, and mortality according to VARC-3 (Supplementary Table 1).
Statistical analysis
Binary variables are shown as absolute numbers or percentages and were compared using the χ2 test. Continuous variables are shown as median (interquartile range [IQR]) and were compared using the Mann-Whitney U test. All p-values had a significance threshold of <0.05. Statistical analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing)17. As confounding variables were present at baseline, we applied a propensity score (PS)-matching method for adjustment between P-VCD and S-VCD patients. This model was calculated using the genetic matching method, a calliper width of 0.1, a population size of 250, a ratio of 1:2 and without replacement. The propensity score distance was estimated with logistic regression. The target estimand was the average treatment effect in the treated population, and the covariates used for matching were age, sex, body mass index, diabetes, glomerular filtration rate, anaemia, peripheral artery disease (PAD), vascular calcification (moderate/severe), vessel tortuosity (moderate/severe), antithrombotic therapy during TAVI (dual antiplatelet medication, triple antiplatelet medication [anticoagulation as well as dual antiplatelet medication], anticoagulation monotherapy, dual therapy [anticoagulation as well as single antiplatelet medication]), SFAR (categorised/dichotomised as above or below 1), secondary access, left ventricular ejection fraction <30% and ultrasound-guided puncture. The remaining number of observations after removing all observations with missing values in covariates was 5,266. Imputation for missing values was not performed. After matching, 2,700 observations remained. Subsequently, 900 patients with P-VCD were compared to 1,800 patients with different S-VCD strategies. Balance between groups was assessed by the average absolute standardised difference and amounted to 0.15 before matching and 0.02 after matching18. A Cochran-Armitage test was conducted to examine potential trends in major, minor, or no primary vascular access complications over the time intervals. The null hypothesis of no trend in data was tested for each group against the others. P-values were corrected with the Bonferroni method and indicated as a trend if <0.05. To compute odds ratios and 95% confidence intervals, we used a logistic regression outcome model with each variable of interest as an outcome, treatment as a predictor, and the function avg_comparisons from the marginaleffects package with its specifications19.
Results
Baseline characteristics
The median age was 81.8 (IQR 78.3, 85.0) years and the median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 3.5% (IQR 2.1, 5.8) overall. Before PS matching, the P-VCD and S-VCD groups differed significantly regarding several baseline risk factors and procedural characteristics (Supplementary Table 2, Supplementary Table 3). After 1:2 PS matching, comorbidities, clinical and computed tomography-derived variables at baseline were well balanced between the two groups, including the presence of PAD, vascular anatomy, and antithrombotic therapy (Table 1). Significant differences persisted for the mean transvalvular gradients (37.0 [IQR 26.2, 46.0] mmHg vs 39.0 [IQR 29.0, 48.0] mmHg; p=0.002), and effective orifice area (EOA; 0.8 [IQR 0.6, 0.9] cm2 vs 0.7 [IQR 0.6, 0.9] cm2; p=0.001).
Table 1. Baseline characteristics after PS matching.
| Baseline | All (N=2,700) | P-VCD (N=900) |
S-VCD (N=1,800) | p-value |
|---|---|---|---|---|
| Age, years | 81.8 (78.3, 85.0) | 81.7 (78.3, 85.1) | 81.8 (78.4, 85.0) | 0.60 |
| Female sex | 1,253 (46.4) | 420 (46.7) | 833 (46.3) | 0.88 |
| BMI, kg/m² | 26.2 (23.9, 29.3) | 26.0 (23.9, 29.6) | 26.3 (23.8, 29.1) | 0.82 |
| CAD | 1,584 (58.8) | 536 (60.1) | 1,048 (58.2) | 0.38 |
| Prior CABG | 259 (9.7) | 87 (9.9) | 172 (9.6) | 0.80 |
| Prior myocardial infarction | 296 (11.0) | 104 (11.6) | 192 (10.7) | 0.51 |
| COPD | 339 (12.6) | 100 (11.1) | 239 (13.3) | 0.13 |
| PAD | 293 (10.9) | 103 (11.4) | 190 (10.6) | 0.53 |
| Atrial fibrillation | 1,077 (42.5) | 346 (40.7) | 731 (43.4) | 0.20 |
| Prior stroke | 322 (11.9) | 101 (11.2) | 221 (12.3) | 0.46 |
| Diabetes | 568 (21.0) | 197 (21.9) | 371 (20.6) | 0.47 |
| eGFR, ml/min/1.73 m2 | 53.0 (40.0, 69.0) | 54.7 (39.8, 70.0) | 53.0 (40.0, 68.0) | 0.30 |
| Anaemia (haemoglobin <11 g/dl) | 706 (26.1) | 237 (26.3) | 469 (26.1) | 0.91 |
| NYHA Class IV | 177 (6.9) | 59 (7.3) | 118 (6.6) | 0.57 |
| Logistic EuroSCORE II, % | 3.5 (2.1, 5.8) | 3.7 (2.1, 6.2) | 3.4 (2.2, 5.6) | 0.39 |
| Antithrombotic medication | ||||
| Dual antiplatelet medication | 1,192 (44.1) | 386 (42.9) | 806 (44.8) | 0.37 |
| Anticoagulation monotherapy | 371 (13.7) | 128 (14.2) | 243 (13.5) | 0.65 |
| Dual therapy (anticoagulation and single antiplatelet medication) | 762 (28.2) | 249 (27.7) | 513 (28.5) | 0.68 |
| Triple therapy | 43 (1.6) | 14 (1.6) | 29 (1.6) | 1.00 |
| Computed tomography | ||||
| MLD of the common femoral artery at primary access site, mm | 7.6 (6.6, 8.5) | 7.9 (6.8, 8.9) | 7.5 (6.4, 8.4) | <0.001* |
| Vascular calcification – moderate or severe | 1,661 (61.5) | 562 (62.4) | 1,099 (61.1) | 0.51 |
| Vessel tortuosity – moderate or severe | 881 (32.6) | 284 (31.6) | 597 (33.2) | 0.42 |
| Echocardiography | ||||
| LVEF <30% | 125 (4.6) | 46 (5.1) | 79 (4.4) | 0.46 |
| Mean transvalvular gradient, mmHg | 38.0 (28.0, 48.0) | 37.0 (26.2, 46.0) | 39.0 (29.0, 48.0) | 0.002* |
| Effective orifice area, cm2 | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) | 0.7 (0.6, 0.9) | 0.001* |
| Severe aortic regurgitation | 57 (2.2) | 15 (1.7) | 42 (2.4) | 0.35 |
| Binary variables are shown as absolute numbers (percentages) and were compared using the χ2 test. Continuous variables are shown as median (interquartile range) and were compared using the Mann-Whitney U test. *Indicates statistical significance. BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; MLD: minimal lumen diameter; NYHA: New York Heart Association; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; PS: propensity score; P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device | ||||
Procedural characteristics
Procedural characteristics are listed in Table 2. Consistent with similar sheath sizes, SFAR >1 for the primary access was comparable in both groups (148 [16.4%] vs 315 [17.5%]; p=0.53). Transcatheter heart valve (THV) choice was balloon-expandable THV in 33.3% vs 54.4% (P-VCD vs S-VCD; p<0.001). A secondary femoral access (e.g., for pigtail catheter placement) was used in 63.7% vs 64.3% (p=0.79), and ultrasound-guided puncture was performed in 2.0% vs 1.9% (p=0.96). In the S-VCD group, closure was performed with one S-VCD in 173 patients (9.6%), with at least two S-VCD in 1,216 patients (67.6%) and with a combination of one S-VCD and one Angio-Seal VCD in 411 patients (22.8%). Heparin was reversed with protamine in 87.0% of P-VCD and 56.4% of S-VCD patients. TTVC was shorter (8.0 [IQR 5.0, 13.0] min vs 11.0 [IQR 9.0, 16.0] min; p<0.001), but overall procedure duration (57.0 [IQR 47.0, 70.0] min vs 50.0 [IQR 35.0, 65.0] min; p<0.001) and fluoroscopy time (13.4 [IQR 10.0, 18.4] min vs 11.0 [IQR 8.0, 15.6] min; p<0.001) were longer, and more contrast agent was used (170 [IQR 130.0, 210.0] ml vs 102.0 [IQR 76.0, 148.0] ml; p<0.001) in P-VCD versus S-VCD groups.
Table 2. Procedural characteristics after PS matching.
| Procedural characteristics | All (N=2,700) | P-VCD (N=900) | S-VCD (N=1,800) | p-value |
|---|---|---|---|---|
| Primary access sheath size, Fr | 14.0 (14.0, 16.0) | 14.0 (14.0, 14.0) | 14.0 (14.0, 16.0) | 0.62 |
| SFAR (OD/MLD) >1 | 463 (17.1) | 148 (16.4) | 315 (17.5) | 0.53 |
| Vascular closure strategy | ||||
| One S-VCD | 173 (6.4) | 0 (0) | 173 (9.6) | <0.001* |
| Two S-VCD | 1,216 (45.0) | 0 (0) | 1,216 (67.6) | <0.001* |
| One S-VCD and one Angio-Seala | 411 (15.2) | 0 (0) | 411 (22.8) | <0.001* |
| One P-VCD | 900 (33.3) | 900 (100) | 0 (0) | <0.001* |
| Ultrasound-guided puncture | 52 (1.9) | 18 (2.0) | 34 (1.9) | 0.96 |
| Secondary access | ||||
| Femoral | 1,730 (64.1) | 573 (63.7) | 1,157 (64.3) | 0.79 |
| Radial | 970 (35.9) | 327 (36.3) | 643 (35.7) | 0.79 |
| Balloon-expandable THV | 1,279 (47.4) | 300 (33.3) | 979 (54.4) | <0.001* |
| Heparin reversal with protamine | 1,793 (66.6) | 779 (87.0) | 1,014 (56.4) | <0.001* |
| Time to vascular closure, min | 9.0 (6.0, 14.3) | 8.0 (5.0, 13.0) | 11.0 (9.0, 16.0) | <0.001* |
| Procedure duration, min | 53.0 (40.0, 67.0) | 57.0 (47.0, 70.0) | 50.0 (35.0, 65.0) | <0.001* |
| Contrast agent, ml | 123.0 (85.0, 178.0) | 170.0 (130.0, 210.0) | 102.0 (76.0, 148.0) | <0.001* |
| Fluoroscopy time, min | 12.0 (8.3, 16.6) | 13.4 (10.0, 18.4) | 11.0 (8.0, 15.6) | <0.001* |
| Binary variables are shown as absolute numbers (percentages) and were compared using the χ2 test. Continuous variables are shown as median (interquartile range) and were compared using the Mann-Whitney U test. aBy Terumo. *Indicates statistical significance. MLD: minimal lumen diameter; OD: outer diameter; PS: propensity score; P-VCD: plug-based vascular closure device; SFAR: sheath-to-femoral artery ratio; S-VCD: suture-based vascular closure device; THV: transcatheter heart valve | ||||
Clinical outcomes
Large-bore access-related complications for P-VCD versus S-VCD occurred in 14.9% versus 10.3% (p<0.001), respectively, and were considered major in 3.6% versus 4.6% (p=0.218) and minor in 11.3% versus 5.8% (p<0.001) of patients (Central illustration). Bleeding accounted for most of these complications (9.6% vs 7.2%; p=0.028), followed by stenosis/occlusion (1.8% vs 1.1%; p=0.11), pseudoaneurysm (1.6% vs 1.0%; p=0.209), dissection (1.1% vs 0.6%; p=0.167) and other (0.1% vs 0.4%; p=0.244) (Figure 3).
These were treated with prolonged endovascular balloon inflation (5.4% vs 2.6%; p<0.001), stent implantation (4.7% vs 0.7%; p<0.001), surgical repair (0.7% vs 1.7%; p=0.03) or with conservative treatment/prolonged manual compression (1.2% vs 4.2%; p<0.001) in patients with P-VCD versus S-VCD, respectively (Table 3, Figure 4). VCD failure was observed in 10.8% versus 4.7% (p<0.001) of cases. In addition, access-related vascular complications occurred in 0.3% versus 2.4% (p<0.001; major: 0.1 vs 0.9%; p=0.037; minor: 0.2 vs 1.5%; p=0.009) at the secondary access site. Severe bleeding (Type 3/4: 2.1% vs 3.8%; p=0.02) and permanent pacemaker implantation (10.7% vs 13.7%; p=0.023) were observed more frequently in patients receiving S-VCD compared to P-VCD. The remaining VARC-3 outcomes, including stroke, acute kidney injury, myocardial infarction, and all-cause mortality, did not differ between the two groups. However, the length of in-hospital stay was shorter in P-VCD patients (6 [IQR 5.0, 8.0] days vs 7 [IQR 5.0, 9.0] days; p<0.001). Overall, a reduction of major access-related vascular complications from 2016 to 2021 was observed in the unmatched cohort (Figure 5).
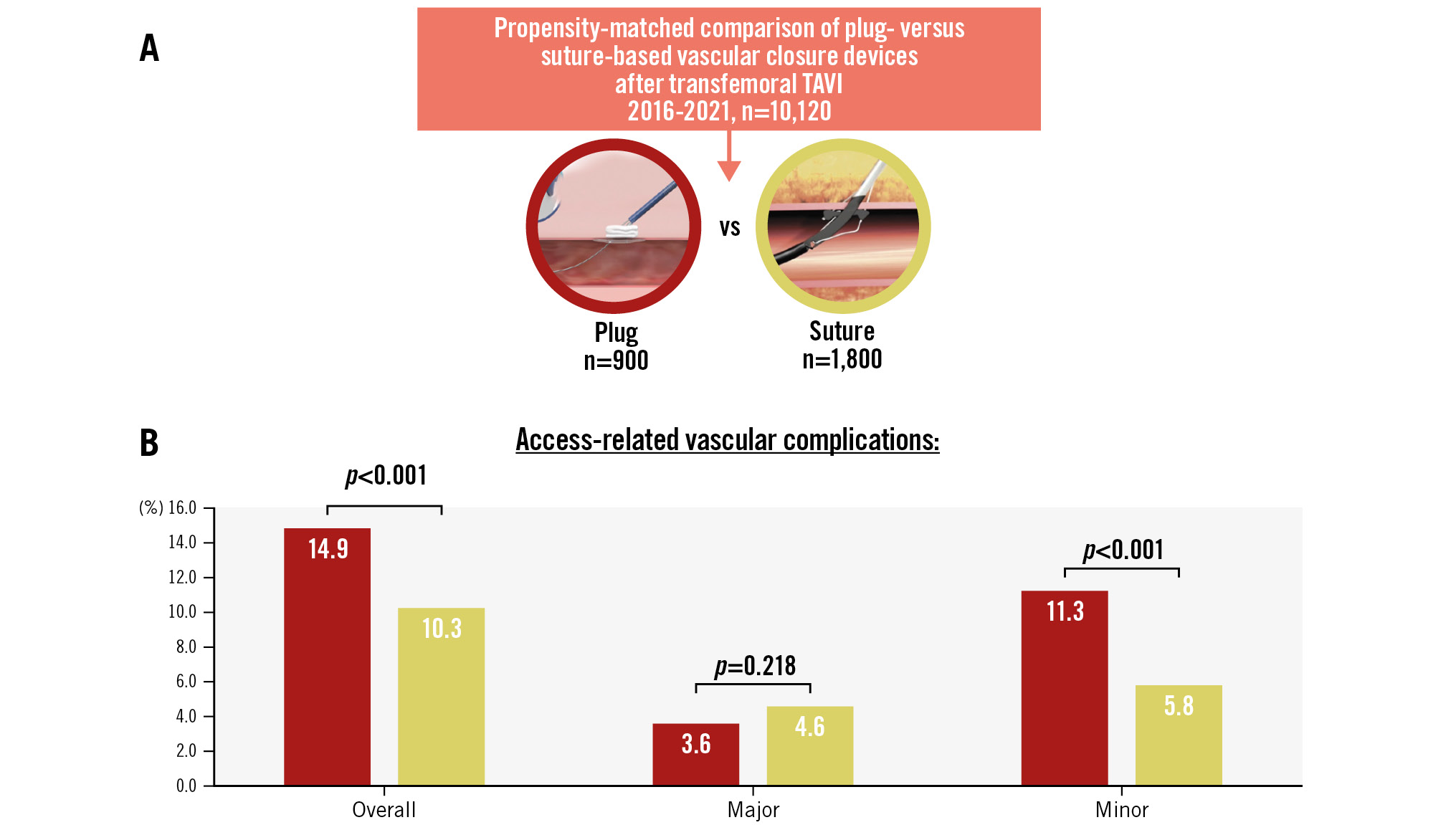
Central illustration. Access-related vascular complications at the primary access site. A) From an initial cohort of 10,120 patients, propensity matching resulted in 900 patients with P-VCD and 1,800 with S-VCD. B) Study results for access-related vascular complications at the primary access site. Reproduced with permission from Teleflex Medical GmbH, Fellbach, Germany (A) and Abbott Vascular International BVBA, Diegem, Belgium. (B). P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device: TAVI: transcatheter aortic valve implantation
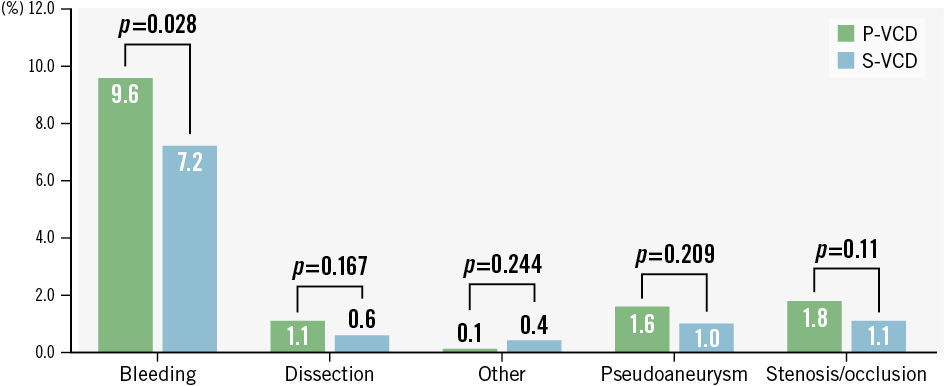
Figure 3. Types of access-related vascular complications at the primary access site. P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device
Table 3. Thirty-day outcomes after PS matching.
| All (N=2,700) | P-VCD (N=900) | S-VCD (N=1,800) | Odds ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Access-related vascular complications (primary large-bore access) | |||||
| All | 320 (11.9) | 134 (14.9) | 186 (10.3) | 1.52 (1.2, 1.92) | <0.001* |
| Major | 114 (4.2) | 32 (3.6) | 82 (4.6) | 0.77 (0.51, 1.16) | 0.218 |
| Minor | 206 (7.6) | 102 (11.3) | 104 (5.8) | 2.08 (1.58, 2.76) | <0.001* |
| Type | |||||
| Bleeding | 215 (8.0) | 86 (9.6) | 129 (7.2) | 1.38 (1.04, 1.84) | 0.028* |
| Dissection | 21 (0.8) | 10 (1.1) | 11 (0.6) | 1.84 (0.78, 4.37) | 0.167 |
| Pseudoaneurysm | 32 (1.2) | 14 (1.6) | 18 (1.0) | 1.58 (0.78, 3.2) | 0.209 |
| Stenosis/occlusion | 35 (1.3) | 16 (1.8) | 19 (1.1) | 1.71 (0.89, 3.29) | 0.11 |
| Other | 8 (0.3) | 1 (0.1) | 7 (0.4) | 0.29 (0.04, 2.34) | 0.244 |
| Treatment | |||||
| Conservative/manual compression | 86 (3.2) | 11 (1.2) | 75 (4.2) | 0.29 (0.15, 0.54) | <0.001* |
| Prolonged endovascular balloon inflation | 94 (3.5) | 48 (5.4) | 46 (2.6) | 2.18 (1.42, 3.33) | <0.001* |
| Stent implantation | 55 (2.1) | 42 (4.7) | 13 (0.7) | 6.81 (3.75, 12.38) | <0.001* |
| Surgical repair | 37 (1.4) | 6 (0.7) | 31 (1.7) | 0.39 (0.16, 0.91) | 0.03* |
| Other | 30 (1.1) | 14 (1.6) | 16 (0.9) | 1.78 (0.86, 3.69) | 0.119 |
| VCD failure | 177 (6.7) | 94 (10.8) | 83 (4.7) | 2.45 (1.8, 3.33) | <0.001* |
| Access-related vascular complications (secondary access) | |||||
| All | 47 (1.7) | 3 (0.3) | 44 (2.4) | 0.13 (0.04, 0.43) | <0.001* |
| Major | 18 (0.7) | 1 (0.1) | 17 (0.9) | 0.12 (0.02, 0.88) | 0.037* |
| Minor | 29 (1.1) | 2 (0.2) | 27 (1.5) | 0.15 (0.03, 0.62) | 0.009* |
| Access-related non-vascular complications | 14 (0.6) | 7 (0.9) | 7 (0.4) | 2.44 (0.85, 7.02) | 0.097 |
| Bleeding (Type 3/4) | 88 (3.3) | 19 (2.1) | 69 (3.8) | 0.54 (0.32, 0.91) | 0.02* |
| Stroke (disabling & non-disabling) | 67 (2.5) | 18 (2.0) | 49 (2.7) | 0.73 (0.43, 1.25) | 0.25 |
| Severe acute kidney injury (AKIN III and AKIN IV) | 60 (2.5) | 23 (2.7) | 37 (2.3) | 1.25 (0.73, 2.14) | 0.418 |
| Myocardial infarction | 9 (0.3) | 2 (0.2) | 7 (0.4) | 0.57 (0.12, 2.76) | 0.485 |
| Permanent pacemaker implantation | 343 (12.7) | 96 (10.7) | 247 (13.7) | 0.75 (0.59, 0.96) | 0.023* |
| Length of in-hospital stay, days | 7.0 (5.0, 8.0) | 6.0 (5.0, 8.0) | 7.0 (5.0, 9.0) | <0.001* | |
| All-cause death | 120 (4.7) | 42 (5.2) | 78 (4.5) | 1.19 (0.81, 1.73) | 0.376 |
| Binary variables are shown as absolute numbers (percentages). Continuous variables are shown as median (interquartile range). OR (95% CI) and p-values were calculated using logistic regression with each variable as an outcome and treatment as a predictor. *Indicates statistical significance. AKIN: Acute Kidney Injury Network; CI: confidence interval; OR: odds ratio; PS: propensity score; P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device; VCD: vascular closure device | |||||
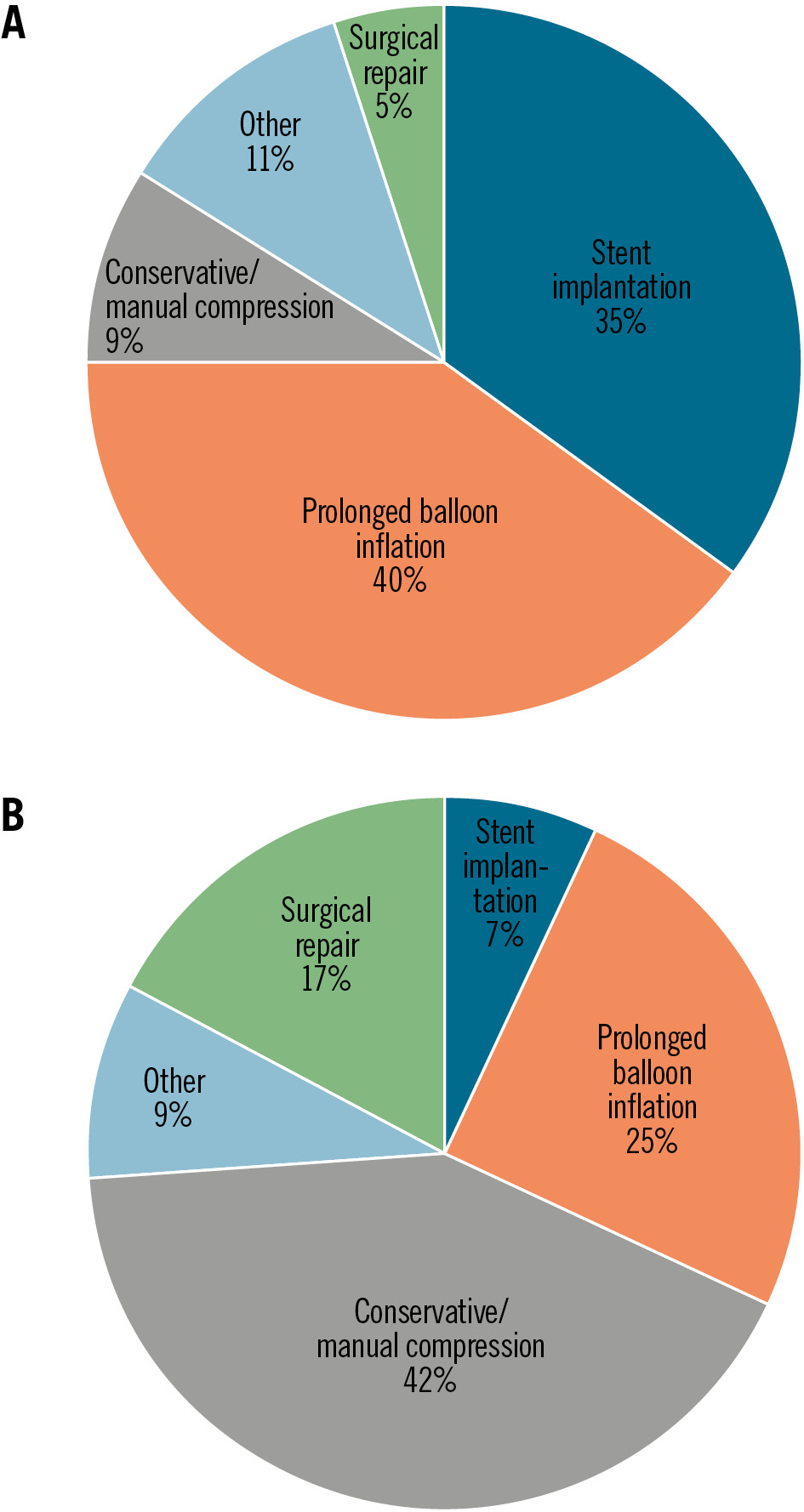
Figure 4. Treatment of access-related vascular complications at the primary TAVI access site. P-VCD (A) and S-VCD (B) groups. P-VCD: plug-based vascular closure device; S-VCD: suture-based vascular closure device; TAVI: transcatheter aortic valve implantation
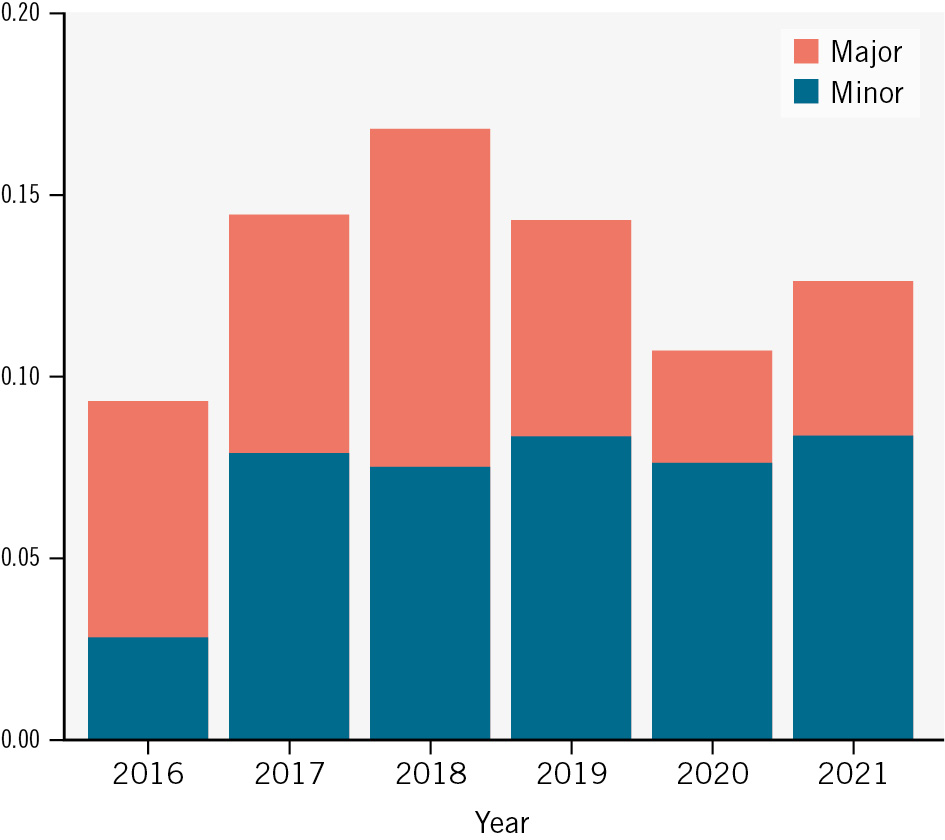
Figure 5. Temporal trend of vascular complications in the PULSE registry. A significant reduction of major access-related vascular complications at the primary TAVI access was observed in the unmatched PULSE registry from 2016 to 2021 (p<0.001 according to the Cochran-Armitage test with Bonferroni-corrected p-value). PULSE: Plug or sUture based vascuLar cloSurE after TAVI; TAVI: transcatheter aortic valve implantation
Discussion
We investigated the results of different vascular closure strategies after TAVI in the large contemporary multicentre PULSE registry according to the updated VARC-3 criteria. Our main findings were the following: (i) a P-VCD strategy was associated with higher rates of (minor) vascular access site complications compared to an S-VCD strategy at the primary access site, (ii) endovascular treatment (e.g., prolonged balloon inflation or stent implantation) was required more often as bailout therapy for complication management in P-VCD patients, and (iii) vascular complications related to the secondary access led to a relevant additional number of events irrespective of the primary VCD strategy.
Despite significant improvements in THV devices and the technical aspects of TAVI procedures, vascular access remains a relevant source of complications in TAVI, and these complications are linked to increased morbidity and mortality20. This emphasises the need for further refinement of vascular access and closure strategies to improve outcomes. Several devices and strategies are currently in use for percutaneous closure of a large-bore access site. While initial retrospective studies suggested favourable outcomes with lower vascular complication rates for P-VCD, two recently published randomised trials demonstrated either comparable results or lower complication rates with S-VCD8910112122. Selection bias, different endpoint definitions (e.g., primary large-bore access vs all vascular complications), follow-up strategies (e.g., routine ultrasound vs clinical follow-up) or learning curve aspects may have led to these conflicting results. Hence, our aim was to validate these findings in a large multicentre registry of 10 high-volume TAVI programmes in Germany. Consistent with the aforementioned randomised controlled trial, we found a higher incidence of primary access site-related vascular complications with P-VCD compared to S-VCD, particularly due to more minor complications. In line with previous publications, bleeding at the large-bore access site was the main driver of events in both groups. On the contrary, we did not find an increased incidence of pseudoaneurysms, which may have been due to the clinical as opposed to systematic ultrasound-based follow-up1011. Differences in procedure duration, fluoroscopy time, contrast agent used, and length of in-hospital stay are most likely due to centre-specific approaches and not caused by the different closure techniques.
Something that discriminates between the strategies is that the P-VCD is a “single-shot” device with limited bailout options after implantation compared to the S-VCD. Accordingly, the mean time to vascular closure was shorter for this strategy, in agreement with previous results, and may reflect the device’s ease of use and prompt haemostasis in the majority of cases51011. However, in cases of access-related vascular complications, particularly residual bleeding due to insufficient P-VCD closure, endovascular bailout manoeuvres including prolonged endovascular balloon inflation or stent implantation were often required81011. In contrast, S-VCD allow for the implantation of additional S-VCD or P-VCD as part of the closure strategy before endovascular bailout therapies need to be considered81011. In the end, prolonged manual compression proved sufficient for most cases with vascular complications in the S-VCD group. Surgical repair at the large-bore access site was mostly performed when staged and was only necessary in a small number of patients in both groups, interestingly with a higher incidence after implantation of S-VCD. In summary, both closure strategies require a different armamentarium of bailout procedures that need to be considered when using these techniques.
Interestingly, primary access was mainly gained without ultrasound guidance in the PULSE registry, even though studies have demonstrated a substantial benefit of ultrasound-guided puncture to gain large-bore access232425. That being said, the observed rates of ultrasound guidance in the PULSE registry were consistent with previous publications and current practice1126. These aspects underline the importance of implementing standardised ultrasound-guided puncture techniques in TAVI procedures to further improve outcomes.
In addition to vascular complications at the large-bore primary access site, a relevant number of vascular complications occurred at the secondary access site. These accounted for almost 20% of all access-related vascular complications in our registry. Despite sufficient matching for radial and femoral secondary access, a higher incidence of vascular complications at the secondary access site was observed in S-VCD patients. The underlying aetiology for this finding remains unclear. However, these additional complications may also have led to more procedural bleeding complications in the S-VCD group. These results emphasise prior observations that the preferred secondary access in TAVI procedures should be radial instead of femoral in order to reduce vascular complications27. Whether different strategies of heparin antagonisation may have influenced these results will require further investigation. Elevated rates of permanent pacemaker implantation in S-VCD patients are likely linked to more frequent implantation of self-expanding valves in this group. The remaining VARC-3 outcomes were comparable among both groups in the PULSE registry, and we observed declining major access-related vascular complications over time28. Learning curve effects, which, as suggested by other studies, differ between P-VCD and S-VCD, could have impacted post-implantation results7222930.
Limitations
Despite the strengths of being a large multicentre registry, several limitations should be discussed. This was a retrospective analysis including different site-specific TAVI protocols to mirror clinical reality in a large sample to detect subtle differences. However, despite well-balanced propensity score matching, both groups differed regarding some aspects, such as different standards for the length of stay post-TAVI and different levels of experience with the studied VCD, which were not assessed. Also, overall low rates of ultrasound guidance, as well as other unknown confounders that may have been left out, could have influenced results. The retrospective evaluation and site-specific adjudication may have led to underreporting of events, even though complication rates were consistent with the current literature. In addition, we focused on vascular complications and short-term 30-day follow-up to evaluate the immediate procedural and periprocedural phases. Patient data were collected over a time span of 6 years, and temporal effects and learning curve aspects cannot be disregarded and may have influenced results.
Conclusions
In this large propensity-matched comparison of patients treated with transfemoral TAVI, P-VCD were associated with higher rates of vascular access site complications compared to S-VCD, primarily driven by minor vascular complications at the large-bore access site. Endovascular balloon inflation or stent implantation were used more commonly as bailout treatment after P-VCD failure. In both groups, secondary access site-related complications led to a relevant number of additional vascular complications. These aspects should be considered when selecting access and closure strategies for TAVI procedures.
Impact on daily practice
In this large, real-world, multicentre registry, a plug-based vascular closure strategy was associated with higher rates of primary access-related vascular complications compared to a suture-based approach, mostly driven by minor complications. Also, endovascular treatment was more common after plug-based vascular closure. In both groups, secondary access-related complications led to a relevant number of additional vascular complications. As access-related complications in transcatheter aortic valve implantation (TAVI) remain a concern and are frequently associated with vascular closure, and since data regarding vascular closure strategies continue to be inconsistent, our results suggest that application of suture-based compared to plug-based vascular closure could provide a beneficial outcome for patients undergoing transfemoral TAVI. Also, the frequency of secondary access complications should not be underestimated, and a transradial compared to a transfemoral approach could lead to a more favourable outcome.
Conflict of interest statement
W. Kim received personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences, and Shockwave Medical. M. Adam received consultancy or speaker fees from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic; and is a proctor for Abbott, JenaValve, and Medtronic. D. Braun received speaker fees from Abbott and Edwards Lifesciences. D. Leistner received speaker and advisory fees from Abbott. H. Dreger received proctoring, speaker and advisory fees from Abbott and Edwards Lifesciences; as well as research support from Abbott. L. Conradi received advisory fees from Abbott, Medtronic, and JenaValve; as well as consulting fees from Edwards Lifesciences, Boston Scientific, MicroPort, Pi-Cardia, and MicroInterventions. A Schäfer received speaker fees and/or travel support from Edwards Lifesciences, Abbott, and Boston Scientific - unrelated to the submitted work. S. Scholtz received speaker fees from Edwards Lifesciences - unrelated to the submitted work. R.S. von Bardeleben received consulting fees from Abbott. T.K. Rudolph received speaker and advisory fees from Abbott, Edwards Lifesciences, JenaValve, Boston Scientific, Medtronic, and Amgen. M. Seiffert received speaker or advisory fees from Abbott, Abiomed, Amgen, AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Daichii Sankyo, Edwards Lifesciences, Inari Medical, Medtronic, Pfizer, Shockwave Medical, and Siemens Healthineers; and a research grant from Boston Scientific - all unrelated to the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

